Introduction
CML is a myeloproliferative disorder that is
characterized by the presence of the reciprocal translocation
t(9;22), which forms the Philadelphia (Ph) chromosome. During this
translocation, the breakpoint cluster region (BCR) gene at
position 22q11.2 is juxtaposed to the c-Abelson (ABL1) gene
at 9q34.1, forming the BCR-ABL1 fusion gene, which encodes a
constitutively active tyrosine kinase (TK) protein (1,2). The
constitutively active protein is associated with increased levels
of erythrocytes, monocytes, megakaryocytes, myelocytes and
platelets in the peripheral blood and marked myeloid hyperplasia in
the bone marrow (3).
CML presents in one of three phases: Chronic phase,
accelerated phase or blast crisis. The latter is of myeloid,
lymphoid or mixed-lineage phenotype (4).
TKIs have markedly changed the approach to CML
management. TKIs have improved patient outcomes to the extent that
they are now currently accepted as the first-line agents for nearly
all patients with CML, regardless of the phase of the disease.
However, certain patients experience resistance to these
medications; this occurs through several mechanisms including the
accumulation of additional cytogenetic abnormalities, which can
confer a survival advantage to the treated myeloid cells. The most
common cytogenetic abnormalities include an additional Ph
chromosome, trisomy 8 and isochromosome 17q (5,6). Several
other less common cytogenetic abnormalities have been reported;
however, to the best of our knowledge, those found in the present
case have not been previously reported.
Case report
Presentation
The present case involves a 27-year-old Saudi male
patient, whose initial presentation was three years prior. At that
time, he presented with pallor and abdominal distension. He was
revealed to have significant splenomegaly and marked leukocytosis,
with a white blood cell (WBC) count of 105×109/l.
Subsequent investigations confirmed the diagnosis of CML. The
patient was initially treated with imatinib; however, due to
myelosuppression, the treatment was changed to dasatinib.
Subsequently, due to a skin reaction, the treatment was changed to
nilotinib (100 mg/day), which the patient clinically responded to
and tolerated well.
At his current presentation, the patient had fever,
bone pain and cytopenia. Investigations confirmed the diagnosis of
precursor B cell acute lymphoblastic leukemia with the presence of
the novel combined chromosomal abnormalities of non-Ph der(22),
i(9), and der(20), carrying the BCR-ABL1 fusion gene.
Complete blood count (CBC)
CBC with differential was performed using an
Automatic Hematological Analyzer Sysmex XE-5000 (Sysmex America,
Inc., Lincolnshire, IL, USA).
Immunophenotyping
Immunophenotyping was performed on the patient's
bone marrow aspirate as follows; upon collection of the bone marrow
aspirate, 0.5 ml of the sample was mixed with 10 ml of red blood
cell lysing solution and centrifuged at 540 × g for 5 min. The
supernatant was discarded and the cell pellet further washed with
PBS. A 100 µl aliquot of cell suspension with an adjusted
concentration of 10×109 cells/l was added to tubes
containing commercial pre-titrated volumes of labelled monoclonal
antibody cocktails to bind several antigens, surface and
cytoplasmic clusters of differentiation (CD) (BD Biosciences, San
Jose, CA, USA) and incubated in the dark for 15 min at room
temperature. These monoclonal antibodies were used in conjunction
with four fluorochromes [fluorescein isothiocyanate (FITC),
phycoerythrin (PE), allophycocyanin (APC) and peridinin chlorophyll
(PerCP)] in each tube where they were diluted by factor of 20. FITC
labelled antibodies bound to CD14 (cat. no. 561712), surface and
cytoplasmic IgG (cat. no. 560952), surface and cytoplasmic CD3
(cat. no. 561806), CD34 (cat. no. 560942), cytoplasmic CD66 (cat.
no. 551479), IgM (cat. no. 562029), CD33 (cat. no. 561818), CD38
(cat. no. 560982), CD2 (cat. no. 561759), CD64 (cat. no. 560970),
terminal deoxynucleotidyl transferase (TdT) (cat. no. 347194), CD45
(cat. no. 560976) and (myeloperoxidase) MPO (cat. no. 340580). PE
labelled antibodies bound to surface and cytoplasmic IgG (cat. no.
