Introduction
Melanoma is one of the deadliest forms of cancer
known to humans and has the highest propensity to metastasize to
the brain. Current therapeutics for metastatic brain tumours
include surgery and radiation therapy but despite technological
advancement on improving treatment safety and efficacy with these
modalities, brain metastases remain a major cause of death in
patients with metastatic melanoma (1,2).
In search of more effective therapeutics, an
oncogenic signalling pathway driven by the transcription factor
Forkhead box M1 (FOXM1) has emerged as a promising anti-metastatic
target in recent years. FOXM1 protein belongs to a class of highly
evolutionary conserved mammalian transcription factors which
underlie the regulation of many fundamental homeostatic and
developmental processes (3,4). In normal cells, FOXM1 serves as a key
regulator of cell cycle progression and cellular development,
mediating G1-S and G2-M phase transition and maintaining a balance
between cell proliferation and apoptosis in developing cells
(4). While its expression is turned
off in terminally differentiated cells, an aberrant gain of
FOXM1 function has been shown to link to tumorigenesis
(3,4).
Emerging evidence has demonstrated that FOXM1 is upregulated
in a multitude of solid tumours including breast, lung, basal cell,
pancreatic, hepatocellular, ovarian and prostate carcinomas,
rendering it one of the most overexpressed genes in human cancers
(5,6).
More importantly, recent studies have indicated that the oncogenic
mechanisms of FOXM1 involve not only cell cycling dysregulation as
predicted from its physiological function but the control of a wide
array of oncogenic pathways and events including inhibition of cell
differentiation, apoptosis and DNA repair and promotion of cell
invasion, migration and angiogenesis, quintessential of the
expression of the metastatic phenotypes (4). Conceivably targeting FOXM1 could
result in an across-the-board inhibition of many of the
pro-metastatic events and combining with existing ablative
treatment with focused irradiation, highly selective and localized
cancer cell death can potentially be induced with low toxicity to
normal tissue.
Recently several lines of evidence have lent support
to the roles of FOXM1 expression in inducing metastatic
melanoma and suppression of FOXM1 via RNA interference,
blocking peptides, or chemotherapeutic agents has proven successful
in countering tumorigenesis (7–10).
Importantly new evidence has indicated that combined treatment of
FOXM1 inhibition with a small molecule inhibitor Siomycin A
(SIOA) and irradiation can achieve a higher rate of cell death in
glioblastoma cell lines, thus raising the possibility of harnessing
FOXM1 inhibitors as a radiosensitizing agent (11). Currently there is a dearth of
information in the role of FOXM1 inhibition in sensitizing
radioresistant melanoma and we therefore sought to elucidate the
cellular effects of irradiation and SIOA on metastatic melanoma
cells and determine the effects of pretreatment with SIOA on
cellular response to irradiation.
Materials and methods
Cell culture and irradiation
A panel of ATCC-derived (SK-MEL-28, A375) or
cultured, short-term patient-derived (WMD009, WMD046, MM200,
SMU027) melanoma cells were kindly provided by Professor H. Rizos
(Macquarie University, Sydney, Australia) to identify a FOXM1
overexpressing strain. All cell lines were previously published
except for WMD046 which is a short term patient derived sample
sourced from Professor Rizos (12–16). Cells
were maintained in Dulbecco's Modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (FBS), 20 mM HEPES and 4 mM L-glutamine and cultured
at 37°C in humidified air with 5% carbon dioxide. Cells were
routinely passaged at 80% confluence with 0.1% Trypsin/EDTA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells were seeded
in 8-well chamber slides, 96- or 6-well plates for irradiation with
X-rays (0–40 Gy) generated by a 6 MV linear accelerator (LINAC;
Elekta Synergy, Crawley, UK) at Macquarie University Hospital
(Sydney, Australia) or for SIOA treatment. SIOA was purchased from
Sigma-Aldrich; Merck KGaA and dissolved in dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Merck KGaA). DMSO constituted a final
concentration of 0.1% in all assays.
Trypan blue viability assay
Viable-to-dead cell ratios were determined 1–5 days
after drug or radiation treatment using the trypan blue viability
assay. Briefly, floating and adherent cells were collected, washed
and stained with trypan blue for 10 min before automated counting
of white (live) or blue (permeable, dead) cells with an automated
cell counter (Countess II FL Automated Cell Counter; Thermo Fisher
Scientific, Inc.).
MTT proliferation assay
Cells were seeded in 96-well plates at
5×103 cells per well in 5% serum-containing medium and
allowed to adhere overnight before treatment with drug or
radiation. Treatment proceeded for 24, 48, 72 or 120 h. At least 8
replicate wells were used for each dose and time point within each
independent experiment. Five h prior to the end of each incubation
period, 20 µl of MTT (Thermo Fisher Scientific, Inc.) was added to
each well (0.5 mg/ml) and the plates incubated at 37°C for a
further 5 h. The medium was then discarded by inversion and the
cells resuspended in 200 µl of DMSO per well to dissolve the
formazan product. The plates were mechanically shaken for 5–10 min
and the absorbance read at 560/670 nm within 1 h using a microplate
reader (BMG Labtech PHERAstar FS; Thermo Fisher Scientific,
Inc,).
Scratch wounding cell migration
assay
A scratch wound assay was used to examine cellular
migration after SIOA and radiation treatment. Cells were seeded in
8-well chamber slides (Nunc; Thermo Fisher Scientific, Inc.) or
6-well culture plates to obtain 90–100% confluence. Cells were
irradiated with single doses of radiation (0–40 Gy) or SIOA (0–5
µM) for 1 h prior to scratch wounding of the cell layer with a 1 ml
pipette tip. The medium was replaced with or without the thiazole
antibiotic to remove floating cells and debris. The scratch wound
area was immediately imaged with an upright inverted microscope and
reimaged at 24, 48 and 72 h post-wounding. When sequential
combination treatments were given, a period of 24 h was given
before addition of the second treatment.
Images were analyzed using Image J (Rasband, W.S.,
ImageJ, National Institutes of Health, Bethesda, Maryland, USA;
https://imagej.nih.gov/ij/, 1997–2016).
