Introduction
The oral tongue, comprising the dorsal, lateral and
ventral two-thirds anterior to the circumvallate papillae, is the
most commonly tumour affected site within the oral cavity and oral
tongue squamous cell carcinoma (OTSCC) is increasing in incidence
(1). Moreover, amongst all oral
subsites, OTSCC shows the most aggressive behaviour and poor
prognosis (2,3). Despite intense research, no improvement
in survival has been seen for patients with OTSCC in recent years.
New knowledge on this tumour is thus of utmost importance. A
complicating factor of large multicentric studies is the ethnic
difference seen between patients with oral squamous cell carcinoma
(OSCC) (4). To explore this in OTSCC,
we analysed groups of patients from two different geographical
locations; one from Sweden in Northern Europe and another from
Italy in Southern Europe. We analysed E-cadherin, β-catenin and
cytokeratins 5 and 19 in 120 OTSCCs from the two geographical
locations to investigate tumour epithelial phenotypes in
correlation to patient outcomes.
The epithelial calcium dependent adhesion molecule
E-cadherin is associated with squamous differentiation in squamous
cell carcinoma (SCC) (5) and oral SCC
(OSCC), where low levels associate with poor prognosis (6), metastasis (7) and local recurrence (8). E-cadherin is a commonly used marker of
epithelial cell differentiation and is expressed at different
levels in individual SCCs. E-cadherin is involved in cell adhesion,
being anchored to the cytoskeleton via β-catenin, a cytoplasmic
plaque protein that maintains cell-cell adhesion in the normal oral
squamous epithelium. Cytoplasmic β-catenin correlates with advanced
stages and poor differentiation in OTSCC (9). Cytokeratins (CK) are also used as
markers of epithelial differentiation and are variably expressed in
SCC. CKs are intermediate filament proteins that act in specific
pair-wise combinations depending on epithelial type and degree of
differentiation (10). CK5 and CK19
are expressed by basal epithelial cells (11). CK5 is paired with CK14 in squamous
epithelium and CK19 can be seen both in basal squamous cells and
simple epithelial cells (10). CK5 is
helpful in detection of cervical micro-metastases in head and neck
cancer tissue (12). CK5 is almost
ubiquitously expressed in head and neck SCC (HNSCC), whereas CK19
is more frequently expressed in tumours from pharynx and larynx
(10) and also correlates with poor
prognosis in OTSCC (13).
Materials and methods
Patients
Formalin fixed and paraffin-embedded (FFPE) biopsies
from 87 consecutive patients with primary OTSCC available at
Clinical Pathology, Umeå University Hospital, Sweden, and 33
patients at Dipartimento Universitario di Anatomia Patologica,
Second University of Naples, Italy were included in the study. All
tumours were derived from the mobile tongue. All Swedish patients
belonged to the Scandinavian ethno-geographical area and all
Italian patients to the South-Italian ethno-geographical area. Of
the 120 patients, 60 were men and 60 women with a mean age of 63.3
years, ranging from 19–93 years. Of all tumours, 68% were localised
on the lateral border of the oral tongue, 19% on the ventral side
and 3% on the dorsal side. Lesions were too widespread to state the
location in 10% of the patients. Most of the Swedish patients (54%)
were treated with radiotherapy followed by surgery, whereas 64%
Italian patients were treated by surgery only (Table I). The majority of tumours (109) had
previously been analysed for HPV16, p16 and podoplanin (14,15). The
mean follow-up time was 47 months (range 1–179 months). Data on
survival and cause of death were obtained from the clinical files.
The study was performed retrospectively on surplus tissues after
diagnosis. The use of redundant tissues for this study was approved
by the local Ethical Committee (dnr 01–057 and 03–201). All patient
data were anonymised and the study was performed in accordance with
European Union regulations and the Declaration of Helsinki. For
clinical information and hospital location see Table I.
 | Table I.Clinical data in relation to
ethnicity. |
Table I.
