Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the leading causes of cancer-related mortality (1). In 2016, there were an estimated 53,000
new cases and 42,000 cases of cancer-related mortality in the
United States. The 5-year relative survival rate of PDAC is only 7%
(2). Due to the difficulty in
diagnosing PDAC at an early stage, >50% of patients present with
metastasis upon diagnosis (3). Thus,
investigating novel diagnostic markers and therapeutic targets for
PDAC is critical.
MircroRNAs (miRNAs/miRs) are small and endogenous
single-stranded RNA molecules. Previous studies revealed that
miRNAs could bind to the 3′untranslated region of their target
genes and regulate their post-transcriptional expression, which led
to targeting the mRNA translational repression or degradation of
genes (4,5). miRNAs have been shown to function as
tumor suppressors or oncogenes in the development and progression
of PDAC. Certain miRNAs, including miR-454 (6), miR-183-5p (7), miR-200a (8) and miR-138-5p (9), have been reported to affect tumor cell
proliferation, migration, invasion and metastasis.
miR-661 has been revealed to function as an oncogene
in certain tumors, including breast cancer (10), ovarian cancer (11) and non-small cell lung cancer (12). However, the role of miR-661 in PDAC
expression remains unknown. The present study aimed to reveal the
clinical significance and functional effects of miR-661 on PDAC.
The aim of the present study was to examine the expression of
miR-661 in PDAC tissues and determine the association between
miR-661 expression and poor prognosis of patients with PDAC.
Furthermore, the effects of ectopic expression of miR-661 on
proliferation of PDAC cells in vitro and the Wnt signalling
pathway were examined.
Materials and methods
Tissue samples and cell lines
PDAC tissues and corresponding adjacent normal
ductal epithelial tissues were obtained following surgery from 59
patients included 39 men and 20 women, aged 34–82 years old, with a
mean age of 53.5 years old. following pancreaticoduodenal resection
at Zhujiang Hospital of Southern Medical University (Guangzhou,
China) between June 2008 and July 2012. The clinical data of the
patients is summarized in Table I.
The study was approved by the Committees for the Ethical Review of
Research at Zhujiang Hospital of Southern Medical University, and
written informed consent was obtained from all patients. All
patients had not received any treatment prior to surgery. The fresh
tissues obtained during surgery were stored at −80°C for further
analysis.
 | Table I.Association between miR-661 expression
and clinical factors. |
Table I.
Association between miR-661 expression
and clinical factors.
|
|
| miR-661 expression
levels |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Patients (n=59) | Lower (n=27) | Higher (n=32) | P-value |
|---|
| Sex |
|
|
| 0.933 |
| Male | 39 | 18 | 21 |
|
|
Female | 20 | 9 | 11 |
|
| Age, years |
|
|
| 0.296 |
| ≤60 | 37 | 15 | 22 |
|
|
>60 | 22 | 12 | 10 |
|
| Differentiation |
|
|
| 0.465 |
| Well and
moderate | 40 | 17 | 23 |
|
| Poor | 19 | 10 | 9 |
|
| Lymph node
metastasis |
|
|
| 0.039a |
|
Negative | 40 | 22 | 18 |
|
|
Positive | 19 | 5 | 14 |
|
| Pathological T
stage |
|
|
| 0.034a |
| T1,
T2 | 20 | 13 | 7 |
|
| T3,
T4 | 39 | 14 | 25 |
|
| Tumor location |
|
|
| 0.942 |
| Head | 29 | 12 | 17 |
|
| Uncinate
process | 16 | 7 | 9 |
|
| Body and
tail | 14 | 8 | 6 |
|
| Tumor size, cm |
|
|
| 0.612 |
| ≤4 | 36 | 16 | 20 |
|
|
>4 | 23 | 11 | 12 |
|
The PDAC ASPC-1, PANC-1 and MIA PaCa-2 cell lines,
and one human pancreatic duct epithelial HPDE6 cell line, were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified 5% CO2 atmosphere at
37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and all cell
lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The RNA
concentrations were determined with a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) at 260 and 280 nm (A260/280). The cDNA
was reverse transcribed using the TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The RT-qPCR was performed using a TaqMan miRNA assay on an
ABI7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The thermocycling
conditions were as follows: Denaturation at 95°C for 5 min followed
by 35 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. The relative mRNA
expression was determined using the comparative 2−ΔΔCq
method (13). The primer sequence for
miR-661 was as follows: miR-661 forward: 5′-GTGCCTGGGTCTCTGGCCT-3′.