For every scratch wound, a series of 4 images were taken along the
length of the wounded area and measured by blinded observer. For
each image, a region of interest (ROI; rectangle) was drawn that
aligned with the upper and lower edges of the image and the
observed division between cell regrowth and denudation. The area
within the ROI was recorded for each image and divided by the area
measured immediately after scratch wounding on day 0 to give wound
area as a percentage of the original scratch wound area. These
wound area percentages for each dose and time point from 3
independent experiments were then averaged (n=3) and plotted.
Western blot analysis
Protein lysates were prepared in immunoprecipitation
buffer (50 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 0.5%
deoxycholate, 0.1% sodium dodecyl sulphate, 1% NP40 substitute, 5
mM EDTA) supplemented with freshly prepared protease inhibitor
cocktail (GE Healthcare, Chicago, IL, USA). Protein concentration
was determined using the BCA protein assay (Pierce Biotechnology
Inc.; Thermo Fisher Scientific, Inc.). Whole cell protein extracts
(30 µg) were resolved by SDS-PAGE, transferred to a PVDF membrane
using the iblot transfer system (Thermo Fisher Scientific, Inc.)
and probed with primary antibodies, species-specific HRP-conjugated
secondary antibodies and detected by enhanced chemiluminescence.
The following antibodies were used: Anti-FOXM1 (H-19 sc-501 rabbit
polyclonal); anti-FOXM1 (G-5 sc-376471 mouse monoclonal; both Santa
Cruz Biotechnology, Inc., Dallas, TX, USA); anti-cleaved poly
(ADP-ribose) polymerase 1 (PARP1; ab2321 rabbit polyclonal; Abcam,
Cambridge, UK); anti-B-cell lymphoma 2 (Bcl2; sc-492 rabbit
polyclonal, Santa Cruz Biotechnology, Inc.); anti-phospho-AKT
(Ser473) (cs-4060S rabbit monoclonal; Cell Signalling
Biotechnology, Inc., Danvers, MA, USA); anti-superoxide dismutase 2
(Sod2; ab13534 rabbit polyclonal; Abcam). All membranes were probed
with antibodies targeting either β-actin (A5060.5 rabbit
polyclonal; Sigma-Aldrich; Merck KGaA) or GAPDH (ab181602 rabbit
monoclonal; Abcam) as protein loading controls.
Statistical analysis
Data are presented as mean ± standard error of the
mean (SEM). Effects of single treatments over time were analyzed by
two-way ANOVA with Dunnett's post-hoc analysis for multiple group
comparisons using Prism 6.04 software (Graphpad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Constitutive FOXM1 expression in
melanoma cell lines
FOXM1 expression has been noted to be
constitutively activated in many cancers including melanoma and to
contribute to tumorigenesis and chemo- and radio-resistance
(5,7–9). A series
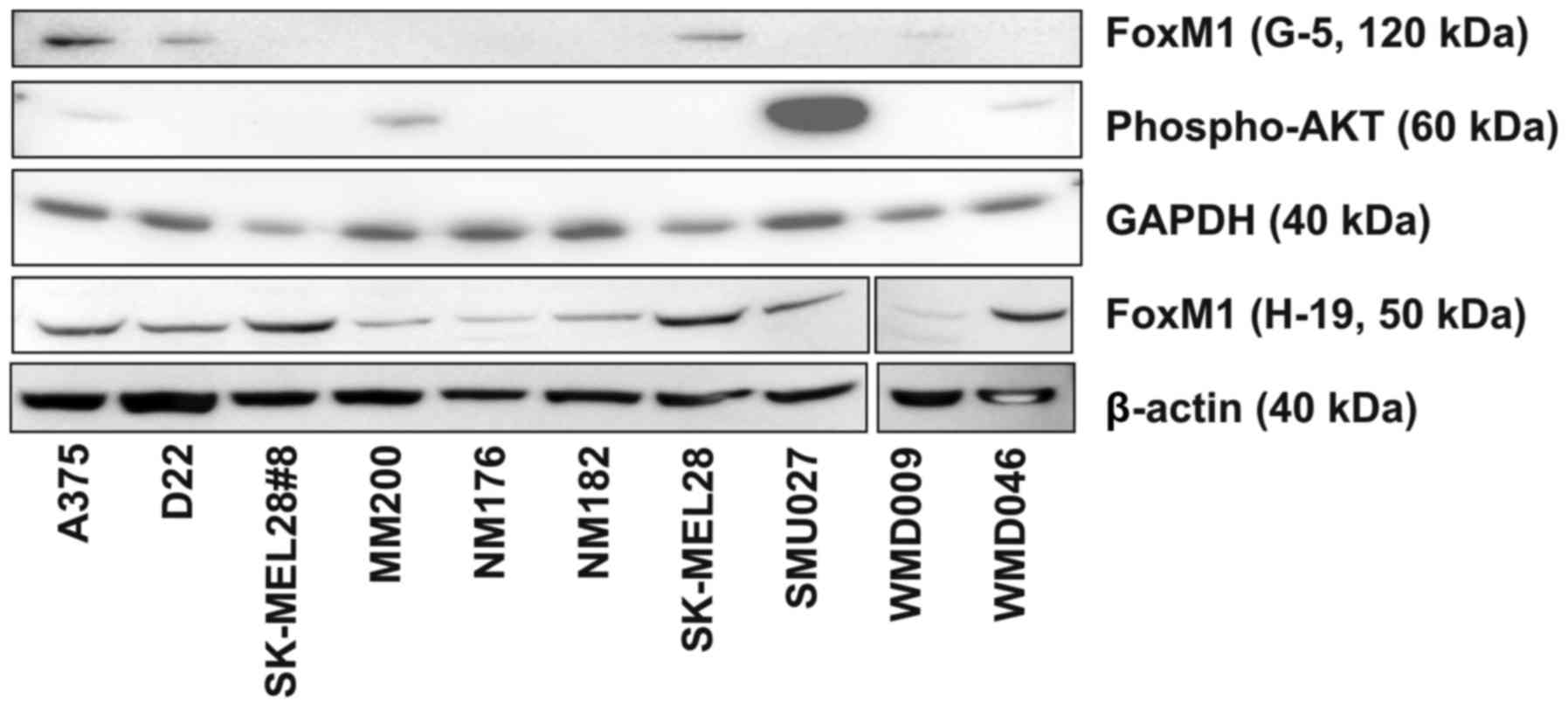
of 10 melanoma cell lines were tested for FOXM1 protein expression
by Western blotting with two different FOXM1 antibodies. A mouse
monoclonal anti-FOXM1 antibody (G-5) recognized a single band of
approximately 120 kDa, consistent with the full length FOXM1
protein, in strains A375, D22, SK-MEL-28 and faintly in WMD009
(Fig. 1). In contrast, a polyclonal
rabbit anti-FoxM1 antibody (H-19) identified a primary band on
western blots at approximately 50 kDa with a faint band at 80 kDa
(Fig. 1). The pattern of expression
of the 80 kDa band in strains A375, D22 and SK-MEL-28 was
consistent with that of the 120 kDa band (G-5). The 50 kDa band was
expressed in all cell lines but was considered non-specific as it
did not respond to SIOA treatment. In view of these, the H-19 mouse
monoclonal antibody was selected for further studies.