560951), cytoplasmic CD11 (cat. no. 560999), CD8 (cat. no. 560959),
CD10 (cat. no. 561002), CD56 (cat. no. 561903), CD117 (cat. no.
561682), CD13 (cat. no. 560998), CD7 (cat. no. 561934), CD58 (cat.
no. 560959) and cytoplasmic CD79a (cat. no. 555935). APC labelled
antibodies bound surface and cytoplasmic IgG (cat. no. 562025),
CD20 (cat. no. 560900), CD4 (cat. no. 561840), CD19 (cat. no.
561742), CD15 (cat. no. 561716), CD22 (cat. no. 562860), CD11b
(cat. no. 340937), CD5 (cat. no. 340583), HLA-DR (cat. no. 560896),
cytoplasmic CD22 (cat. no. 562860), CD25 (cat. no. 340939) and CD34
(cat. no. 560940). PerCP labelled antibodies bound CD45 (cat. no.
561086) and cytoplasmic CD3 (cat. no. 347344).
For intracellular staining (i.e., for CD79a, CD3,
TdT and MPO), lymphocyte permeabilization preceded the addition of
cytoplasmic and nuclear antibodies. This was achieved by adding 0.5
ml FACS Permeabilizing Solution to 100 µl of the lysed sample
followed by 10-minute dark incubation at room temperature. The FACS
Permeabilizing Solution was prepared by diluting 1 ml of BD
Permeabilizing stock solution (cat. no. 340973; BD Biosciences) in
9 ml of distilled water.
The samples were processed with a FACSCanto II cell
analyzer and the analysis was performed using FACS Diva Software
(version 8.0.1; BD Biosciences). The flow cytometry data was
analyzed with a threshold of 25,000 events. For gating, forward
scatter, side scatter, CD45, CD3, CD19 in addition to other lineage
specific markers were used. The lineages of the blasts were
determined in each case depending upon the expression of
lineage-specific markers where an expression for a certain marker
was considered positive if the percentage of the cells expressing
that marker was ≥20% and negative if <20%, except for TdT and
MPO where the threshold was 10% as recommended (7).
Cytogenetic analysis
Chromosomal analysis using GTG banding was performed
as described previously (8).
Karyotyping was performed in 20 metaphases from the patient's
unstimulated bone marrow sample according to the nomenclature of
the International System for Human Cytogenetics (9).
Fluorescence in situ hybridization
(FISH)
FISH was performed using Vysis BCR-ABL1
Tri-Color Dual-Fusion FISH Probes (Abbott Pharmaceutical Co. Ltd.,
Lake Bluff, IL, USA) to detect the BCR-ABL1 translocation,
as described previously (10).
Molecular analysis
EDTA whole blood samples from the patient were used
for quantification of the BCR-ABL1 P210 transcript by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). This was performed using the GeneXpert® Dx
System (Roche Diagnostics GmbH, Mannheim, Germany) as described
previously (11).
Initial presentation
At initial presentation, the patient's CBC with
differential revealed a hemoglobin (Hb) level of 8.6 g/dl, a
platelet count of 188×109/l and a WBC count of
105×109/l, comprised of 52% neutrophils, 1% lymphocytes,
3% monocytes, 0% eosinophils, 0% basophils, 1% promyelocytes, 2%
myelocytes and 5% blast cells. The patient's peripheral blood smear
revealed normocytic hypochromic anemia with anisocytosis and
schistocytosis. There was marked leukocytosis with a significant
number of immature myeloid precursors, indicating
leukoerythroblastosis. The patient's bone marrow aspirate was
hemodiluted, but revealed moderate cellularity with the presence of
myeloid, erythroid and megakaryocytic lineages and ~5% blast cells.
