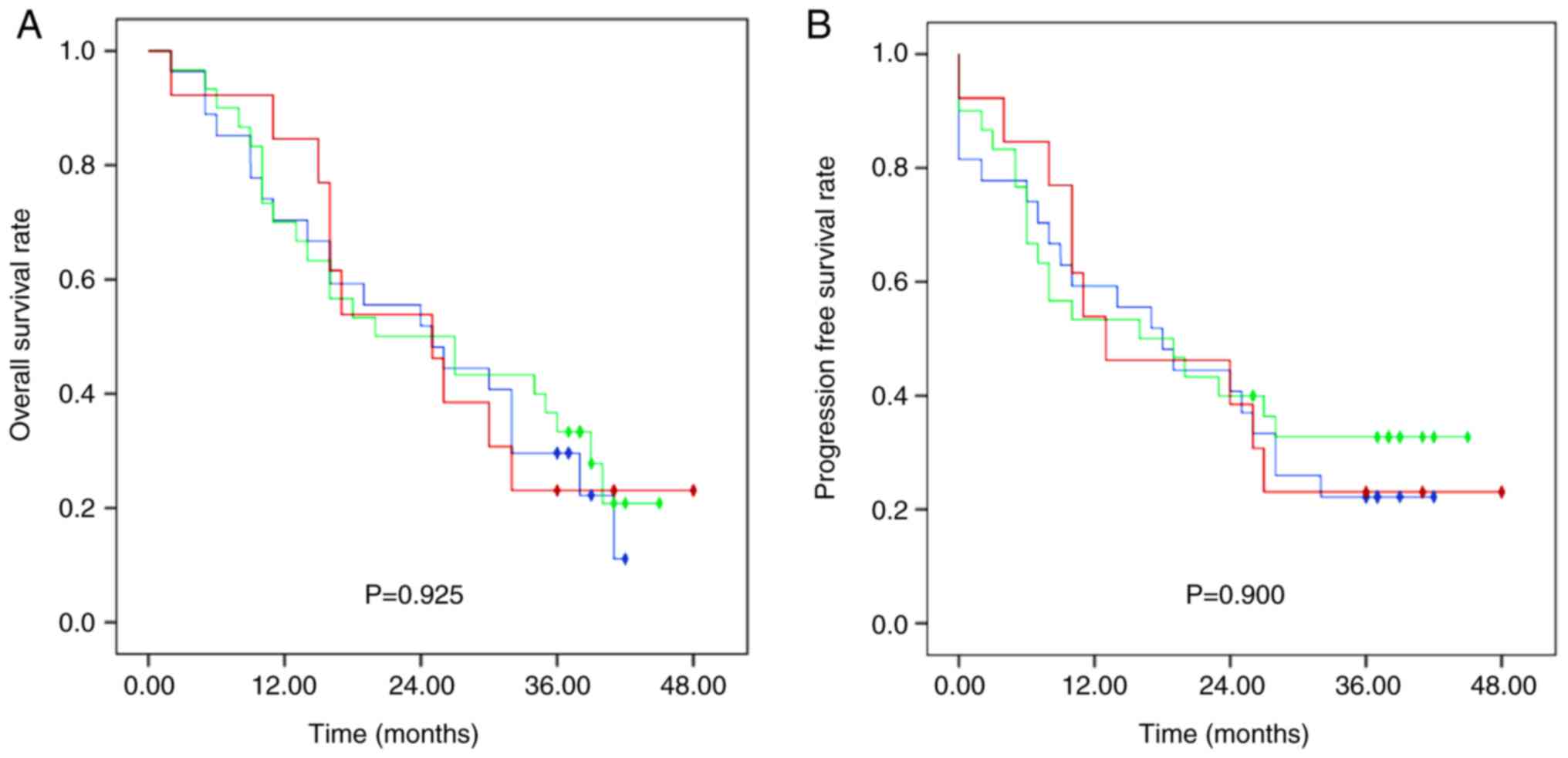
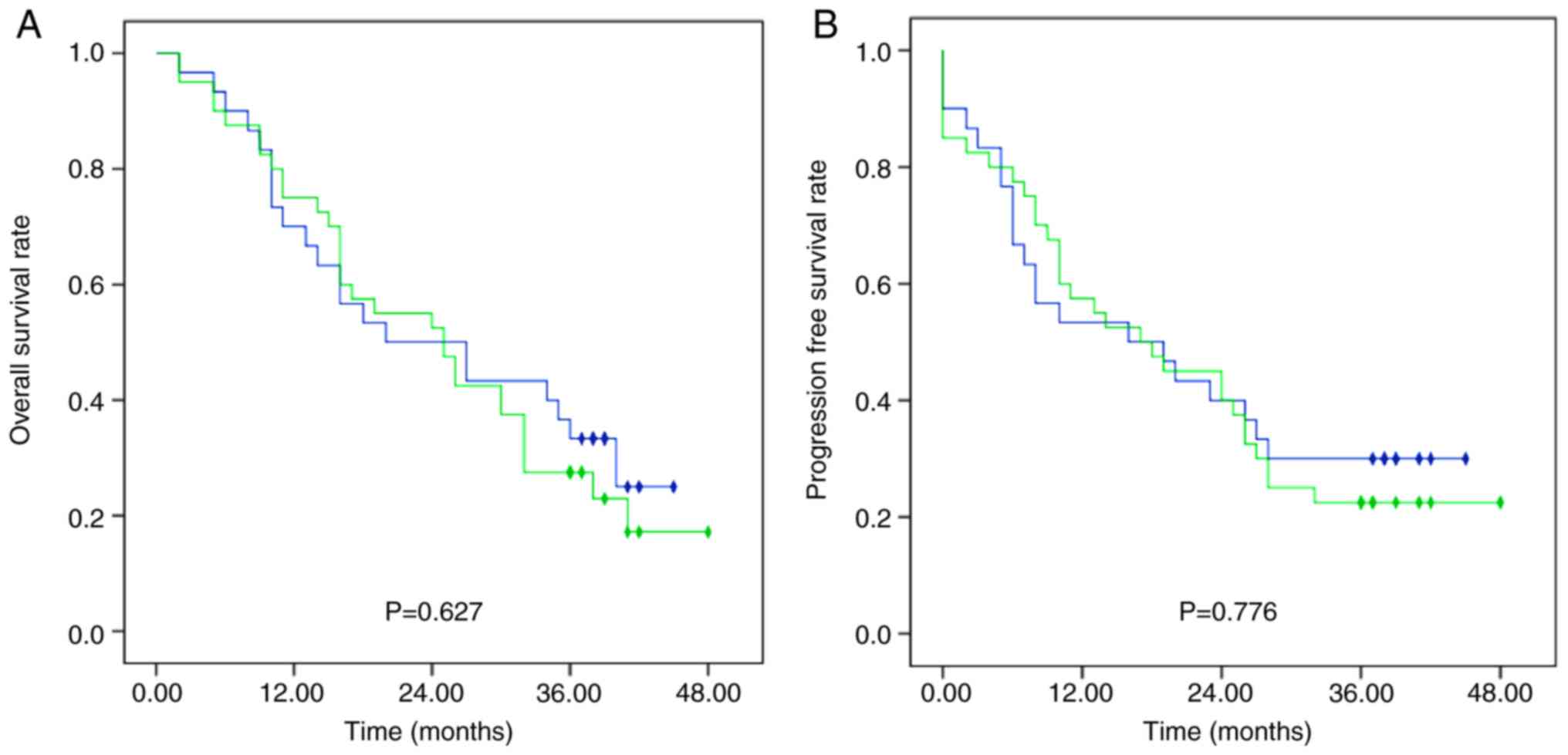
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Pardo BJ, Bronson NW, Diggs BS, Thomas
CR Jr, Hunter JG and Dolan JP: The global burden of esophageal
cancer: A disability-adjusted life-year approach. World J Surg.