As constitutive activation of the AKT pathway can
influence FOXM1 expression (8), we also examined the relative levels of
expression of phosphorylated AKT in the 10 melanoma cell lines.
Expression of phosphorylated AKT (Ser473) was observed only in
A375, MM200, SMU027 and WMD046 (Fig.
1). There appeared to be no correlation between the relative
expression levels of FOXM1 and phospho-AKT. Collectively the
SK-MEL-28 cell line was chosen for further studies given its high
expression levels of FOXM1.
SIOA inhibits SK-MEL-28 proliferation
and induces cellular apoptosis
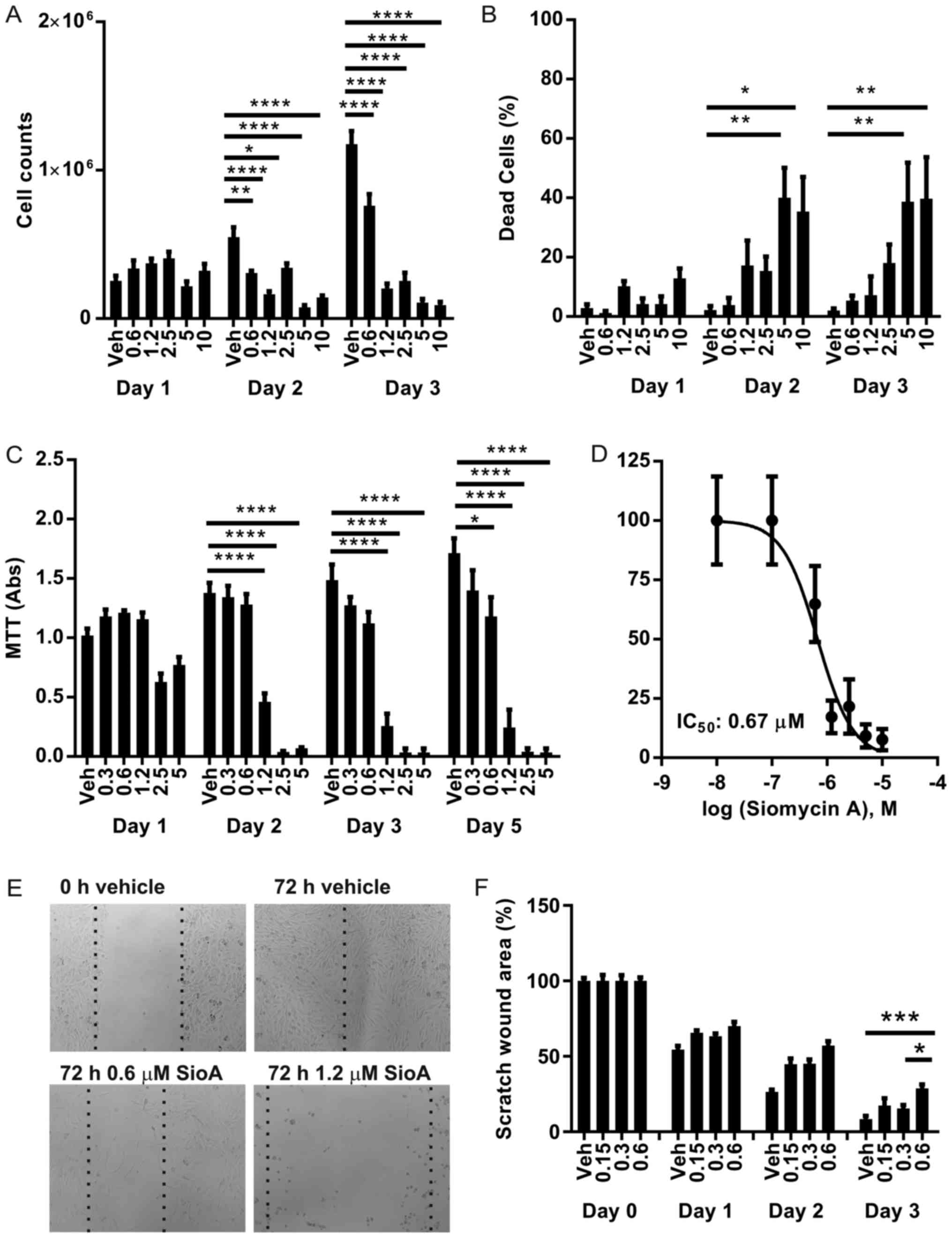
SK-MEL-28 cells were treated with SIOA at
concentrations of 0–10 µM for up to 5 days. Cell proliferation and
death were measured using trypan blue viability assays (Fig. 2A and B) and MTT proliferation assays
(Fig. 2C). Vehicle controls (0.1%
DMSO) continued to grow over the 72 h period. At a dose of 0.6 µM
SIOA, cell growth was inhibited 1.5-fold by 72 h (P<0.0001)
while doses >1.2 µM completely inhibited growth and
significantly increased the proportion of dead cells in the first
48 h period. The IC50 was determined as 0.67 µM
(Fig. 2D).
SIOA inhibits SK-MEL-28 cell
migration
Cellular migration was examined in a scratch wound
assay. At concentrations >1.2 µM, SIOA primarily induced cell
death across the entire plate of cells (see phase-contrast images,
Fig. 2E). At sub-lethal
concentrations (≤0.6 µM), cellular migration was inhibited without
significant cell death after 3 days (Fig.
2E and F).