U6 forward: 5′-CTCGCTTCGGCAGCACA-3′ and U6 reverse:
5′-AACGCTTCACGAATTTGCGT-3′. The mRNA expression was normalized to
U6.
Cell transfection
ASPC-1 and PANC-1 cells were transfected with 100 nM
miRNA-negative control (miR-NC), miR-661 mimic (100 nM) or miR-661
inhibitor (100 nM) (Chang Jing Bio-Tech, Ltd., Changsha, China)
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Following cell transfection for 48 h, the
cells were harvested for RT-qPCR or western blot analysis to assess
the mRNA and protein expression.
Cell proliferation assay
A cell proliferation assay was performed using Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen,
China). Briefly, a 96-well plate was seeded with ASPC-1 and PANC-1
cells (1×103 cells per well) and incubated for 24 h.
Cells were transfected with miR-NC, miR-663 mimic or miR-663
inhibitor as aforementioned. Cell counting kit solution (10 µl) was
added to each well according to the manufacturer's protocol, and
cell proliferation was detected at 0, 1, 2 and 3 days. The cell
proliferation was measured using a microplate spectrophotometer
(Molecular Devices, LLC, Sunnyvale, CA, USA) at 450 nm.
Cell colony assay
ASPC-1 and PANC-1 cells (500 cells per well) were
seeded in 6-well plates and cultured for 7 days. Cell colonies were
fixed in 100% methanol at room temperature for 24 h and
subsequently stained with 1% crystal violet at room temperature for
1 h under a light microscope and the magnification was ×200. All
experiments were performed in triplicate.
Western blot analysis
Transfected cells were lysed in
radioimmunoprecipitation assay buffer (Pierce; Thermo Fisher
Scientfic, Inc.). The concentration of protein was detected by
Bradford assay protein quantitation kit (Abcam, Cambridge, UK).
Equal quantities of protein (30 µg) were separated on 10% SDS-PAGE
gels and transferred to polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were
blocked with 5% skimmed milk in TBS/0.1% Tween (TBST) for 1 h at
room temperature. The membranes were probed with primary antibodies
against cyclin D1 (catalog no. sc-8396; 1:500), β-catenin (catalog
no. sc-376841; 1:1,000; Santa Cruz Biotechnology) and transcription
factor 4 (TCF4; catalog no. sc-166699, 1:500) and GAPDH (catalog
no. sc-69778; 1:1,000) (all Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at 4°C overnight. The membranes were incubated with
horseradish peroxidase (HRP)-conjugated bovine, anti-mouse
secondary antibody (catalog no. sc-2380, 1:500, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
The blots were detected using an enhanced chemiluminescence system
(GE Healthcare, Chicago, IL, USA) and analysed with Image Lab
software (version 3.0; Bio-Rad Laboratories, Inc.). GAPDH was used
as an internal control for protein expression.
