Introduction
Esophageal cancer (EC) is the eighth most common
cancer worldwide and the sixth leading cause of cancer-associated
mortality (5-year survival, 15–25%) (1,2). Over
one-half of all newly diagnosed EC cases occur in China, where the
incidence rate is 100-times that in western countries (3). Squamous cell carcinoma (SCC) accounts
for ~95% of all cases (4).
Since the landmark results of the RTOG85-01 clinical
trial (5), concurrent
chemoradiotherapy (CCRT) has become the standard treatment for
cases of EC which are not amenable to surgery. Previous studies
have demonstrated the clinical utility of involved field
irradiation to deliver a dose of 50.4 Gy to the clinical target
volume (CTV) (6–9). In China, debate remains regarding the
appropriate radiation dose and volume, and elective nodal
irradiation (ENI) with a dose of ≥60 Gy has been the standard
treatment in the majority of hospitals over the past decade.
Controversy also exists regarding the chemotherapy
regimen for CCRT. When used in CCRT, cisplatin (DDP) combined with
5-fluorouracil (5-FU) may evoke a tumor response and improve
patient survival (10–12). However, adverse events (AEs),
including nausea and vomiting are an issue, and the renal toxicity
of DDP and cardiotoxicity of 5-FU limit their use in elderly
patients. Other drug combinations have been reported to have
similar therapeutic effects to DDD/5-FU, whilst also being better
tolerated (13–16). Nedaplatin (NDP) is an alternative
platinum-based drug for the treatment of EC (17,18).
Furthermore, docetaxel (DOC) and tegafur-gimeracil-oteracil
potassium (S-1) have demonstrated therapeutic efficacy in EC when
used in combination with a platinum-based drug (16,19–23).
There is no current consensus regarding which of DOC
or S-1 is the preferred choice for use in combination with NDP. The
present study was designed in order to compare survival, treatment
responses and toxicities between different NDP-based CCRT regimens,
to identify the most suitable regimen for the treatment of EC.
Patients and methods
Patients
Patients with EC were retrospectively collected at
The First Affiliated Hospital of Nanjing Medical University
(Jiangsu, China) between January 1st 2012 and May 31st
2016. The inclusion criteria were: i) Histologically-confirmed SCC;
ii) clinical stage II–III, diagnosed according to the criteria of
the International Union Against Cancer 2009, 7th edition; iii) no
previously treated thoracic disease; iv) age 20–80 years; v)
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
score of 0–2; and vi) lesion length and depth of tumor invasion
measured by ultrasound gastroscopy (EUS) prior to treatment. The
exclusion criteria were: i) Patient scheduled for surgery; ii) poor
liver, kidney or bone marrow function, or diseases that may
increase treatment-associated organ dysfunction; iii) severe
cardiopulmonary diseases; iv) esophageal perforation or deep
ulceration; v) considerable esophageal bleeding; and vi)
contraindications to radiotherapy or chemotherapy. The Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University approved this study, and patients were enrolled with
their informed consent.
Data collection
Baseline characteristics, including demographic data
and ECOG PS scores were collected from patient medical records.
Upper gastrointestinal barium radiography and endoscopy were used
to confirm lesion length and upper/lower boundaries, and to exclude
esophageal fistulas. Each patient underwent an enhanced
64-multislice computed tomography (CT) scan of the cervix, chest
and abdomen for tumor node metastasis (TNM) staging, and EUS to
obtain an accurate T stage. Two titanium alloy clips placed at the
upper and lower tumor boundaries during EUS helped to delineate the
gross tumor volume (GTV) for radiotherapy.
Grouping
Patients were divided into SNR (S-1 plus NDP
concurrent with radiotherapy), DNR (DOC plus NDP concurrent with
radiotherapy) or NR (NDP alone concurrent with radiotherapy)
groups.