SIOA decreases FOXM1 and BCL2 protein
expression in SK-MEL-28 melanoma cells with a concomitant increase
in PARP-1 cleavage
Protein expression of FOXM1 and two FOXM1-regulated
genes, the anti-oxidant enzyme, SOD2, and the anti-apoptotic
protein, BCL2, were examined in response to SIOA (Fig. 3). FOXM1 (full length protein, 120 kDa)
was abruptly reduced at concentrations between 1.2 and 2.5 µM SIOA.
Levels of SOD2 increased dose-dependently, however BCL2 decreased
between 1.2 and 2.5 µM SIOA, in line with the decreases in FOXM1
expression observed. Expression of the p65 subunit of the NFKB
transcription factor decreased in a linear fashion with SIOA dose.
The late apoptotic marker, cleaved PARP1, was evident at
concentrations above 1.2 µM SIOA up to 48 h, consistent with the
level of cell death observed.
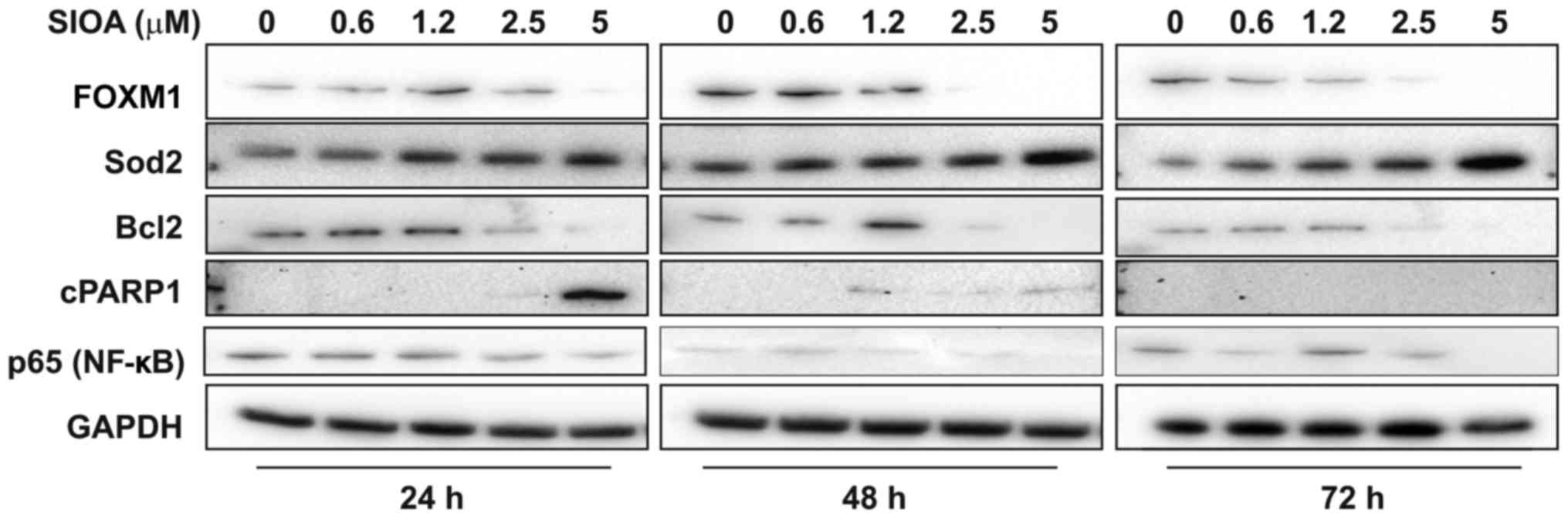 | Figure 3.SIOA inhibits FoxM1 expression in
SK-MEL-28 melanoma cells. SIOA (0.6–5 µM) or vehicle (0.1% DMSO)
was added to SK-MEL-28 cells and total protein harvested after 24,
48 and 72 h. Western analysis was performed with antibodies
targeting FoxM1 (120 kDA), Sod2 (25 kDa), Bcl2 (26 kDa), cleaved
PARP1 (85 kDa), p65 (NFκB) (65 kDa), and GAPDH (40 kDa). Blots are
representative of 3 independent experiments. SIOA, Siomycin A;
DMSO, dimethyl sulfoxide; FOXM1, Forkhead box M1; Sod2, superoxide
dismutase 2; Bcl2, B-cell lymphoma 2; PARP-1, polymerase 1. |
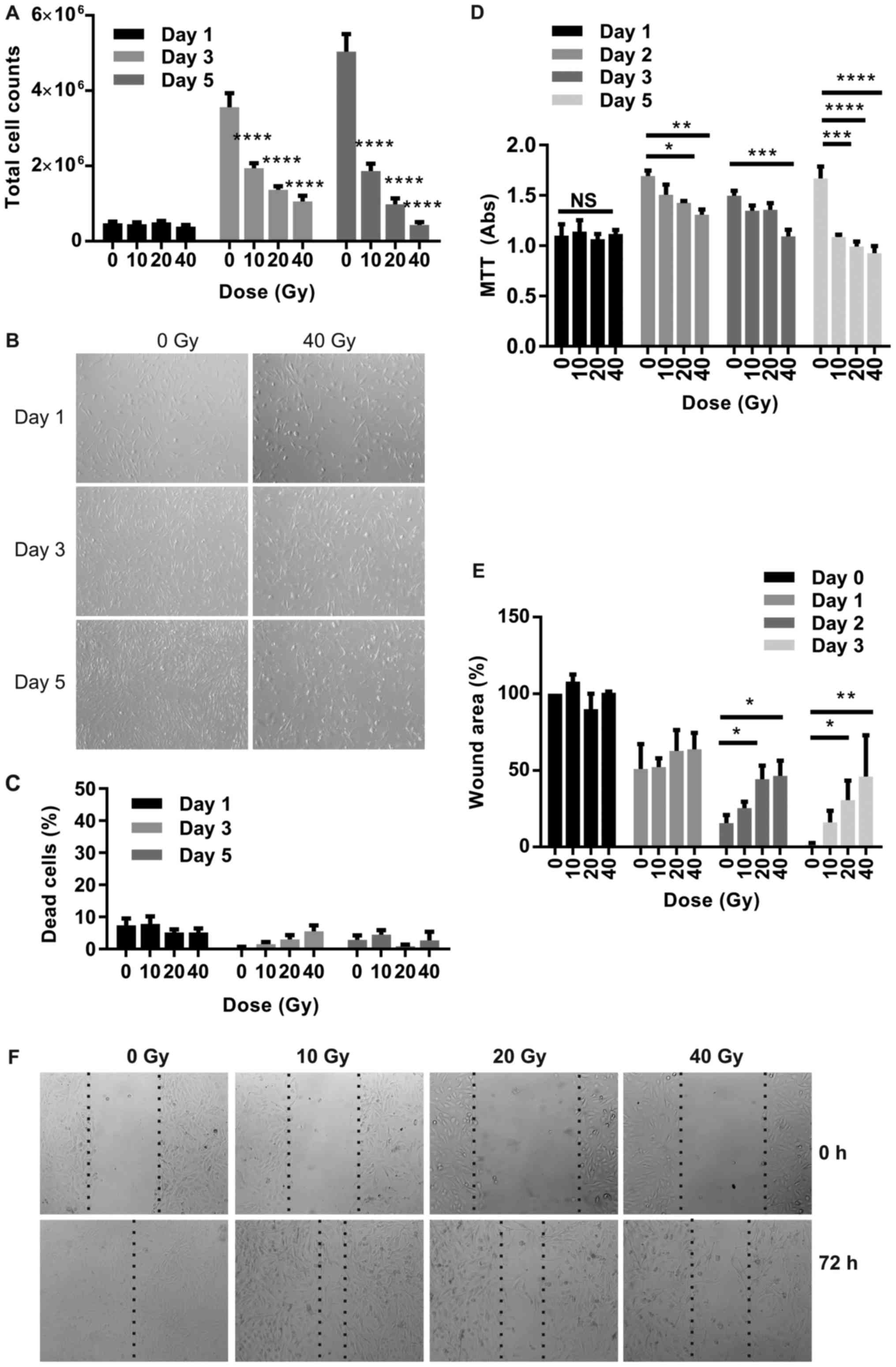
Radiation inhibits SK-MEL-28 growth
with minimal effects on cell death
Total cell counts were determined over a period of 5
days for SK-MEL-28 melanoma cells exposed to radiation doses up to
40 Gy. Cells continued to grow in the absence of irradiation up to