Clinical data in relation to
ethnicity.
|
| No. of patients
(%) |
|---|
|
|
|
|---|
| Characteristics | Swedish | Italian | Total |
|---|
| Sex |
| Male | 43 (49) | 17 (52) | 60 (100) |
|
Female | 44 (51) | 16 (48) | 60 (100) |
| Age, years |
| ≤40 | 14 (16) | 2 (6) | 16 (13) |
|
41–65 | 34 (39) | 11 (33) | 45 (38) |
|
>65 | 39 (45) | 20 (61) | 59 (49) |
| T1/T2 | 56 (64) | 24 (73) | 80 (67) |
| N+ | 19 (22) | 13 (39) | 32 (27) |
| Survival |
|
2-year | 46/87 (53) | 24/26 (92) | 113 (94) |
|
5-year | 35/80 (44) | 11/13 (85) | 93 (78) |
| Treatment |
| RT
followed by surgery | 47 (54) | 1 (3) | 48 (40) |
| RT
only | 17 (20) | 7 (21) | 24 (20) |
| Surgery
followed by RT | 8 (9) | 4 (12) | 12 (10) |
| Surgery
only | 12 (14) | 21 (64) | 33 (28) |
|
None | 3 (3) | 0 (0) | 3 (2) |
| Total no. | 87 | 33 | 120 |
At the end of the study, 54% of patients were cancer
free, either alive disease free (ADF) or disease free but dead from
another cause (DDF). The remaining 46% were still affected by
cancer, dead of disease (DOD), alive with disease (AWD) or dead
with disease (DWD) but from a cause other than their OTSCC. Of
these latter patients, 44% showed tumour relapse. Two years after
treatment (available for 113 patients, 94%) 70 were alive and 43
dead, and after five years (available for 93 patients, 82%) 46 were
alive and 47 dead (Table I).
Immunohistochemistry
Sections were pretreated in CC1-buffer (Cell
Conditioner 1; Ventana Medical Systems, Inc., Tucson, AZ, USA) at
95°C for 36 min (E-cadherin, β-catenin), at 95°C for 64 min (CK19)
and at 100°C for 36 min (CK5). Slides were then incubated with
primary antibodies diluted in Ventana antibody diluent for 32 min
at 36°C and detected using Ultra View Universal DAB Detection kit
using a Bench Mark Ultra (Ventana Medical Systems, Inc.). For
slides stained with CK5 an extra step adding an Opti View HQ Linker
(Ventana Medical Systems, Inc.) was added before detection. Slides
were counterstained with Hematoxylin and Bluing Reagent (Ventana
Medical Systems, Inc.). The antibody against E-cadherin (M3612,
DAKO; Agilent Technologies, Inc., Santa Clara, CA, USA) was diluted
1:25, anti-β-catenin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) 1:1,500, anti-CK-5 (Novocastra; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA) 1:100 and anti-CK-19 (M0888, DAKO; Agilent
Technologies, Inc.) 1:50.
Scoring
The Quick Score (QS) method was used to assess the
overall levels of staining for each antibody. Staining was
evaluated by combining the proportion of positive tumour cells
(1=0–4%, 2=5–19%, 3=20–39%, 4=40–59%, 5=60–79% and 6=80–100%) with
intensity of staining (0=negative, 1=weak, 2=intermediate and
3=strong). The final QS was achieved by multiplying these two
scores, ranging between 0–18 (16).
LB and KN scored E-cadherin and β-catenin, 88 samples stained for
CK5 and CK19 were scored by LL, PH and KN and the remaining 32
samples by KN alone.
Statistical analysis
Tumours were grouped according to geographical
distribution and level of immunostaining, where low/medium and high
tumours were defined as a QS of 0–10 and 12–18 respectively. SPSS
v.24 (IBM Corp., Armonk, NY, USA) was used for statistical
analyses. Pearson chi-squared test was used to calculate P-values.
Kaplan-Meier curves were plotted to perform survival analysis and
differences among groups was explored with Log Rank (Mantel-Cox)
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical data
Data on two-year survival were available for all 87
Swedish and 26 of the 33 (79%) Italian patients, where the latter
group showed a better 2-year survival (P=0.0005).