Radiotherapy
Radiotherapy began within a week of completing the
first chemotherapy cycle. Intensity-modulated radiation therapy
with a 6-MV X-ray was used to deliver a total dose of 60–66 Gy
(1.8–2.0 Gy per fraction) to the primary tumor and 45–50 Gy to the
subclinical region. GTV was defined as the total volume of the
primary tumor (GTVp) and involved lymph nodes (GTVnd). Positive
lymph nodes were defined as: Shortest axis diameter ≥1 cm or ≥0.5
cm if beside the recurrent laryngeal nerve; or high standardized
uptake values on positron emission tomography/CT images. Primary
tumor CTV was defined as GTVp plus a 3 cm craniocaudal margin and a
1 cm lateral margin. CTV of the involved lymph nodes was defined as
GTVnd plus 1 cm all directional margins. The elective lymph nodes
included group 1, 2, 4, 5 and 7 thoracic and supraclavicular nodes
for cervical, upper- and middle-thoracic EC, and group 2, 4, 5 and
7 thoracic, left gastric and paracardial nodes for lower-thoracic
EC. The planning target volume was defined as CTV plus a 5 mm
margin in all directions. Based on the dose-volume histogram, the
organ dose limits were set as follows: i) Mean lung dose (MLD) ≤16
Gy, V20 ≤30%; ii) mean heart dose (MHD) ≤40 Gy; and iii) maximum
spinal cord dose ≤45 Gy. If these constraints were not satisfied,
the plan was altered to: MLD <20 Gy, lung V20 <40%, and MHD
<45 Gy.
Chemotherapy
Chemotherapy began concurrently with the first week
of radiotherapy and was repeated 3 weeks later. The regimen was one
of the following: i) 100 mg/m2 NDP intravenously on day
1 and 70 mg/m2 S-1 orally twice daily for 2 weeks; ii)
50 mg/m2 NDP intravenously on day 1 and DOC
intravenously on days 1 (2 mg/m2) and 8 (35
mg/m2); or iii) 60 mg/m2 NDP intravenously on
days 1 and 2. Complete blood count and hepatic and renal functions
were examined 24 h following completion of intravenous chemotherapy
and 1 week later. Myocardial zymogram examination and
electrocardiography were used to detect treatment-induced heart
damage. The chemotherapy dose was reduced by 20% in the subsequent
cycle if grade 4 hematological or grade ≥3 non-hematological
toxicity occurred, and chemotherapy and radiotherapy were suspended
until bone marrow/other organ functions normalized. Chemotherapy
was terminated if the patient was unable to tolerate the toxicity
or withdrew. Two additional cycles of chemotherapy with the same
regimen as CCRT were performed following CCRT in patients who had
residual tumors, N2 stage and good chemotherapy tolerance.
Follow-up and evaluation
Outpatient-based follow-ups (every 3 months for the
first 12 months, then biannually) ran between the end of
chemotherapy/radiotherapy and June 30th 2017 or patient
mortality. Tumor response, recurrence and metastasis were evaluated
through systematic examinations, including physical examination,
enhanced CT of the cervix, chest and abdomen, gastroscopy and upper
gastroenterography. In accordance with the Response Evaluation
Criteria in Solid Tumors version 1.1 (24), the clinical response was classified as
complete remission (CR), partial remission (PR), stable disease
(SD) or progressive disease (PD). CR was defined in the majority of
cases as pathological CR confirmed by gastroscopy. Local recurrence
(LR) following CR was confirmed by endoscopy. Overall response rate
(ORR) was defined as CR plus PR. Regional lymph node recurrence was
diagnosed by imaging as: i) Nodes that reappear in the original
position following CR; or ii) regional nodes that newly appear
after prophylactic irradiation. Locoregional recurrence was defined
as LR plus regional node recurrence. Overall survival (OS) was
calculated from the first day of treatment to the date of mortality
from any cause or end of follow-up. Progression-free survival (PFS)
was calculated from the first day of treatment to the date of
mortality from any cause or disease progression. Acute
hematological toxicities were classified according to the National
Cancer Institute Common Toxicity Criteria version 4.0. Acute and
late toxicities of the lung and esophagus were evaluated according
to RTOG criteria.
Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Continuous variables are expressed as median +
range and categorical variables as frequencies and percentages.
Fisher's exact tests, χ2 tests, and Wilcoxon rank sum
tests were used in comparisons of patient and tumor
characteristics, toxicities and first failure patterns. Survival
data were estimated using the Kaplan-Meier method and log-rank
test. Two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
A total of 70 patients (median age, 66 years; range,
50–81 years), including 56 males (80%) with thoracic ESCC were
enrolled. Median tumor length was 5 cm (range, 2–12 cm). The
baseline characteristics are presented in Table I.