day 5 until growth reached 100% confluence, while radiation
inhibited cell growth at all doses (Fig.
4A and B). In the irradiated cells, cell numbers increased
between day 1 and day 3 at all doses, but declined moderately
between days 3 and 5 at the higher doses (10 and 20 Gy). The
percentage of dead, trypan blue-positive cells did not change
significantly over the time period in response to radiation at all
doses and did not reach >8% at any time (Fig. 4C), consistent with the inability to
detect the apoptotic marker, cleaved PARP-1, on western blots (not
shown). An MTT proliferation assay confirmed the predominance of
growth inhibition rather than cell death at radiation doses of
10–40 Gy (Fig. 4D).
Radiation inhibits SK-MEL-28 migration
in a scratch wound assay
Wound area after scratch injury was measured
consecutively in live cells over 3 days in response to radiation.
Wound area (as a percentage of denuded area measured immediately
after scratch injury) continued to decline at all doses over the
time period as cells repopulated the denuded area (Fig. 4E and F). Inhibition of regrowth was
seen at all doses however this reached significance at doses of 20
Gy (P<0.05) and 40 Gy (P<0.01).
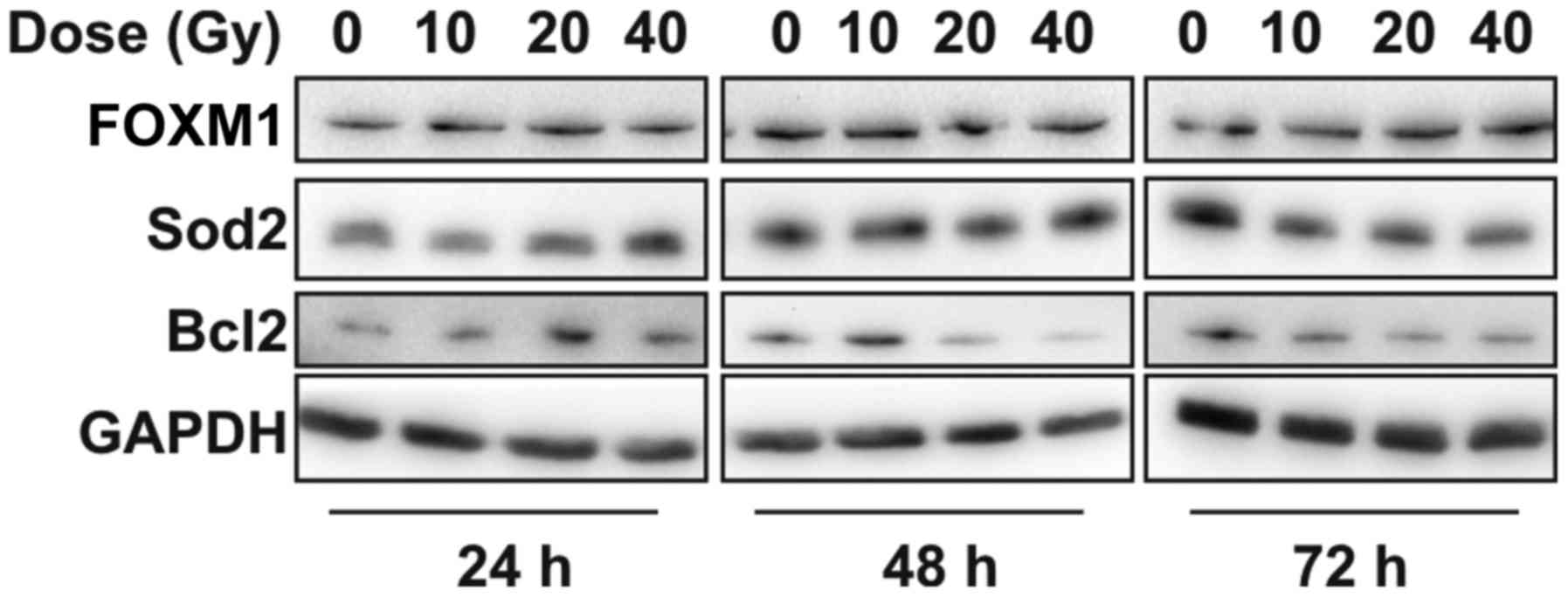
Radiation does not alter FOXM1
expression in SK-MEL-28 melanoma cells
No significant change in FOXM1 protein expression
was observed in SK-MEL-28 cells in response to radiation (Fig. 5). SOD2 and BCL2 protein expression
increased transiently with radiation dose at the 24 h time point
relative to the non-irradiated control. BCL2 expression was
moderately reduced at the higher doses (20 and 40 Gy) at later time
points, however SOD2 expression was not different to controls.