The karyotype of each of the 10 metaphases obtained from the bone
marrow aspirate was 46,XY,t(9;22)(q34.1;q11.2). FISH analysis
confirmed the presence of the BCR-ABL1 fusion in 95% of
nuclei in the aspirate. Molecular studies of the patient's
peripheral blood determined a BCR-ABL1 fusion transcript
level of 100% International Scale (IS) units. Based on these
results, the patient was diagnosed with CML.
Current status
At the current presentation, the patient's CBC with
differential revealed an Hb level of 10.5 g/dl, a platelet count of
37×109/l and a WBC count of 0.9×109/l,
comprised of 48% neutrophils, 25% lymphocytes, 6% variant
lymphocytes, 2% monocytes, 0% eosinophils, 0% basophils and 14%
blast cells. The peripheral blood smear revealed pancytopenia with
the presence of blasts.
The bone marrow aspirate revealed markedly increased
blasts with markedly decreased myeloid, erythroid and
megakaryocytic cell lineages. Immunophenotyping analysis of the
aspirate revealed that the blast cells were positive for CD34
(partial, i.e., only a subset of the population of interest was
positive), cytoplasmic CD79a, CD19, CD10, cytoplasmic TdT, CD20,
HLA-DR, CD73 (partial), CD58, CD44, CD200, CD24, cytoplasmic CD66
and CD72 antigens. The cells were negative for cytoplasmic
myeloperoxidase, cytoplasmic CD3, surface CD3, CD7, surface IgM,
CD45 (negative to low), CD117, cytoplasmic CD22, CD33, CD13, CD38,
CD123 and CD86 antigens, consistent with the precursor B-cell
lymphoblastic nature of these blast cells.
A bone marrow biopsy revealed 99% cellularity with
95–99% blasts. The number of morphologically normal myeloid,
erythroid and megakaryocytic cells was markedly decreased to
absent. Karyotyping performed on 20 metaphases from the bone marrow
revealed the following:
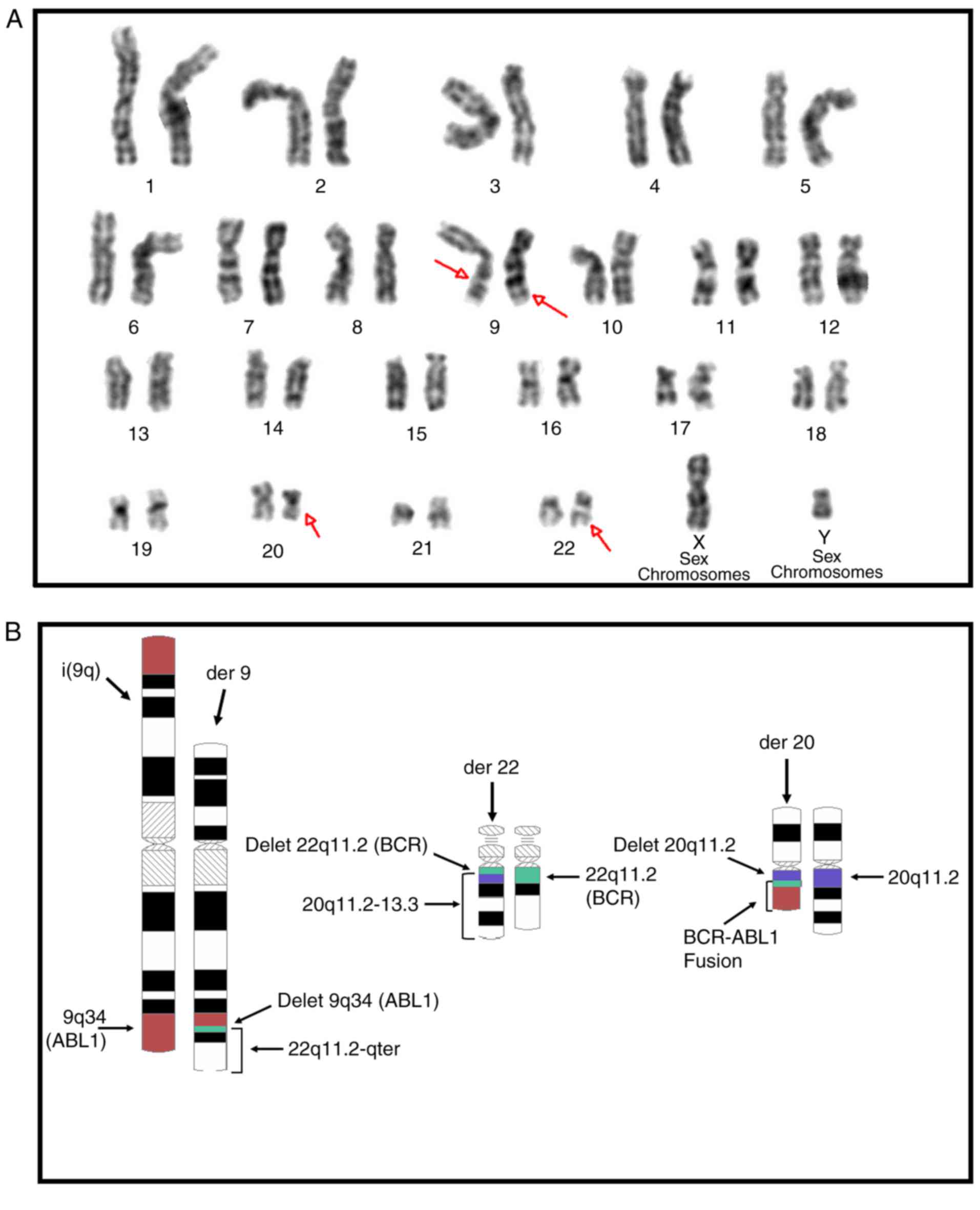
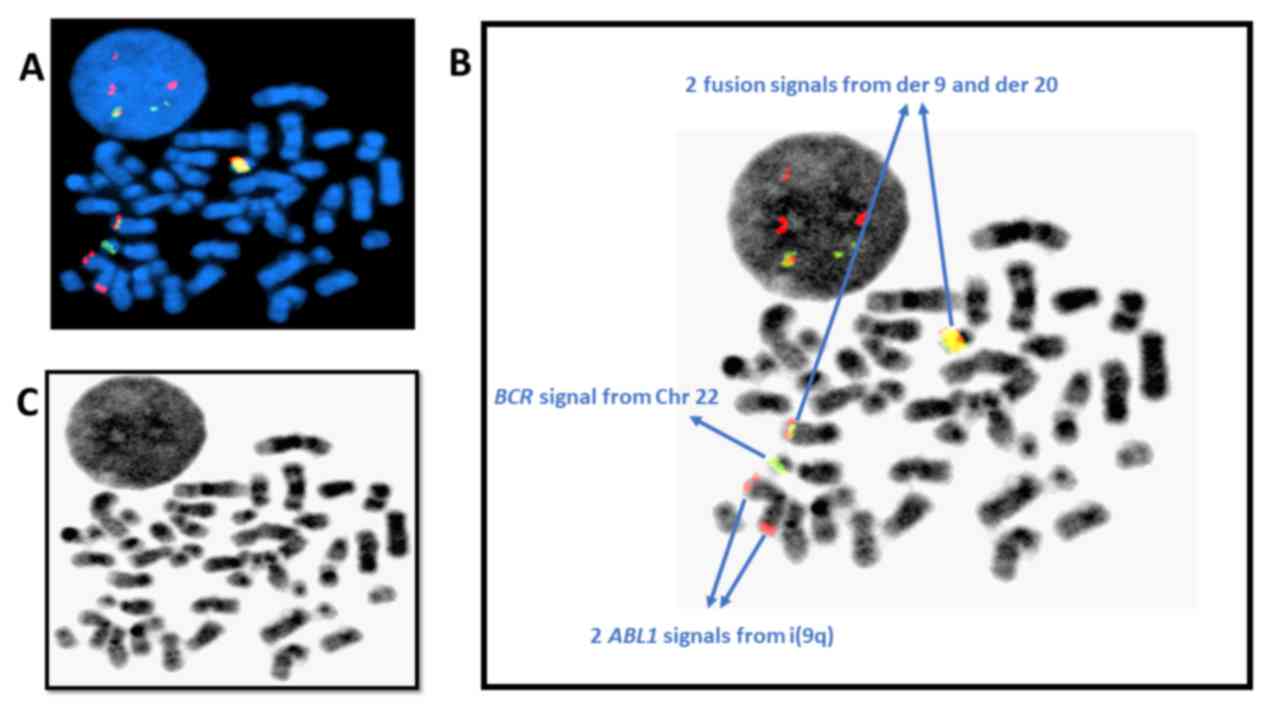
46,XY,i(9)(q10),der(22)t(9;22)(q34.1;q11.2)t(20;22)(q11.2;q11.2)[18]/46,XY[2](Fig.