40:395–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W: Cancer statistics: Updated cancer
burden in China. Chin J Cancer Res. 27:12015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen DJ and Ajani J: An expert opinion on
esophageal cancer therapy. Expert Opin Pharmacother. 12:225–239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation therapy oncology group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji K, Zhao L, Yang C, Meng M and Wang P:
Three-dimensional conformal radiation for esophageal squamous cell
carcinoma with involved-field irradiation may deliver considerable
doses of incidental nodal irradiation. Radiat Oncol. 7:2002012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Li M, Meng X, Kong L, Zhang Y,
Wei G, Zhang X, Shi F, Hu M, Zhang G and Yu J: Involved-field
irradiation in definitive chemoradiotherapy for locally advanced
esophageal squamous cell carcinoma. Radiat Oncol. 9:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HJ, Suh YG, Lee YC, Lee SK, Shin SK,
Cho BC and Lee CG: Dose-response relationship between radiation
dose and loco-regional control in patients with stage II-III
esophageal cancer treated with definitive chemoradiotherapy. Cancer
Res Treat. 49:669–677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herskovic A, Martz K, al-Sarraf M,
Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L
and Emami B: Combined chemotherapy and radiotherapy compared with
radiotherapy alone in patients with cancer of the esophagus. N Engl
J Med. 326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suntharalingam M, Moughan J, Coia LR,
Krasna MJ, Kachnic L, Haller DG, Willett CG, John MJ, Minsky BD and
Owen JB: 1996-1999 Patterns of CareStudy: The national practice for
patients receiving radiation therapy for carcinoma of the
esophagus: Results of the 1996-1999 Patterns of Care Study. Int J
Radiat Oncol Biol Phys. 56:981–987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu CH, Yeh KH, Lui LT, Lee YC, Bu CF,
Wang HP, Lin JT and Cheng AL: Concurrent chemoradiotherapy for
locally advanced esophageal cancer-a pilot study by using daily
low-dose cisplatin and continuous infusion of 5-fluorouracil.
Anticancer Res. 19:4463–4467. 1999.PubMed/NCBI
|
|
13
|
Tu L, Sun L, Xu Y, Wang Y, Zhou L, Liu Y,
Zhu J, Peng F, Wei Y and Gong Y: Paclitaxel and cisplatin combined
with intensity-modulated radiotherapy for upper esophageal
carcinoma. Radiat Oncol. 8:752013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu HT, Ai DS, Tang HR, Badakhshi H, Fan
JH, Deng JY, Zhang JH, Chen Y, Zhang Z, Xia Y, et al: Long-term
results of paclitaxel plus cisplatin with concurrent radiotherapy
for loco-regional esophageal squamous cell carcinoma. World J
Gastroenterol. 23:540–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo JF, Zhang B, Wu F, Wang B, Xing H, Zhu
GY, Nie XY and Peng J: A phase II trial of docetaxel plus
nedaplatin and 5-fluorouracil in treating advanced esophageal
carcinoma. Chin J Cancer. 29:321–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao T, Chen H and Zhang T: Docetaxel and
cisplatin concurrent with radiotherapy versus 5-fluorouracil and
cisplatin concurrent with radiotherapy in treatment for locally
advanced oesophageal squamous cell carcinoma: A randomized clinical
study. Med Oncol. 29:3017–3023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang F, Wang Y, Wang ZQ, Sun P, Wang DS,
Jiang YX, Zhang DS, Wang FH, Xu RH and Li YH: Efficacy and safety
of cisplatin-based versus nedaplatin-based regimens for the
treatment of metastatic/recurrent and advanced esophageal squamous
cell carcinoma: A systematic review and meta-analysis. Dis
Esophagus. 30:1–8. 2017. View Article : Google Scholar
|
|
18
|
Sato Y, Takayama T, Sagawa T, Okamoto T,
Miyanishi K, Sato T, Araki H, Iyama S, Abe S, Murase K, et al: A
phase I/II study of nedaplatin and 5-fluorouracil with concurrent
radiotherapy in patients with esophageal cancer. Cancer Chemother
Pharmaco. 58:570–576. 2006. View Article : Google Scholar
|
|
19
|
Tanaka Y, Yoshida K, Tanahashi T, Okumura
N, Matsuhashi N and Yamaguchi K: Phase II trial of neoadjuvant
chemotherapy with docetaxel, nedaplatin, and S1 for advanced
esophageal squamous cell carcinoma. Cancer Sci. 107:764–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka Y, Yoshida K, Osada S, Yamaguchi K
and Takahashi T: Docetaxel, nedaplatin, and S-1 (DGS) chemotherapy
for advanced esophageal carcinoma: A phase I dose-escalation study.
Anticancer Res. 31:4589–4597. 2011.PubMed/NCBI
|
|
21
|
Ohba A, Kato K, Ito Y, Katada C, Ishiyama
H, Yamamoto S, Ura T, Kodaira T, Kudo S and Tamaki Y:
Chemoradiation therapy with docetaxel in elderly patients with
stage II/III esophageal cancer: A phase 2 trial. Adv Radiat Oncol.