Statistical analysis
Statistical analyses were performed using SPSS
statistical software 18.0 (IBM, Corp., Armonk, NY, USA). Data are
presented as the mean ± standard deviation from at least three
independent experiments. Difference between two groups or multiple
groups were evaluated by Student's t-test or one-way analysis of
variance with Student-Newman-Keuls (SNK) test as the post hoc test,
respectively. To validate the association between miR-661
expression and clinical factors, the χ2 test was
performed. Kaplan-Meier survival analysis and a log rank test was
used to examine the survival plots for disease-free survival rate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-661 expression is upregulated in
PDAC tissues and is associated with a poor survival rate
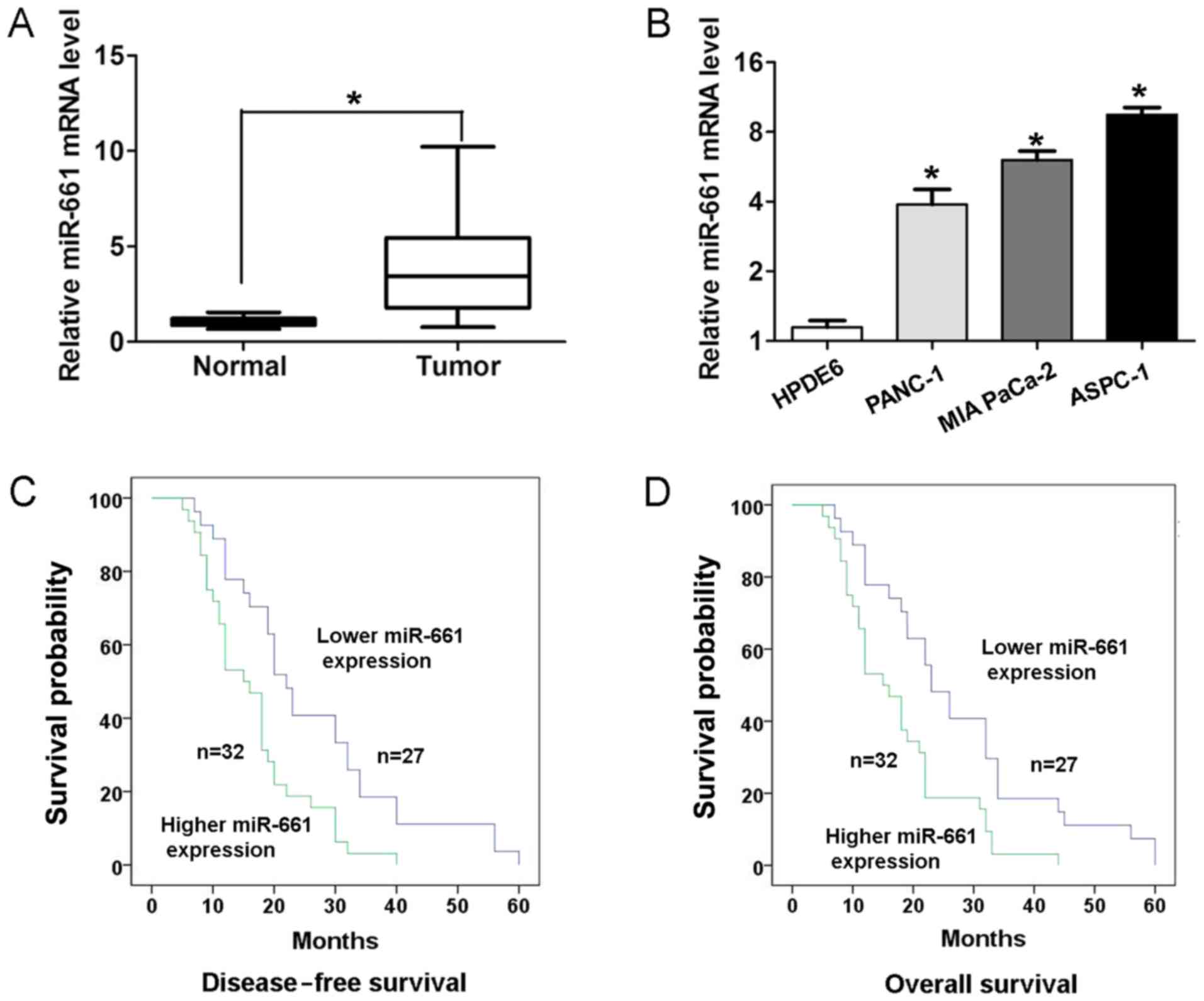
To determine the expression of miR-661 expression in
PDAC tissues and adjacent normal tissues, RT-qPCR analyses were
performed. As presented in Fig. 1A,
PDAC tissues exhibited higher expression of miR-661 compared with
adjacent normal tissues (P<0.05). In addition, the expression of
miR-661 was higher in three human PDAC ASPC-1, PANC-1 and MIA
PaCa-2 cell lines compared with that in the human pancreatic duct
epithelial HPDE6 cell line (Fig. 1B).
To validate the association between miR-661 expression and clinical
factors, the χ2 test was performed. Results revealed
that higher miR-661 expression demonstrated a positive association
with lymph node metastasis (P<0.05; Table I) and advanced pathological T stage
(P<0.05; Table I) in patients with
PDAC. Kaplan-Meier survival analysis revealed that the patients
with higher miR-661 expression had a significantly worse
disease-free survival rate (log-rank, 9.388; P<0.05; Fig. 1C) and overall survival rate (log-rank,
9.629; P<0.05; Fig. 1D) than those
with lower miR-661 expression.