1A). This result, combined with that of the FISH analysis,
confirmed the presence of a clone with the concurrent cytogenetic
abnormalities of i(9)(q10), non-Philadelphia der(22) and der(20)
carrying the BCR-ABL1 fusion gene (Figs. 1B and 2A-C).
Discussion
The blast crisis demonstrated in this case was of
the precursor B cell lymphoblastic type. It is well established
that ~30% of blast crises in CML are of the lymphoid rather than
the myeloid phenotype. The aberrant cytogenetic abnormalities
observed in this case, in addition to the BCR-ABL1 fusion
during Ph chromosome formation, were i(9q)(q10) formation and the
reciprocal translocation between the Ph chromosome and 20q11.2. To
the best of our knowledge, this is the first case reported to
combine these cytogenetic aberrations. This case report also links
these cytogenetic aberrations to the precursor B cell lymphoblast
phenotype. Ascertaining the blast phenotype has its own therapeutic
implications, since the treatment protocol of lymphoid blast crisis
is different to that of the myeloid type. In other words, linking
these abnormalities to lymphoid crisis, in this case, had
therapeutic implications since this directed the treatment toward
vincristine- and prednisone-based protocols (i.e., lymphoid blast
crisis protocol).
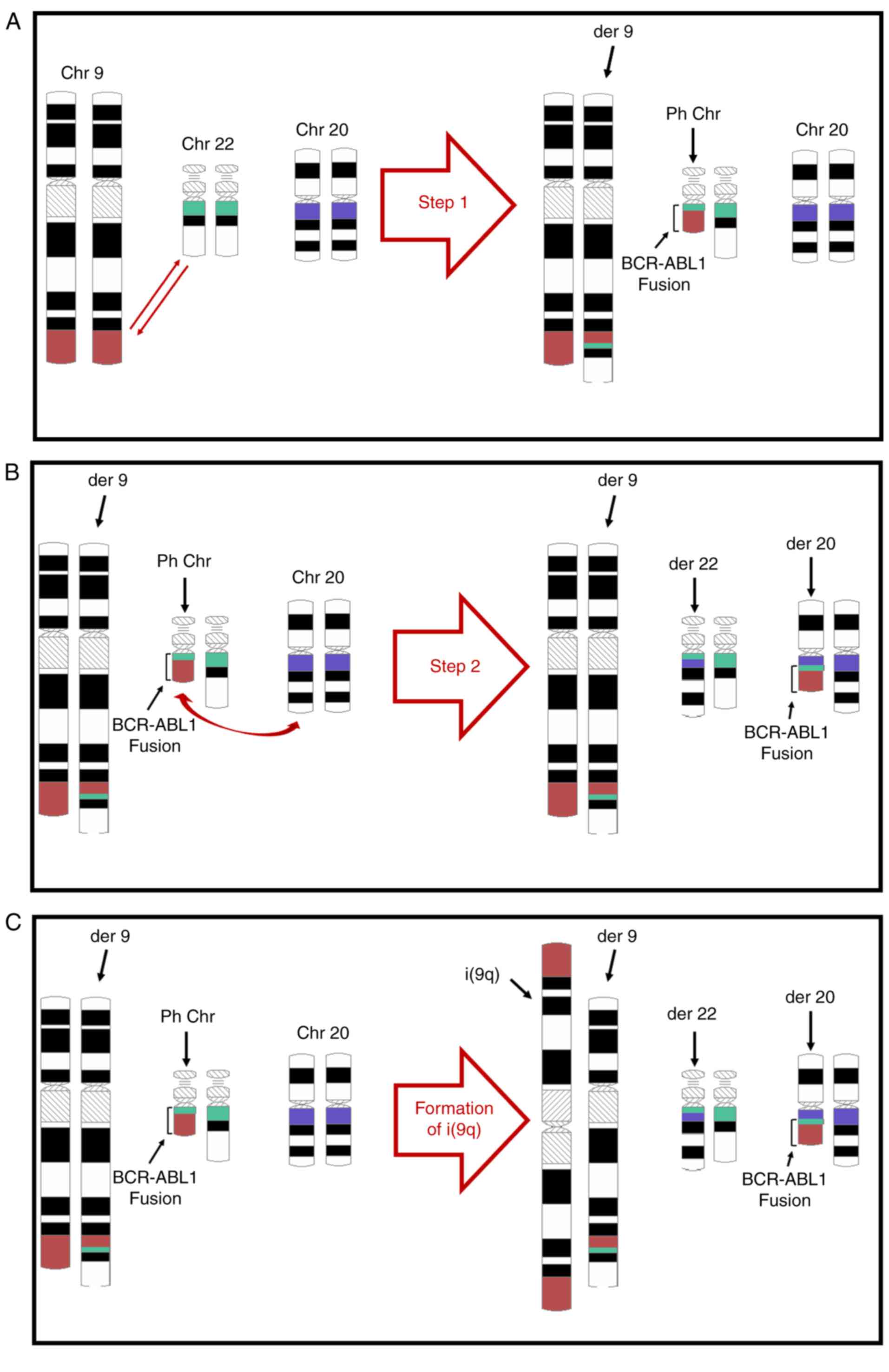
From the patient's history, initial karyotyping and
FISH results, it may be concluded that these cytogenetic
abnormalities did not occur simultaneously. The first abnormality