Immunohistochemistry
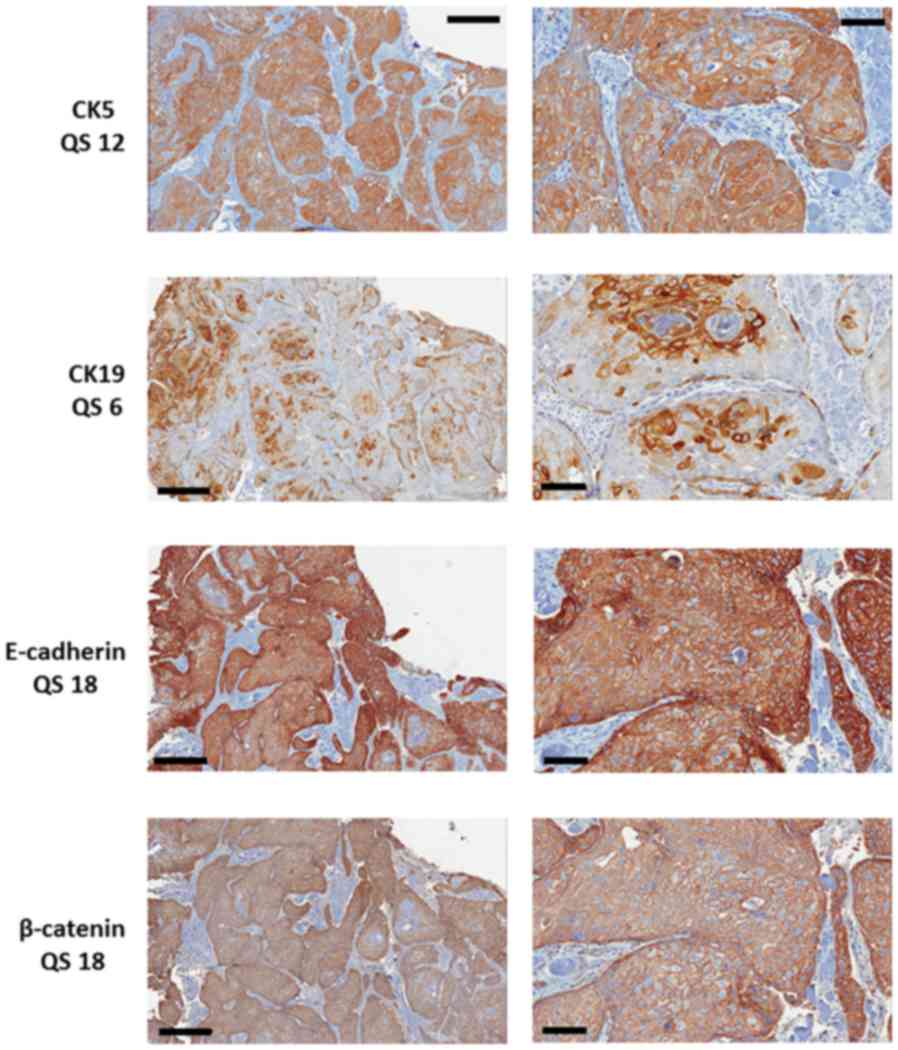
All cases were stained for E-cadherin, β-catenin,
CK5 and CK19 (Fig. 1). Positive
staining for E-cadherin and β-catenin was seen in 118 of the 120
cases. All 120 cases expressed CK5, whereas only 76 (63%) contained
CK19 positive tumour cells.
To study potential differences in markers based on
ethnicity, patients were sub-divided into Italian and Swedish
origins. Patients in the Swedish cohort showed a higher proportion
of high E-cadherin tumours (QS 12–18) than Italian patients
(P=0.039; Table II).
 | Table II.Expression levels of E-cadherin,
β-catenin, CK5 and CK19 in relation to ethnicity. |
Table II.