Upregulation of miR-661 promotes
proliferation of PDAC cell lines in vitro
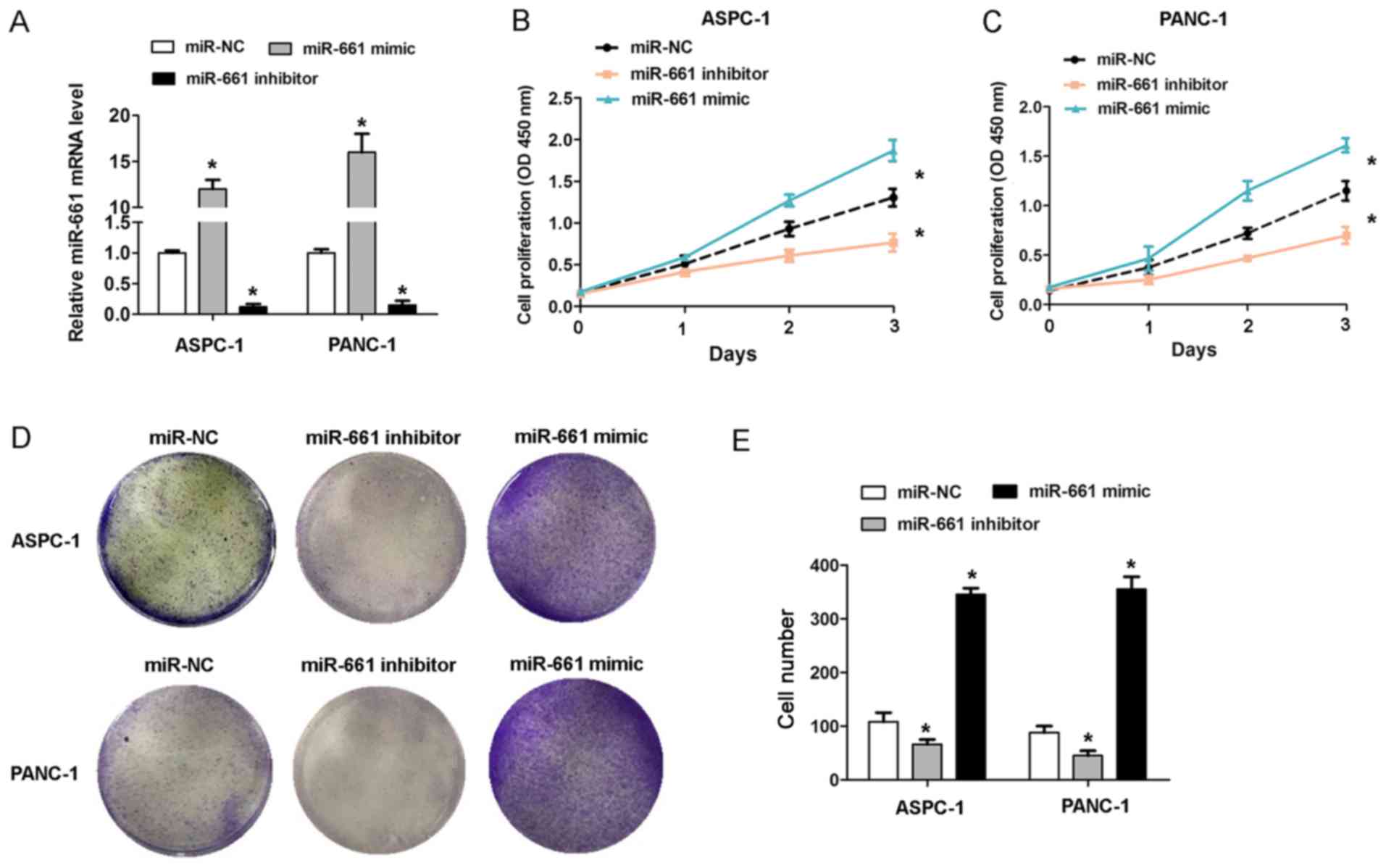
To investigate the effect of miR-661 expression on
PDAC cell growth, gain of function and loss of function assays were
performed in ASPC-1 and PANC-1 cells. The RT-qPCR analysis revealed
that the miR-661 mimic caused an increase in miR-661 expression,
while the miR-661 inhibitor caused a decrease in miR-661 expression
in the ASPC-1 and PANC-1 cells (Fig.