was the classical t(9;22)(q34.1;q11.2), resulting in Ph chromosome
formation (Fig. 3A). The other two
abnormalities were the i(9)(q10) formation and the reciprocal
translocation between Ph and 22 chromosomes, transferring the
BCR-ABL1 fusion gene to the latter (Fig. 3B and C). These two events must have
followed the Ph chromosome formation during the clonal evolution
that conferred TKI resistance. It is not possible to tell whether
these two latter events occurred simultaneously, or their order of
occurrence if they did occur sequentially. For demonstration
purposes only, i(9)(q10) formation is indicated in Fig. 3C to be the final event.
It is likely that the B cell lymphoid nature of the
blast crisis observed in this patient is attributed to the loss of
9p during i(9q) formation. This possibility stems from the fact
that 9p loss is a known recurrent cytogenetic abnormality observed
in chronic lymphocytic leukemia and mantle cell lymphoma (12). This arm contains genes that are
important for B cell differentiation and cell cycle regulation,
including PAX5 and CDKN2A. The paired box gene
PAX5 was altered in 32% of precursor B cell acute
lymphocytic leukemia (ALL) cases (13). In Ph-positive-ALL patients, an
additional 9p abnormality [including i(9)(q10)] had a negative
impact on disease-free survival (14–16). This
predicts a poor prognosis for the patient discussed in the present
study; however, extended follow-up is required to confirm this.