Expression levels of E-cadherin,
β-catenin, CK5 and CK19 in relation to ethnicity.
|
| No. of patients
(%) |
|---|
|
|
|
|---|
| Expression | Swedish, (%) | Italian, (%) | P-value |
|---|
| E-cadherin |
| QS
0–10 | 54 (62) | 27 (82) | 0.039 |
| QS
12–18 | 33 (38) | 6 (18) |
|
| β-catenin |
| QS
0–10 | 70 (80) | 31 (94) | 0.071 |
| QS
12–18 | 17 (20) | 2 (6) |
|
| CK5 |
| QS
0–10 | 48 (55) | 22 (67) | 0.254 |
| QS
12–18 | 39 (45) | 11 (33) |
|
| CK19 |
| QS
0–10 | 78 (90) | 31 (94) | 0.468 |
| QS
12–18 | 9 (10) | 2 (6) |
|
| Total no. | 87 | 33 | 120 |
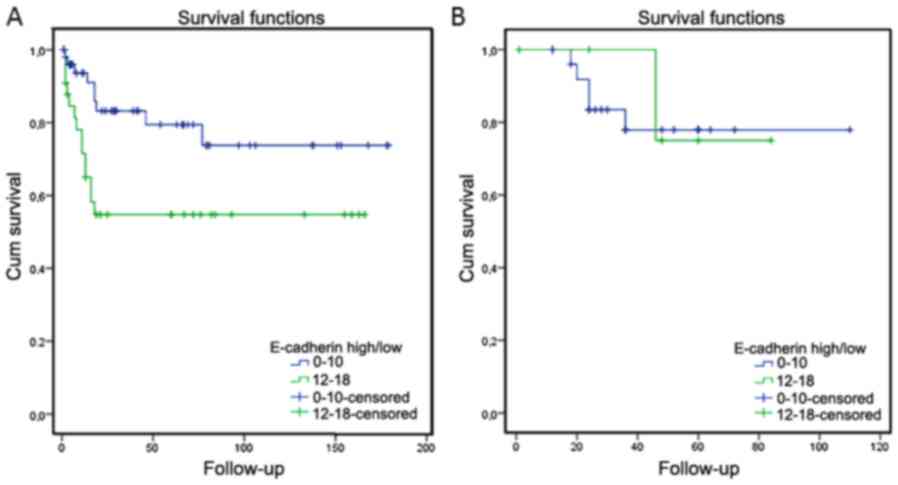
In Swedish patients that had died of their disease
(23 patients) high levels of E-cadherin correlated with poor
survival (P=0.016), whereas no correlation was seen for the six
Italian patients that were dead of their disease (P=0.842; Fig. 2). Of the 23 Swedish patients that died
of disease, 17 (74%) had received preoperative radiotherapy (RT)
and surgery was then performed on 10 of these; two had received
post operative RT and four no RT. Of these latter four, two had
received surgery and two no treatment at all. For the six Italian
patients, five had received RT only and one also post operative
surgery No correlation with survival was seen for β-catenin, CK5 or
CK19 in either group of patients.
Discussion
Recent advances in diagnosis, surgical management
and chemoradiotherapy regimens have only minimally improved the
five-year survival for patients with OSCC (17). The present results contribute the
important point that E-cadherin levels vary according to
ethno-geographical area.
E-cadherin plays a key role in establishing and
maintaining intercellular connections and is the main protein of
adherens junctions anchoring oral epithelial cells to each other.
Dysfunctional E-cadherin-mediated cell adhesion is associated with
cancer invasion and metastasis. Many immunohistochemical studies
have shown aberrant E-cadherin expression in SCC of the head and
neck (HNSCC), and downregulation of E-cadherin has been reported to
indicate poor prognosis in OTSCC (18). Those results contrast with our data
showing that higher expression of E-cadherin correlates with poor
disease-free survival. However, the results from these two studies
are not directly comparable as not only different antibodies were
used, which is known to affect results (19), but also different methods to evaluate
staining. On the other hand, our results are in concordance with a
recent study of laryngeal SCC, using a similar analysis with
calculation of percent as well as staining intensity of E-cadherin
positive tumour cells (20).
Variability in the previously published results of E-cadherin in
HNSCC, OSCC and OTSCC probably also depend on sample size, sample
types included and their geographical location.
In the present study, we investigated patients from
Sweden and Italy to examine the potential geographic variation in
phenotype and phenotype-related clinical outcome in OTSCC patients.