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
|
Characteristics | Total (n=70) | SNR (n=27) | DNR (n=30) | NR (n=13) | P-value |
|---|
| Age, years (%) |
|
|
|
| 0.203 |
|
<70 | 49 (70.0%) | 18 (66.7%) | 24 (80.0%) | 7 (53.8%) | – |
|
≥70 | 21 (30.0%) | 9 (33.3%) | 6 (20.0%) | 6 (46.2%) | – |
| Sex (%) |
|
|
|
| 0.880 |
|
Male | 56 (80.0%) | 21 (77.8%) | 24 (80.0%) | 11 (84.6%) | – |
|
Female | 14 (20.0%) | 6 (22.2%) | 6 (20.0%) | 2 (15.4%) | – |
| ECOG PS score
(%) |
|
|
|
| 0.112 |
| 0 | 21 (30.0%) | 10 (37.0%) | 9 (30.0%) | 2 (15.4%) | – |
| 1 | 35 (50.0%) | 14 (51.9%) | 16 (53.3%) | 5 (38.4%) | – |
| 2 | 14 (20.0%) | 3 (11.1%) | 5 (16.7%) | 6 (46.2%) | – |
| Tumor location
(%) |
|
|
|
| 0.091 |
| Upper
thoracic | 21 (30.0%) | 5 (18.5%) | 13 (43.3%) | 3 (23.1%) | – |
| Middle
thoracic | 34 (48.6%) | 13 (48.1%) | 12 (40.0%) | 9 (69.2%) | – |
| Lower
thoracic | 15 (21.4%) | 9 (33.3%) | 5 (16.7%) | 1 (7.7%) | – |
| Tumor
length, cm (range) | 5 (2–12) | 5 (2–9) | 6 (2–12) | 5 (3–8) | 0.727 |
| Clinical stage
(%) |
|
|
|
| 0.367 |
| II | 20 (28.6%) | 9 (33.3%) | 6 (20.0%) | 5 (38.5%) | – |
|
III | 50 (71.4%) | 18 (66.7%) | 24 (80.0%) | 8 (61.5%) | – |
| T stage (%) |
|
|
|
| 0.059a |
| T1 | 2 (2.9%) | 1 (3.7%) | 1 (3.3%) | 0 | – |
| T2 | 5 (7.1%) | 4 (14.8%) | 1 (3.3%) | 0 | – |
| T3 | 18 (25.7%) | 8 (29.6%) | 4 (13.3%) | 6 (46.2%) | – |
| T4 | 45 (64.3%) | 14 (62.9%) | 24 (80%) | 7 (53.8%) | – |
| N stage (%) |
|
|
|
| 0.167 |
| N0 | 32 (45.7%) | 13 (48.1%) | 20 (66.7%) | 5 (38.5%) | – |
|
N1-3 | 38 (54.3%) | 14 (51.9%) | 10 (33.3%) | 8 (61.5%) | – |
| Radiation dose, Gy
(%) |
|
|
|
| 0.113 |
| 60 | 30 (42.9%) | 8 (29.6%) | 17 (56.7%) | 5 (38.5%) | – |
|
>60 | 40 (57.1%) | 19 (70.4%) | 13 (43.3%) | 8 (61.5%) | – |
| Chemotherapy cycle
(%) |
|
|
|
| 0.239 |
| 2 | 39 (55.7%) | 12 (44.4%) | 20 (66.7%) | 7 (53.8%) | – |
| ≥3 | 31 (44.3%) | 15 (55.6%) | 10 (33.3%) | 6 (46.2%) | – |
All 70 patients completed their concurrent
radiotherapy course with a total dose of ≥60 Gy for GTV; median
dose was 64 Gy (range, 60–66 Gy). ENI was used in all patients
(total dose, 45–50 Gy). A total of 16 (22.9%) patients extended
radiotherapy for a short period due to an acute radiation reaction
and no patients discontinued treatment. All patients received 1–2
cycles of concurrent chemotherapy with NDP/S-1 (27/70, 38.6%),
NDP/DOC (30/70, 42.8%) or NDP alone (13/70, 18.6%). Baseline
characteristics were similar between the SNR, DNR and NR groups
(Table I). Following CCRT, 31 (44.3%)
patients received additional cycles of adjuvant chemotherapy (21
received the same regimen as in CCRT).
Tumor response and survival
Median follow-up was 32 (range, 2–48) months. Median
OS was 25 [95% confidence interval (95% CI), 16.80–33.20] months.
Estimated 1-, 2- and 3-year OS rates for all patients were 82.9,
53.9, and 31.4%, respectively. A total of 53 (75.7%) patients
succumbed during follow-up, including 29 (41.4%) from EC or its
complications (e.g. esophageal fistula or bleeding) and 24 (34.3%)
from non-treatment-associated diseases (e.g. bacterial pneumonia or
cardiovascular diseases).