Due to patient being <40 years old, they were
subjected to a pediatric Ph-positive-ALL treatment protocol, as
recommended (17). He received
imatinib (400 mg daily) and 4 drugs as induction therapy:
Daunorubicin (25 mg/m2 weekly for 4 doses), vincristine
[2 mg/week intravenously for 4 doses], prednisone (60
mg/m2 for 28 days) and asparaginase (2,500
U/m2 on day 6). He also received intrathecal central
nervous system prophylaxis chemotherapy with cytarabine on day 1
and methotrexate on days 8 and 29. A complete remission was
achieved on day 29, which was confirmed by flow
cytometry, cytogenetic and molecular testing
results, with minimal residual disease (<0.1%) and undetectable
levels of BCR-ABL1 mRNA. The patient then continued to
receive 400 mg imatinib daily.
Consolidation therapy commenced on day 36 of
induction. Phase one consisted of etoposide (100 mg/m2)
and ifosfamide (1,800 mg/m2), daily for 5 days.
Intrathecal therapy was administered on days 8 and 15 of
consolidation therapy, and consisted of methotrexate (15 mg),
hydrocortisone (15 mg) and cytarabine (30 mg). Phase two of
consolidation consisted of high-dose methotrexate (5,000
mg/m2) on day 1 and high-dose cytarabine (3,000
mg/m2) every 12 h on days 2 and 3 (4 doses in total).
Triple intrathecal therapy was administered on day 1. The patient
has recently undergone tissue-matched stem cell
transplantation.
In conclusion, the present study reports a case of
TKI-resistant Ph-positive-CML presenting with lymphoblastic crisis
wherein the blast cells, in addition to the Ph chromosome,
exhibited additional novel combined cytogenetic abnormalities. This
report adds to the literature on newly identified
TKI-resistance-conferring cytogenetic abnormalities, and also links
them to precursor B cell lymphoblastic crisis. This also has its
own therapeutic implications since the blast phenotype determines
the treatment protocol.
Acknowledgements
The authors would like to thank Ms. Amal Al-Khattaf
(Flow Cytometry Section, King Fahad Specialist Hospital) for her
assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HAK wrote the manuscript, drew the figures, designed
the study, interpreted the data, contributed to addressing all
questions related to the accuracy and integrity of this study, and
given his final approval of the version to be published. HA
initiated this research project and interpreted the cytogenetic
results. AA followed up the patient and provided the test results.
All authors read and approved the final manuscript.
Ethics approval
Approval from Johns Hopkins Aramco Healthcare (JHAH)
Institutional Review Board and Ethics Committee was obtained to
publish this case report.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
figures.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CML
|
chronic myeloid leukemia
|
|
Ph
|
Philadelphia
|
|
TK
|
tyrosine kinase
|
|
TKI
|
tyrosine kinase inhibitor
|
|
BCR
|
breakpoint cluster region gene
|
|
ABL1
|
c-Abelson gene
|
|
WBC
|
white blood cell
|
|
CBC
|
complete blood count
|
|
CD
|
cluster of differentiation
|
|
FITC
|
fluorescein isothiocyanate
|
|
PE
|
phycoerythrin
|
|
APC
|
allophycocyanin
|
|
PerCP
|
peridinin chlorophyll
|
|
TdT
|
terminal deoxynucleotidyl
transferase
|
|
MPO
|
myeloperoxidase
|
|
FISH
|
fluorescence in situ hybridization
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
Hb
|
hemoglobin
|
|
IS
|
International Scale
|
|
ALL
|
acute lymphocytic leukemia
|
References
|
1
|
Kuru D, Argüden Tarkan Y, Ar M, Çırakoğlu
A, Öngören Ş, Yılmaz Ş, Eşkazan A, Deviren A, Soysal T,
Hacıhanefioğlu S and Ülkü B: Variant Philadelphia translocations
with different breakpoints in six chronic myeloid leukemia
patients. Turk J Haematol. 28:186–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aliano S, Cirmena G, Fugazza G, Bruzzone
R, Palermo C and Sessarego M: Standard and variant Philadelphia
translocation in a CML patient with different sensitivity to
imatinib therapy. Leuk Res Rep. 2:75–78. 2013.PubMed/NCBI
|
|
3
|
Sawyers CL: Chronic myeloid leukemia. N
Engl J Med. 17:1330–1340. 1999. View Article : Google Scholar
|
|
4
|
Cervantes F, Villamor N, Esteve J, Montoto
S, Rives S, Rozman C and Montserrat E: ‘Lymphoid’ blast crisis of
chronic myeloid leukaemia is associated with distinct
clinicohaematological features. Br J Haematol. 100:123–128. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milojkovic D and Apperley J: Mechanisms of
resistance to imatinib and second-generation tyrosine inhibitors in
chronic myeloid leukemia. Clin Cancer Res. 15:7519–7527. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johansson B, Fioretos T and Mitelman F:
Cytogenetic and molecular genetic evolution of chronic myeloid
leukemia. Acta Haematol. 107:76–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdulsalam AH, Nadal-Melsio E and Naresh
KN: Complementarity of evaluation of myeloperoxidase expression by
flow cytometry and immunohistochemistry on bone marrow trephine
biopsy sections in acute myeloid leukemia. Cytometry B Clin Cytom.