Better survival was seen in Italian patients, even though more of
the Italian patients had nodal metastasis at diagnosis (39% vs. 22%
of the Swedish patients). As survival is influenced by treatment
and most Swedish patients had received neo-adjuvant radiotherapy in
contrast to the Italian group, no conclusions can be drawn from the
difference in survival data seen in this study. Nonetheless,
differences in protein expression between Swedish and Italian
patients show biological variation between tumours in different
patient populations. Whilst we have not studied the underlying
reasons for these variations, there are some obvious potential
factors that may influence tumour phenotypes between Sweden and
Italy, including the effects of different diets, where the
Mediterranean diet typical for our Italian cohort is known to
influence the incidence and nature of oral cancer (21,22). An
alternative and non-exclusive factor would be the use of different
tobacco products, where snus usage is common in Sweden and
influences the oral microbiome (23,24), known
to be important for OTSCC (25).
In summary, the present study shows that levels of
E-cadherin vary between patients based on ethno-geographical
distribution. This finding can help explain the inconsistencies
seen in studies from different parts of the World that often use
the same markers as surrogates for cancer cell phenotypes and their
association with clinical outcome. Further studies are required to
explain the reasons for the different phenotypes of OTSCC in
Northern and Southern Europe, but, similar to other worldwide
geographical cancer variations, factors including diet and
lifestyle such as smoking habits are prime candidates to account
for the differences we have observed (4).
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
Cancer Research Foundation in Northern Sweden, The Swedish Cancer
Society (grant no. 17 06 63), Västerbotten County Council, Umeå
University (grant no. MEYS-NPSI-LO1413) and The Grant Agency of the
Czech Republic (grant no. P206/12/G151).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon request.
Authors' contributions
NS, TW, PJC, LL, RoF, LC, LLM, LNS, ReF. GC, KD, KN
designed the experiments, NS, TW, LB, LL, XG, PH, PJC, RoF, GT, FA,
GC, KN performed data analysis. NS, TW, PJC, LB, LL, XG, PH, LC,
LLM, RoF LNS, ReF, GT, GC, MS, GDO, FC, KD, GT, FA, KN interpreted
the data, wrote and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The project was approved by the local Ethical
Committee (dnr 01-057 and 03-201) and the use of surplus archived
tissue after diagnosis was granted by the Ethical Committee,
waiving the requirement for informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bello IO, Soini Y and Salo T: Prognostic
evaluation of oral tongue cancer: Means, markers and perspectives
(I). Oral Oncol. 46:630–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bello IO, Soini Y and Salo T: Prognostic
evaluation of oral tongue cancer: Means, markers and perspectives
(II). Oral Oncol. 46:636–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scully C and Bedi R: Ethnicity and oral
cancer. Lancet Oncol. 1:37–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu H, Lotan R, Menter D, Lippman SM and Xu
XC: Expression of E-cadherin is associated with squamous
differentiation in squamous cell carcinomas. Anticancer Res.
20:1385–1390. 2000.PubMed/NCBI
|
|
6
|
Foschini MP, Leonardi E, Eusebi LH,
Farnedi A, Poli T, Tarsitano A, Cocchi R, Marchetti C, Gentile L,
Sesenna E, et al: Podoplanin and E-cadherin expression in
preoperative incisional biopsies of oral squamous cell carcinoma is
related to lymph node metastases. Int J Surg Pathol. 21:133–141.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foschini MP, Cocchi R, Morandi L, Marucci
G, Pennesi MG, Righi A, Tosi AL, de Biase D, Pession A and
Montebugnoli L: E-cadherin loss and Delta Np73L expression in oral
squamous cell carcinomas showing aggressive behavior. Head Neck.
30:1475–1482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosch FX, Andl C, Abel U and Kartenbeck J:
E-cadherin is a selective and strongly dominant prognostic factor
in squamous cell carcinoma: A comparison of E-cadherin with
desmosomal components. Int J Cancer. 114:779–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang P, Cao HY, Bai LL, Li WN, Wang Y,
Chen SY, Zhang L, Yang LH, Xu HT and Wang EH: The high expression
of TC1 (C8orf4) was correlated with the expression of β-catenin and
cyclin D1 and the progression of squamous cell carcinomas of the
tongue. Tumour Biol. 36:7061–7067. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van der Velden LA, Schaafsma HE, Manni JJ,
Ramaekers FC and Kuijpers W: Cytokeratin expression in normal and
(pre)malignant head and neck epithelia: An overview. Head Neck.