1:230–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ajani JA, Buyse M, Lichinitser M,
Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha
R, Carrato A, et al: Combination of cisplatin/S-1 in the treatment
of patients with advanced gastric or gastroesophageal
adenocarcinoma: Results of noninferiority and safety analyses
compared with cisplatin/5-fluorouracil in the First-Line Advanced
Gastric Cancer Study. Eur J Cancer. 49:3616–3624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang H, Shin SK, Cho BC, Lee CG, Kim CB,
Kim DJ, Lee JG, Hur J, Lee CY, Bae MK, et al: A prospective phase
II trial of S-1 and cisplatin-based chemoradiotherapy for
locoregionally advanced esophageal cancer. Cancer Chemother
Pharmacol. 73:665–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takigawa N, Segawa Y, Ueoka H, Kiura K,
Tabata M, Shibayama T, Takata I, Miyamoto H, Eguchi K and Harada M:
Combination of nedaplatin and vindesine for treatment of relapsed
or refractory non-small-cell lung cancer. Cancer Chemother
Pharmacol. 46:272–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hartmann JT and Lipp HP: Toxicity of
platinum compounds. Expert Opin Pharmacother. 4:889–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takechi T, Nakano K, Uchida J, Mita A,
Toko K, Takeda S, Unemi N and Shirasaka T: Antitumor activity and
low intestinal toxicity of S-1, a new formulation of oral tegafur,
in experimental tumor models in rats. Cancer Chemother Pharmacol.
39:205–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakata B, Mitachi Y, Tsuji A, Yamamitsu S,
Hirata K, Shirasaka T and Hirakawa K: Combination Phase I trial of
a novel oral fluorouracil derivative S-1 with low-dose cisplatin
for unresectable and recurrent gastric cancer (JFMC27-9902). Clin
Cancer Res. 10:1664–1669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akutsu Y, Shuto K, Kono T, Uesato M,
Hoshino I, Shiratori T, Miyazawa Y, Isozaki Y, Akanuma N and
Matsubara H: A phase 1/11 study of second-line chemotherapy with
fractionated docetaxel and nedaplatin for 5-FU/cisplatin-resistant
esophageal squamous cell carcinoma. Hepatogastroenterology.
59:2095–2098. 2012.PubMed/NCBI
|
|
32
|
Miyazaki T, Ojima H, Fukuchi M, Sakai M,
Sohda M, Tanaka N, Suzuki S, Ieta K, Saito K, Sano A, et al: Phase
II study of docetaxel, nedaplatin, and 5-fluorouracil combined
chemotherapy for advanced esophageal cancer. Ann Surg Oncol.
22:3653–3658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suh YG, Lee IJ, Koom WS, Cha J, Lee JY,
Kim SK and Lee CG: High-dose versus standard-dose radiotherapy with
concurrent chemotherapy in stages II-III esophageal cancer. Jpn J
Clin Oncol. 44:534–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamashita H, Okuma K, Wakui R,
Kobayashi-Shibata S, Ohtomo K and Nakagawa K: Details of recurrence
sites after elective nodal irradiation (ENI) using 3D-conformal
radiotherapy (3D-CRT) combined with chemotherapy for thoracic
esophageal squamous cell carcinoma-a retrospective analysis.
Radiother Oncol. 98:255–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu FM, Lee JM, Huang PM, Lin CC, Hsu CH,
Tsai YC, Lee YC and Chia-Hsien Cheng J: Retrospective analysis of
outcome differences in preoperative concurrent chemoradiation with
or without elective nodal irradiation for esophageal squamous cell
carcinoma. Int J Radiat Oncol Biol Phys. 81:593–599. 2011.
View Article : Google Scholar
|
|
36
|
Onozawa M, Nihei K, Ishikura S, Minashi K,
Yano T, Muto M, Ohtsu A and Ogino T: Elective nodal irradiation
(ENI) in definitive chemoradiotherapy (CRT) for squamous cell
carcinoma of the thoracic esophagus. Radiother Oncol. 92:266–269.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim HW, Kim JH, Lee IJ, Kim JW, Lee YC,
Lee CG, Park JJ, Youn YH and Park H: Local control may be the key
in improving treatment outcomes of esophageal squamous cell
carcinoma undergoing concurrent chemoradiation. Digestion.
90:254–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita H, Takenaka R, Omori M, Imae T,
Okuma K, Ohtomo K and Nakagawa K: Involved-field radiotherapy
(IFRT) versus elective nodal irradiation (ENI) in combination with
concurrent chemotherapy for 239 esophageal cancers: A single
institutional retrospective study. Radiat Oncol. 10:1712015.
View Article : Google Scholar : PubMed/NCBI
|