2A). The CCK-8 cell proliferation assays revealed that
transfection of miR-661 mimic promoted cell proliferation in ASPC-1
and PANC-1 cells, whereas miR-661 inhibitor inhibited cell
proliferation (Fig. 2B and C). The
results of cell colony formation assays revealed that transfection
of miR-661 mimic in ASPC-1 and PANC-1 cells caused an increased
cell colony number, whereas miR-661 inhibitor reduced the cell
colony number (Fig. 2D and E). These
results indicated that upregulation of miR-661 promoted the cell
proliferation capacity in vitro.
Upregulation of miR-661 activates the
Wnt signaling pathway in vitro
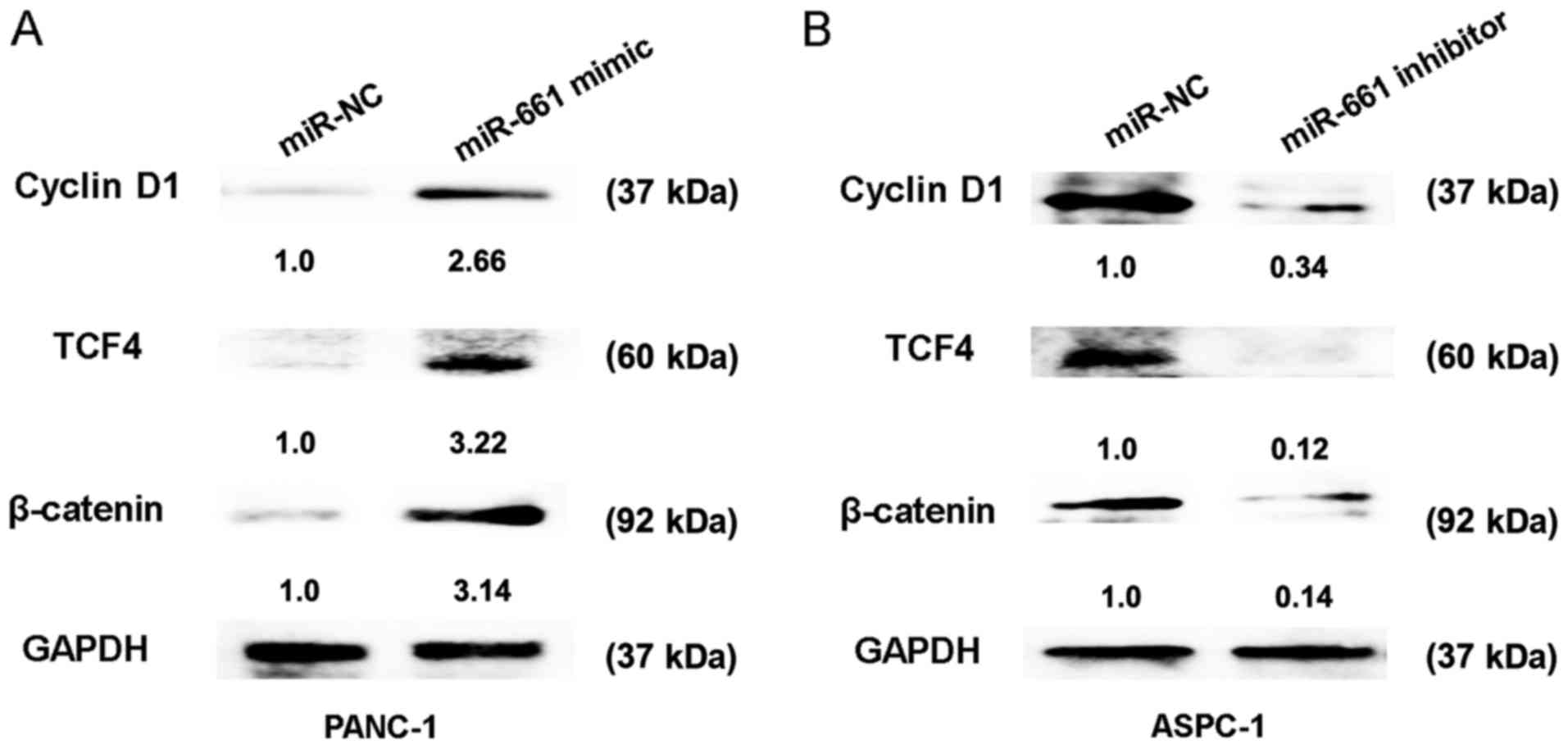
The Wnt signaling pathway has been identified as an
important regulating signalling pathway in PDAC (14). To further confirm the regulatory
association between miR-661 expression and the Wnt signaling
pathway, the relative protein expressions of cyclin D1, TCF4 and
β-catenin were detected. The results of the present study indicated
that transfection of miR-661 mimic in PANC-1 cells caused an
increase in protein expression of cyclin D1, TCF4 and β-catenin,
whereas miR-661 inhibitor reduced the protein expression of cyclin
D1, TCF4 and β-catenin in ASPC-1 cells (Fig. 3A and B). Thus, these results indicated
that upregulation of miR-661 could activate the Wnt signaling
pathway in vitro.
Discussion
Understanding the molecular mechanisms underlying
PDAC progression is important for investigating novel diagnostic
markers and treatment targets (15).
miRNAs have emerged as a class of gene regulators involved in PDAC.
For example, miR-545 inhibits PDAC growth by targeting retinoic
acid-inducible gene-I (16). miR-337
regulates proliferation and invasion in PDAC by targeting homeobox
protein B7 (HOXB7) (17), and miR-429
exhibited a poor outcome in patients with PDAC and inhibits PDAC
growth by targeting TANK-binding kinase 1 (18). However, the role of miR-661 in PDAC
progression remains unknown.
In the present study, it was revealed that miR-661