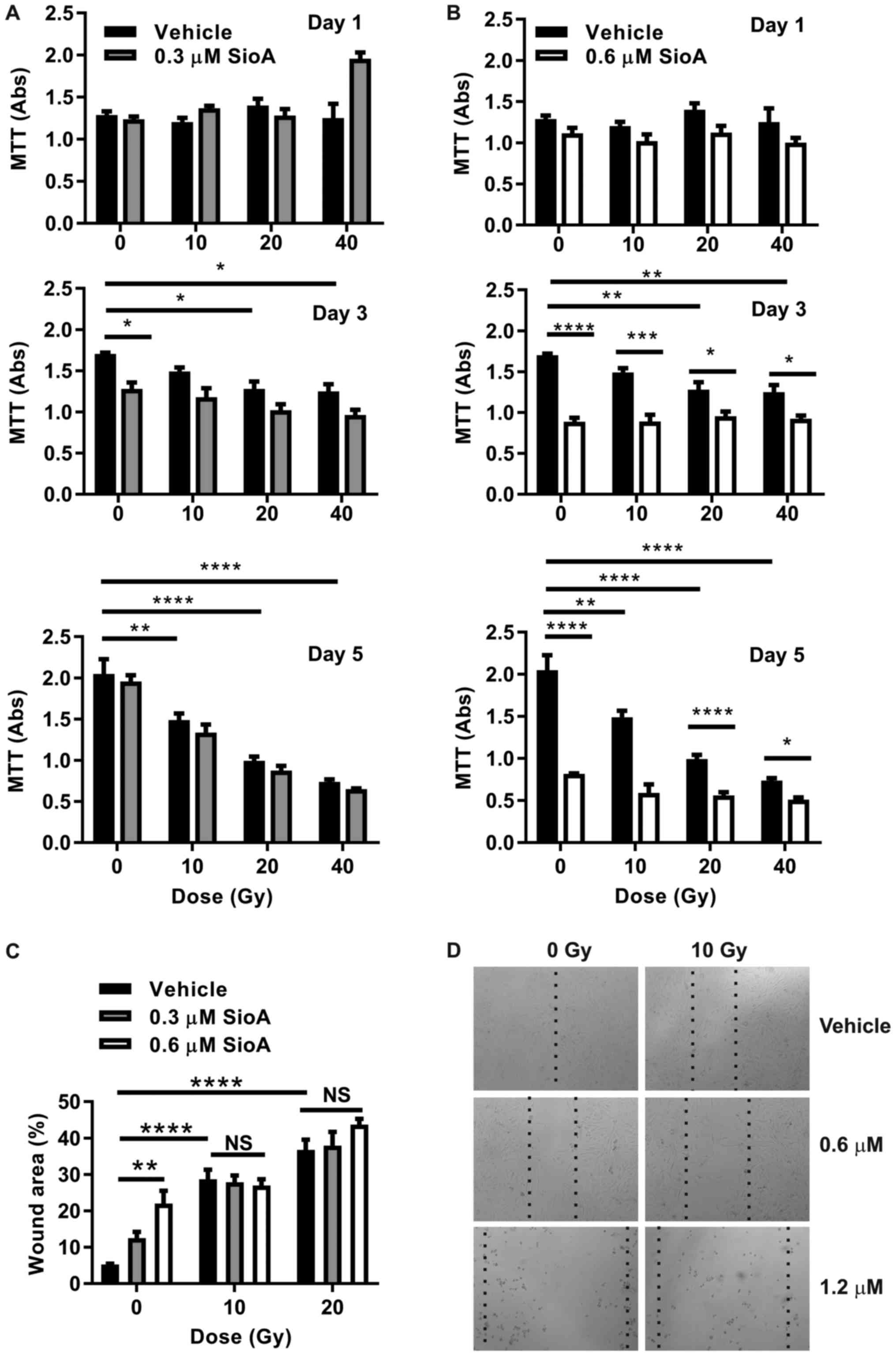
Pretreatment with SIOA at sub-lethal
doses has no radio-sensitizing effect on SK-MEL-28 melanoma
cells
To examine whether radiation and SIOA could act
synergistically on inhibition of SK-MEL-28 cell growth and
migration, cells were treated with a mid-range dose of radiation
(10 Gy) combined with SIOA. Cells were treated with Siomycin A 24 h
prior to radiation treatment to allow FoxM1 knockdown prior to
irradiation. As earlier experiments showed complete cell loss after
2 days with concentrations of SIOA >1.2 µM, cells were examined
at sub-lethal concentrations of 0.3 and 0.6 µM SIOA with 10–40 Gy
doses of radiation.
MTT proliferation assays were performed to establish
any synergistic effects of SIOA and radiation on cell
proliferation. At a dose of 0.3 µM (Fig.
6A), Siomycin A had minimal effect on proliferation in these
assays, while at 0.6 µM, SIOA significantly reduced proliferation
from day 3 onwards (Fig. 6B),
consistent with earlier results (Fig.
2). In combination with radiation, no further reduction in
proliferation was observed over the time period at either dose
(Fig. 6A and B). Similarly, no
synergistic effects of SIOA and radiation were observed on
migration (Fig. 6C and D). At high
doses (>1 µM), apoptosis was absolute within 2 days in the
8-well chamber slides and no further contribution from radiation
could be observed. At sub-lethal concentrations (0.3–0.6 µM), there
were no synergistic effects on migration at radiation doses of 10
and 20 Gy measured at 72 h after radiation (Fig. 6C and D).
Discussion
Overexpression of the pleiotropic transcription
factor FOXM1 has been observed in many human malignancies and
regulates multiple pathways important for tumorigenesis (5,6). Recent
studies demonstrate FOXM1 overexpression in malignant
melanoma and an association with cancer stage and prognosis
suggesting FOXM1 knockdown may be a novel therapeutic target
(7–9).