86:70–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Claussen U, Michel S, Mühlig P, Westermann
M, Grummt UW, Kromeyer-Hauschild K and Liehr T: Demystifying
chromosome preparation and the implications for the concept of
chromosome condensation during mitosis. Cytogenet Genome Res.
98:136–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simons A, Shaffer LG and Hastings RJ:
Cytogenetic nomenclature: Changes in the ISCN 2013 compared to the
2009 edition. Cytogenet Genome Res. 141:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siu LL, Ma ES, Wong WS, Chan MH and Wong
KF: Application of tri-colour, dual fusion fluorescence in situ
hybridization (FISH) system for the characterization of BCR-ABL1
fusion in chronic myelogenous leukaemia (CML) and residual disease
monitoring. BMC Blood Disord. 9:42009.PubMed/NCBI
|
|
11
|
Jobbagy Z, van Atta R, Murphy KM, Eshleman
JR and Gocke CD: Evaluation of the Cepheid GeneXpert BCR-ABL assay.
J Mol Diagn. 9:220–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beà S, López-Guillermo A, Ribas M, Puig X,
Pinyol M, Carrió A, Zamora L, Soler F, Bosch F, Stilgenbauer S, et
al: Genetic imbalances in progressed B-cell chronic lymphocytic
leukemia and transformed large-cell lymphoma (Richter's syndrome).
Am J Pathol. 161:957–968. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mullighan CG, Goorha S, Radtke I, Miller
CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds
SB, et al: Genome-wide analysis of genetic alterations in acute
lymphoblastic leukaemia. Nature. 446:758–764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haidar MA, Cao XB, Manshouri T, Chan LL,
Glassman A, Kantarjian HM, Keating MJ, Beran MS and Albitar M:
p16INK4A and p15INK4B gene deletions in primary leukemias. Blood.
86:311–315. 1995.PubMed/NCBI
|
|
15
|
Mrózek K, Harper DP and Aplan PD:
Cytogenetics and molecular genetics of acute lymphoblastic
leukemia. Hematol Oncol Clin North Am. 23:991–1010. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yanada M, Takeuchi J, Sugiura I, Akiyama
H, Usui N, Yagasaki F, Kobayashi T, Ueda Y, Takeuchi M, Miyawaki S,
et al: Karyotype at diagnosis is the major prognostic factor
predicting relapse-free survival for patients with Philadelphia
chromosome-positive acute lymphoblastic leukemia treated with
imatinib-combined chemotherapy. Haematologica. 93:287–290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kliman D, Barnett M, Broady R, Forrest D,
Gerrie A, Hogge D, Nantel S, Narayanan S, Nevill T, Power M, et al:
Pediatric-based versus adult treatment protocols in young adults
(18-40 years) with standard risk acute lymphoblastic leukemia: The
BC cancer agency experience. Blood. 126:37702015.
|