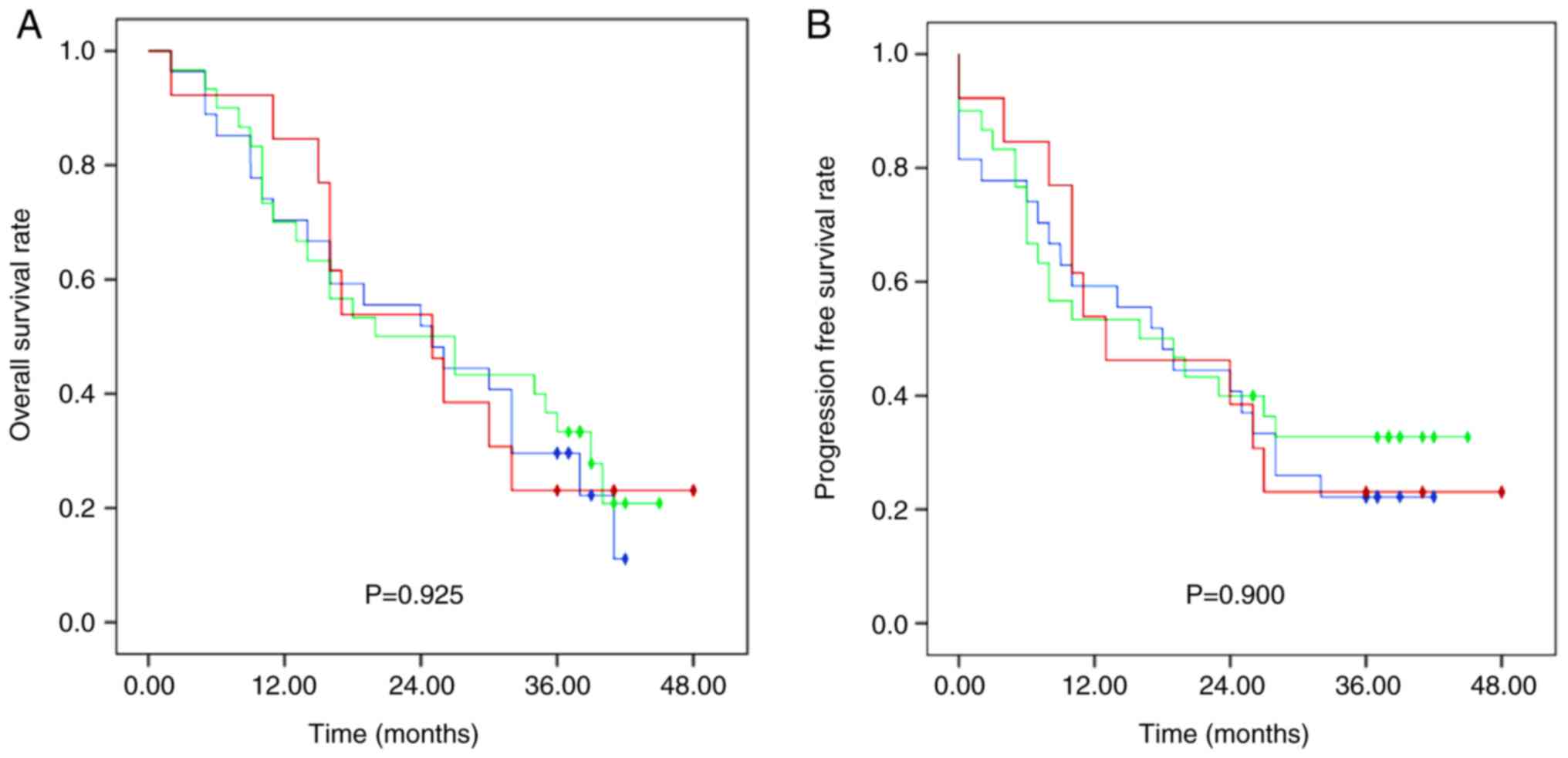
Median OS in the SNR, DNR and NR groups was 25 (95%
CI, 13.13–36.87), 20 (95% CI, 5.24–34.76) and 25 (95% CI,
13.26–36.74) months, respectively, with no significant difference
between groups (Fig. 1). The 1-, 2-
and 3-year OS rates were 70.0, 51.9 and 29.6% in group SNR, 70.0,
50.0 and 33.3% in group DNR and 84.6, 53.8 and 23.1% in group NR,
respectively. Furthermore, no significant differences were observed
in OS rates between patients treated with docetaxel (DNR group) and