Consistent with these studies, we observed reduction in FOXM1 at
micromolar Siomycin A doses in the metastatic melanoma cell line,
SK-MEL-28, with a rather rapid drop-off in FOXM1 expression
at concentrations above 1 µM. BCL2 was similarly inhibited by SIOA
in the same dose range and this is of particular importance as BCL2
is a well-known anti-apoptotic factor and a downstream target of
FOXM1 (17). BCL2 and
FOXM1 inhibition occurred simultaneously with an increase in
expression of the apoptotic marker, cleaved PARP-1. BCL2 is
also a target of the NFκB transcription factor (18), and downregulation may have been
associated with the simultaneous decrease observed in constitutive
NFκB (RELA/P65) expression. In other studies,
proteasome inhibitor-induced apoptosis is at least partly mediated
via suppression of the NFκB signalling pathway (19). Our results illustrated a rapid
induction of apoptosis at doses between 1–2 µM Siomycin A and are
in agreement with the regulatory role of FOXM1 and BCL2.
SOD2 has been demonstrated to be both a FOXM1
(20,21) and NFκB (22) transcriptional target but our data
suggested an uncoupling of this relationship in response to
Siomycin A-we found that SOD2 expression was unchanged
despite a dramatic loss of FOXM1 and NFκB
expression. In addition we observed a transient induction of
SOD2 in response to radiation but this did not correlate
with FOXM1 expression, which remained unchanged. SOD2 is an
important scavenger enzyme controlling intracellular reactive
oxygen species (ROS), and overexpression is a known contributor to
both chemo- and radioresistance in various tumor types (23). Silencing FOXM1 was shown to
decrease SOD2 expression, increase intracellular ROS levels
and in turn the sensitivity to ROS inducers (not radiation),
enhancing apoptosis in vitro and in vivo (21). We think that the observed
FOXM1-SOD2 uncoupling may have contributed to the inability
of SIOA to sensitize melanoma cells to radiation. In line with this
observation, others have shown that radiosensitivity can be
enhanced by downregulation of NFκB and SOD2
(24) and increased chemosensitivity
can be induced by simultaneous BCL2 and SOD2
knockdown in resistant melanoma (25). Collectively further studies on
FOXM1-SOD2 interactions in tumor cells with different
radiosensitivity would shed light on the underpinnings of
radioresistance.
To our knowledge, this is the first study to examine
the effects of combining SIOA as a FOXM1 inhibitor with radiation
in melanoma cells. Combination of FOXM1 suppression and
radiation was previously investigated in various tumor cell lines,
which overall suggested a potential synergistic treatment effect
(11,17,26). Our
results contrast with these findings, however, in that while the
basal expression of FOXM1 was significantly induced by radiation in
various non-melanoma cancer cells types, radiation appeared to have
no effect on FOXM1 expression. Similarly, robust apoptosis
was induced by radiation in various non-melanoma cells but only
cell stasis was observed here, even up to doses of 40 Gy. This dose
range is well above that used in other combination studies and
valid in the clinical setting, hence the radiation dose range does
not appear a limiting factor in this study (11,17,26).
Rather, it appears that the basal level of sensitivity of
individual cell types to radiation and the ability to further
induce FOXM1 may predict the efficacy of combination
treatment. Such notion of cell specificity is echoed by a lymphoma
study in which synergistic induction of apoptosis in several cell
lines treated with combined proteasome inhibitor and radiation was
noted to be dependent on the p53 status with mutant or null p53
cells exhibiting a lack of sensitisation and vice versa (27). On this note, the observed resistance
to radiosensitization of SK-MEL-28, being p53 mutant (28), corroborates with these findings.
It should be noted that in this study we used the
MTT assay as a surrogate for the use of clonogenic survival assays.
Good correlation has been demonstrated between clonogenic survival
and MTT assays, especially when multiple and extended time points
are examined to account for the acute and post-acute phases of the
radiation response (29). However it
should be made clear that our findings of lack of synergism refer
to the properties of proliferation and migration and relationships
to clonal survival and invasion are inferred from the important
role they play in these phenotypes.
We demonstrated that FOXM1-overexpressing melanoma
cells, SK-MEL-28, were highly susceptible to SIOA-induced apoptosis
which in turn was associated with FOXM1, BCL2 and NFκB inhibition.
SIOA treatment, however, did not further sensitize these
radioresistant cells to combined radiation treatment. The
combination of FOXM1 inhibition and radiation therapy may be
ineffective for radioresistant melanoma.
Acknowledgements
The authors would like to thank Professor H. Rizos
(Macquarie University) for supplying the melanoma strains used in
the present study.
Funding
The present study was supported by a research grant
provided by Brain Foundation, Australia (grant no. 9201200696).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VSL was involved in data acquisition (all
techniques), analysis and interpretation of data, manuscript
drafting and revision, and the final approval of the manuscript.
LSM was involved in the conception and design of the study
(methodology), data acquisition (migration, western blotting),
analysis and interpretation of data, manuscript drafting and
revision, and the final approval of the manuscript. VM and EDS were
involved in the conception and design of the study (radiation),
manuscript revision, and the final approval of the manuscript. TLS
was involved in the conception and design of the whole study,
analysis and interpretation of data, manuscript drafting and
revision, the final approval of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan MK, Khan N, Almasan A and Macklis R:
Future of radiation therapy for malignant melanoma in an era of
newer, more effective biological agents. Onco Targets Ther.
4:137–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bullard DE, Cox EB and Seigler HF: Central
nervous system metastases in malignant melanoma. Neurosurgery.
8:26–30. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: A novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu XS, Miao RC, Wan Y, Zhang LQ, Qu K and
Liu C: FoxM1 as a novel therapeutic target for cancer drug therapy.