expression was significantly upregulated in PDAC tissues and cells.
Higher miR-661 expression revealed a positive association with
lymph node metastasis, an advanced T stage and a poor prognosis of
patients with PDAC. Furthermore, ectopic expression of miR-661
significantly promoted cell proliferation in PDAC cells in
vitro. These findings indicated that miR-661 may serve a
tumor-promoting role in PDAC. Lower expression of miR-661 has been
revealed in certain cancers in previous studies. For example,
miR-661 contributed to the proliferation of human ovarian cancer
cells by repressing inositol polyphosphate-5-phosphatase J
expression (11). miR-661 expression
in SNAI1-induced epithelial-to-mesenchymal transition contributes
to breast cancer cell invasion via targeting of nectin-1 and
starD10 messengers (10). miR-661
also promotes tumor cell invasion and metastasis by directly
inhibiting retinoblastoma protein in non-small cell lung cancer
(12). Thus, the results of the
present study indicated that miR-661 exhibited tumor proliferation
promoting effects in PDAC.
Furthermore, the present study also demonstrated
that transfection of the miR-661 mimic caused increased expression
of TCF4 and β-catenin, whereas miR-661 inhibitor reduced the
expression of TCF4 and β-catenin in PDAC cells. These results
indicated that upregulation of miR-661 activated the Wnt signaling
pathway in vitro. In a previous study, Wnt signaling was
shown to serve a crucial role in pancreatic cancer progression;
activation of Wnt/β-Catenin signaling enhanced pancreatic cancer
development and the malignant potential via upregulation of
cysteine-rich angiogenic inducer 61 (19). The long non-coding RNA HOX transcript
antisense RNA affects the radiosensitivity of PDAC by regulating
the expression of Wnt inhibitory factor 1 (20). The present study revealed that
upregulation of miR-661 could activate the Wnt signaling pathway
in vitro, which revealed the importance of miR-661 in PDAC
progression.