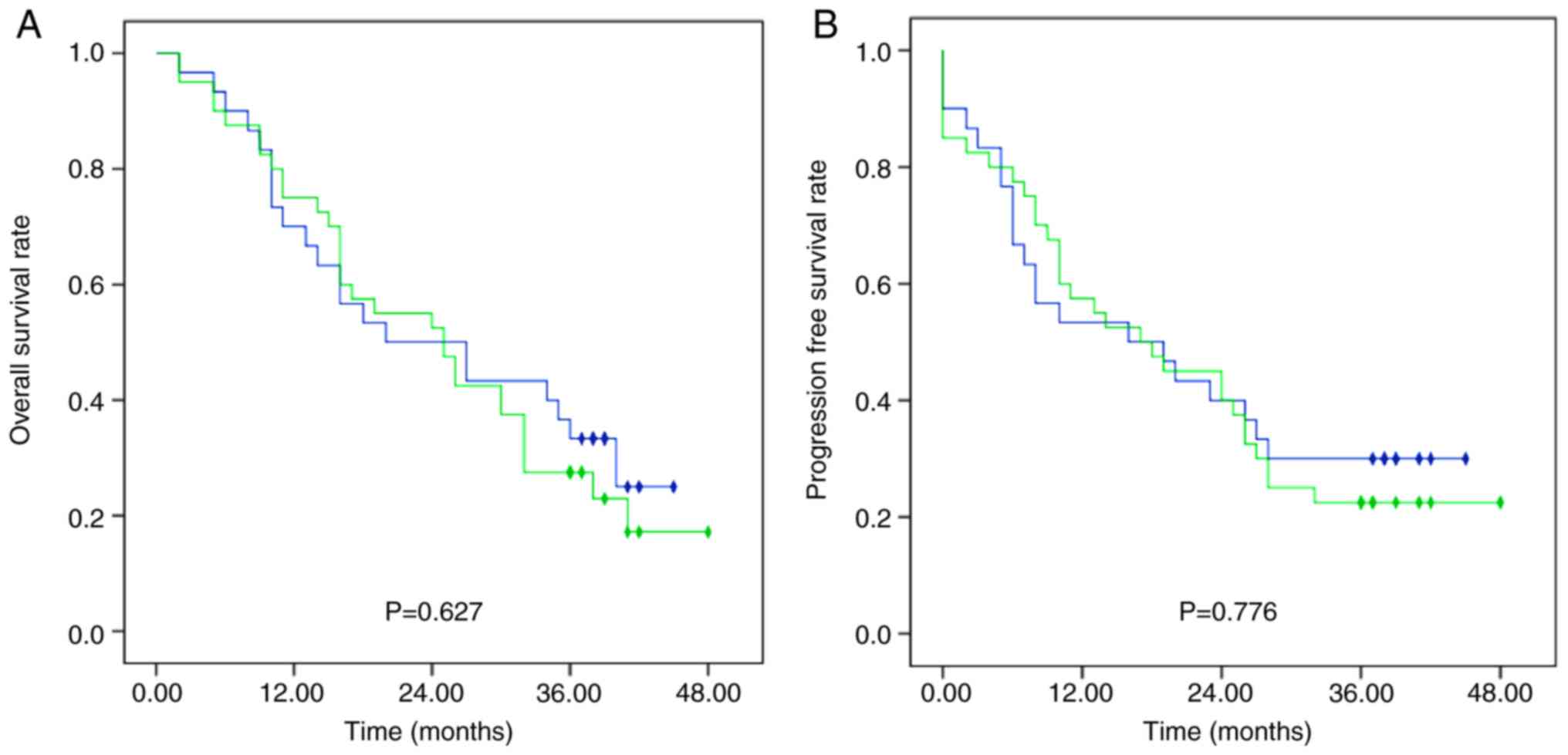
without docetaxel (SNR plus NR group) (Fig. 2).
Median PFS was 18 months (range, 0–48), and the 1-,
2- and 3-year PFS rates were 55.7, 40.0 and 25.7%, respectively.
The 1-, 2-, and 3-year PFS rates were 59.3, 40.7 and 22.2% in the
SNR group, 53.3, 40.0 and 32.7% in the DNR group, and 53.8, 38.5
and 23.1% in the NR group, respectively, with no significant
difference between groups (Fig. 1).
Additionally, PFS rates differed, although not significantly
between the DNR group and the SNR plus NR group (Fig. 2).