Asian Pac J Cancer Prev. 16:23–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wierstra I: FOXM1 (Forkhead box M1) in
tumorigenesis: overexpression in human cancer, implication in
tumorigenesis, oncogenic functions, tumor-suppressive properties,
and target of anticancer therapy. Adv Cancer Res. 119:191–419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito T, Kohashi K, Yamada Y, Maekawa A,
Kuda M, Furue M and Oda Y: Prognostic significance of forkhead box
M1 (FoxM1) expression and antitumour effect of FoxM1 inhibition in
melanoma. Histopathology. 69:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyashita A, Fukushima S, Nakahara S,
Yamashita J, Tokuzumi A, Aoi J, Ichihara A, Kanemaru H, Jinnin M
and Ihn H: Investigation of FOXM1 as a potential new target for
melanoma. PLoS One. 10:e01442412015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kruiswijk F, Hasenfuss SC, Sivapatham R,
Baar MP, Putavet D, Naipal KA, van den Broek NJ, Kruit W, van der
Spek PJ, van Gent DC, et al: Targeted inhibition of metastatic
melanoma through interference with Pin1-FOXM1 signaling. Oncogene.
35:2166–2177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhat UG, Zipfel PA, Tyler DS and Gartel
AL: Novel anticancer compounds induce apoptosis in melanoma cells.
Cell Cycle. 7:1851–1855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maachani UB, Shankavaram U, Kramp T,
Tofilon PJ, Camphausen K and Tandle AT: FOXM1 and STAT3 interaction
confers radioresistance in glioblastoma cells. Oncotarget.
7:77365–77377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carlino MS, Todd JR, Gowrishankar K,
Mijatov B, Pupo GM, Fung C, Snoyman S, Hersey P, Long GV, Kefford
RF and Rizos H: Differential activity of MEK and ERK inhibitors in
BRAF inhibitor resistant melanoma. Mol Oncol. 8:544–554. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carlino MS, Gowrishankar K, Saunders CA,
Pupo GM, Snoyman S, Zhang XD, Saw R, Becker TM, Kefford RF, Long GV
and Rizos H: Antiproliferative effects of continued
mitogen-activated protein kinase pathway inhibition following
acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol
Cancer Ther. 12:1332–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long GV, Fung C, Menzies AM, Pupo GM,
Carlino MS, Hyman J, Shahheydari H, Tembe V, Thompson JF, Saw RP,
et al: Increased MAPK reactivation in early resistance to
dabrafenib/trametinib combination therapy of BRAF-mutant metastatic
melanoma. Nat Commun. 5:56942014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XD, Franco A, Myers K, Gray C,
Nguyen T and Hersey P: Relation of TNF-related apoptosis-inducing
ligand (TRAIL) receptor and FLICE-inhibitory protein expression to
TRAIL-induced apoptosis of melanoma. Cancer Res. 59:2747–2753.
1999.PubMed/NCBI
|
|
16
|
Pupo GM, Boyd SC, Fung C, Carlino MS,
Menzies AM, Pedersen B, Johansson P, Hayward NK, Kefford RF,
Scolyer RA, et al: Clinical significance of intronic variants in
BRAF inhibitor resistant melanomas with altered BRAF transcript
splicing. Biomark Res. 5:172017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halasi M and Gartel AL: Suppression of
FOXM1 sensitizes human cancer cells to cell death induced by
DNA-damage. PLoS One. 7:e317612012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fahy BN, Schlieman MG, Mortenson MM,
Virudachalam S and Bold RJ: Targeting BCL-2 overexpression in
various human malignancies through NF-kappaB inhibition by the
proteasome inhibitor bortezomib. Cancer Chemother Pharmacol.
56:46–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Almond JB and Cohen GM: The proteasome: A
novel target for cancer chemotherapy. Leukemia. 16:433–443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park HJ, Carr JR, Wang Z, Nogueira V, Hay
N, Tyner AL, Lau LF, Costa RH and Raychaudhuri P: FoxM1, a critical
regulator of oxidative stress during oncogenesis. EMBO J.
28:2908–2918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halasi M, Pandit B, Wang M, Nogueira V,
Hay N and Gartel AL: Combination of oxidative stress and FOXM1
inhibitors induces apoptosis in cancer cells and inhibits xenograft
tumor growth. Am J Pathol. 183:257–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinugasa H, Whelan KA, Tanaka K,
Natsuizaka M, Long A, Guo A, Chang S, Kagawa S, Srinivasan S, Guha
M, et al: Mitochondrial SOD2 regulates epithelial-mesenchymal
transition and cell populations defined by differential CD44
expression. Oncogene. 34:5229–5239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hosoki A, Yonekura S, Zhao QL, Wei ZL,
Takasaki I, Tabuchi Y, Wang LL, Hasuike S, Nomura T, Tachibana A,
et al: Mitochondria-targeted superoxide dismutase (SOD2) regulates
radiation resistance and radiation stress response in HeLa cells. J
Radiat Res. 53:58–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, St Clair DK, Fang F, Warren GW,
Rangnekar VM, Crooks PA and St Clair WH: The radiosensitization
effect of parthenolide in prostate cancer cells is mediated by
nuclear factor-kappaB inhibition and enhanced by the presence of
PTEN. Mol Cancer Ther. 6:2477–2486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benlloch M, Mena S, Ferrer P, Obrador E,
Asensi M, Pellicer JA, Carretero J, Ortega A and Estrela JM: Bcl-2
and Mn-SOD antisense oligodeoxynucleotides and a glutamine-enriched
diet facilitate elimination of highly resistant B16 melanoma cells
by tumor necrosis factor-alpha and chemotherapy. J Biol Chem.
281:69–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee Y, Kim KH, Kim DG, Cho HJ, Kim Y,
Rheey J, Shin K, Seo YJ, Choi YS, Lee JI, et al: FoxM1 promotes
stemness and radio-resistance of glioblastoma by regulating the
master stem cell regulator Sox2. PLoS One. 10:e01377032015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurland JF and Meyn RE: Protease
inhibitors restore radiation-induced apoptosis to Bcl-2-expressing
lymphoma cells. Int J Cancer. 96:327–333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avery-Kiejda KA, Zhang XD, Adams LJ, Scott
RJ, Vojtesek B, Lane DP and Hersey P: Small molecular weight
variants of p53 are expressed in human melanoma cells and are
induced by the DNA-damaging agent cisplatin. Clin Cancer Res.
14:1659–1668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buch K, Peters T, Nawroth T, Sänger M,
Schmidberger H and Langguth P: Determination of cell survival after
irradiation via clonogenic assay versus multiple MTT Assay-a
comparative study. Radiat Oncol. 7:12012. View Article : Google Scholar : PubMed/NCBI
|