15:133–146. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JM, Jung CK, Choi YJ, Lee KY, Kang
JH, Kim MS and Hu HJ: The use of an immunohistochemical diagnostic
panel to determine the primary site of cervical lymph node
metastases of occult squamous cell carcinoma. Hum Pathol.
41:431–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Becker MT, Shores CG, Yu KK and Yarbrough
WG: Molecular assay to detect metastatic head and neck squamous
cell carcinoma. Arch Otolaryngol Head Neck Surg. 130:21–27. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ernst J, Ikenberg K, Apel B, Schumann DM,
Huber G, Studer G, Rordorf T, Riesterer O, Rössle M, Korol D and
Bredell MG: Expression of CK19 is an independent predictor of
negative outcome for patients with squamous cell carcinoma of the
tongue. Oncotarget. 7:76151–76158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sgaramella N, Coates PJ, Strindlund K,
Loljung L, Colella G, Laurell G, Rossiello R, Muzio LL, Loizou C,
Tartaro G, et al: Expression of p16 in squamous cell carcinoma of
the mobile tongue is independent of HPV infection despite presence
of the HPV-receptor syndecan-1. Br J Cancer. 113:321–326. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sgaramella N, Jonsson Lindell E, Boldrup
L, Califano L, Coates PJ, Tartaro G, Lo Muzio L, Fåhraeus R,
Colella G, Orabona Dell'Aversana G, et al: High expression of
podoplanin in squamous cell carcinoma of the tongue occurs
predominantly in patients ≤40 years but does not correlate with
tumour spread. J Pathol Clin Res. 2:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Detre S, Jotti Saccani G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massano J, Regateiro FS, Januário G and
Ferreira A: Oral squamous cell carcinoma: Review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang HW, Chow V, Lam KY, Wei WI and Yuen
A: Loss of E-cadherin expression resulting from promoter
hypermethylation in oral tongue carcinoma and its prognostic
significance. Cancer. 94:386–392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strindlund K, Troiano G, Sgaramella N,
Coates PJ, Gu X, Boldrup L, Califano L, Fahraeus R, Muzio LL,
Ardito F, et al: Patients with high c-MYC-expressing squamous cell
carcinomas of the tongue show better survival than those with low-
and medium-expressing tumours. J Oral Pathol Med. 46:967–971.
2017.PubMed/NCBI
|
|
20
|
Greco A, de Virgilio A, Rizzo MI, Pandolfi
F, Rosati D and de Vincentiis M: The prognostic role of E-cadherin
and β-catenin overexpression in laryngeal squamous cell carcinoma.
Laryngoscope. 126:E148–E155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giraldi L, Panic N, Cadoni G, Boccia S and
Leoncini E: Association between Mediterranean diet and head and
neck cancer: Results of a large case-control study in Italy. Eur J
Cancer Prev. 26:418–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Filomeno M, Bosetti C, Garavello W, Levi
F, Galeone C, Negri E and La Vecchia C: The role of a Mediterranean
diet on the risk of oral and pharyngeal cancer. Br J Cancer.
111:981–986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee PN and Hamling J: Systematic review of
the relation between smokeless tobacco and cancer in Europe and
North America. BMC Med. 7:362009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Hebshi NN, Alharbi FA, Mahri M and Chen
T: Differences in the bacteriome of smokeless tobacco products with
different oral carcinogenicity: Compositional and predicted
functional analysis. Genes (Basel). 8:pii: E1062017. View Article : Google Scholar
|
|
25
|
Winn DM, Lee YC, Hashibe M and Boffetta P;
INHANCE consortium, : The INHANCE consortium: Toward a better
understanding of the causes and mechanisms of head and neck cancer.
Oral Dis. 21:685–693. 2015. View Article : Google Scholar : PubMed/NCBI
|