In conclusion, the results of the present study
revealed that miR-661 expression was higher in PDAC tissues and
associated with prognosis. In vitro, miR-661 promoted cell
proliferation and activated Wnt signaling. These results indicated
that miR-661 may function as a prognostic biomaker and provide
insight for the treatment of PDAC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL, KZ and JY conceived and designed the study, and
drafted the manuscript. FL, KZ, JY and ZH and collected, analysed
and interpreted the experiment data, and revised the manuscript
critically for important intellectual content. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Committees for the
Ethical Review of Research at Zhujiang Hospital of Southern Medical
University, and written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Verbeke C, Löhr M, Karlsson JS and Del
Chiaro M: Pathology reporting of pancreatic cancer following
neoadjuvant therapy: Challenges and uncertainties. Cancer Treat
Rev. 41:17–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verbeke C: Morphological heterogeneity in
ductal adenocarcinoma of the pancreas-Does it matter?
Pancreatology. 16:295–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan Y, Xu LL, Shi CY, Wei W, Wang DS and
Cai DF: MicroRNA-454 regulates stromal cell derived factor-1 in the
control of the growth of pancreatic ductal adenocarcinoma. Sci Rep.
6:227932016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Wu G, Wu Z, Yao X and Li G: MiR-200a
suppresses the proliferation and metastasis in pancreatic ductal
adenocarcinoma through downregulation of DEK gene. Transl Oncol.
9:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X,
Deng Y, Jiang J and Sun C: MicroRNA-138-5p regulates pancreatic
cancer cell growth through targeting FOXC1. Cell Oncol (Dordr).
38:173–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vetter G, Saumet A, Moes M, Vallar L, Le
Béchec A, Laurini C, Sabbah M, Arar K, Theillet C, Lecellier CH and
Friederich E: miR-661 expression in SNAI1-induced epithelial to
mesenchymal transition contributes to breast cancer cell invasion
by targeting Nectin-1 and StarD10 messengers. Oncogene.
29:4436–4448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu T, Yuan J, Wang Y, Gong C, Xie Y and
Li H: MiR-661 contributed to cell proliferation of human ovarian
cancer cells by repressing INPP5J expression. Biomed Pharmacother.
75:123–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Cai Y, Rong X, Chen J, Zheng D,
Chen L, Zhang J, Luo R, Zhao P and Ruan J: MiR-661 promotes tumor
invasion and metastasis by directly inhibiting RB1 in non small
cell lung cancer. Mol Cancer. 16:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arensman MD, Kovochich AN, Kulikauskas RM,
Lay AR, Yang PT, Li X, Donahue T, Major MB, Moon RT, Chien AJ and
Dawson DW: WNT7B mediates autocrine Wnt/β-catenin signaling and
anchorage-independent growth in pancreatic adenocarcinoma.
Oncogene. 33:899–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wald P, Liu XS, Pettit C, Dillhoff M,
Manilchuk A, Schmidt C, Wuthrick E, Chen W and Williams TM:
Prognostic value of microRNA expression levels in pancreatic
adenocarcinoma: A review of the literature. Oncotarget.
8:73345–73361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song B, Ji W, Guo S, Liu A, Jing W, Shao
C, Li G and Jin G: miR-545 inhibited pancreatic ductal
adenocarcinoma growth by targeting RIG-I. FEBS Lett. 588:4375–4381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang R, Leng H, Huang J, Du Y, Wang Y,
Zang W, Chen X and Zhao G: miR-337 regulates the proliferation and
invasion in pancreatic ductal adenocarcinoma by targeting HOXB7.
Diagn Pathol. 9:1712014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Zheng K, Ma H, Liu A, Jing W, Shao
C, Li G and Jin G: miR-429 determines poor outcome and inhibits
pancreatic ductal adenocarcinoma growth by targeting TBK1. Cell
Physiol Biochem. 35:1846–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sano M, Driscoll DR, DeJesus-Monge WE,
Quattrochi B, Appleman VA, Ou J, Zhu LJ, Yoshida N, Yamazaki S,
Takayama T, et al: Activation of WNT/β-catenin signaling enhances
pancreatic cancer development and the malignant potential via
Up-regulation of Cyr61. Neoplasia. 18:785–794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Li Z, Zheng S, Chen H, Zhao X,
Gao W, Bi Z, You K, Wang Y, Li W, et al: The long non-coding RNA
HOTAIR affects the radiosensitivity of pancreatic ductal
adenocarcinoma by regulating the expression of Wnt inhibitory
factor 1. Tumour Biol. 37:3957–3967. 2016. View Article : Google Scholar : PubMed/NCBI
|