Overall, 32 patients (45.7%) achieved CR while 25
patients (35.7%) achieved PR (Table
II); the ORR was 81.4%. Median survival was 30 months (95% CI,
24.45–35.55) in patients experiencing disease response and 6 months
(95% CI, 2.48–9.52) in those with no response (P<0.001). The ORR
was 77.7, 83.3 and 84.6% in the SNR, DNR and NR groups,
respectively, with no significant difference between groups
(Table II).
 | Table II.Survival and clinical response |
Table II.
Survival and clinical response
| Parameter | Total (n=70)
(%) | SNR (n=27) (%) | DNR (n=30) (%) | NR (n=13) (%) | P-value |
|---|
| Status at analysis
(%) |
|
|
|
| 0.776 |
|
Alive | 18 (25.7) | 6 (22.2) | 9 (30.0) | 3 (23.1) | – |
|
Deceased | 52 (72.3) | 21 (77.8) | 21 (70.0) | 10 (76.9) | – |
| Response (%) |
|
|
|
| 0.936 |
| CR | 32 (45.7) | 11 (40.7) | 15 (50.0) | 6 (46.2) | – |
| PR | 25 (35.7) | 10 (37.0) | 10 (33.3) | 5 (38.5) | – |
| SD | 4 (5.7) | 1 (3.7) | 2 (6.7) | 1 (7.7) | – |
| PD | 9 (12.9) | 5 (18.5) | 3 (10.0) | 1 (7.7) | – |
Treatment failure
At the end of follow-up, 43/59 patients (72.9%)
experienced treatment failure; 11 patients (15.7%) were not
assessed due to mortality or disease progression before the first
therapeutic evaluation. The most common failure pattern was LR
followed by regional node recurrence and locoregional failure
(Table III). Four patients (6.8%)
had distant metastasis alone and 12 (20.3%) had local and distant
disease. Distant metastasis alone occurred in the organs of 12
patients, distant nodes in four patients, and distant nodes and
organs in two patients. Notably, the distant metastasis rate in the
NR group (41.7%) was much higher compared with the SNR group
(22.7%) and DNR group (24.0%). However, treatment failure rates did
not differ significantly between the SNR, DNR and NR groups
(Table III).
 | Table III.Treatment failure. |
Table III.
Treatment failure.
| Parameter | Total (n=59)
(%) | SNR (n=22) (%) | DNR (n=25) (%) | NR (n=12) (%) | P-value |
|---|
| First failure after
CR/PR/SD | 43 (72.9) | 16 (72.7) | 18 (72.0) | 9 (75.0) | 0.982 |
| Local | 35 (59.3) | 13 (59.1) | 14 (56.0) | 8 (66.7) | 0.826 |
| Regional | 22 (37.3) | 9
(40.9) | 8
(32.0) | 5 (41.7) | 0.771 |
| Locoregional | 20 (33.9) | 7
(31.8) | 8
(32.0) | 5 (41.7) | 0.816 |
| Distant | 16 (27.1) | 5
(22.7) | 6
(24.0) | 5 (41.7) | 0.444 |
|
Distant/local/regional | 12 (20.3) | 4
(18.2) | 4
(16.0) | 4 (33.3) | 0.448 |
Acute toxicities
Overall, grade ≥3 hematological toxicities were
observed in 35 (50.0%) patients (Table
IV). There were three cases of grade 4 leukopenia in the DNR
group, one in the SNR group and zero in the NR group; there were no
cases of grade 4 anemia or thrombocytopenia. The overall grade 3
and 4 hematological toxicity incidence rates were 66.7% in the DNR
group and 37.5% in the SNR plus NR group, and this difference was
significant (P=0.029). However, grade ≥3 hematologic toxicity
incidence rates did not differ significantly between the SNR and NR
groups (P=0.730).
 | Table IV.Radio/chemotherapy-associated
toxicities of grade ≥3. |
Table IV.
Radio/chemotherapy-associated
toxicities of grade ≥3.
| Parameter | Total (n=70)
(%) | SNR (n=27) (%) | DNR (n=30) (%) | NR (n=13) (%) |
|---|
| Acute toxicity |
|
|
|
|
|
Hematological toxicities
(grade ≥3) | 35 (50.0) | 11 (40.7) | 20 (66.7) | 4 (30.8) |
|
Leukopenia | 31 (44.3) | 8 (29.6) | 19 (63.3) | 4 (30.8) |
|
Anemia | 7 (10.0) | 2 (7.4) | 5 (16.7) | 0 (0.0) |
|
Thrombocytopenia | 10 (14.3) | 5 (18.5) | 5 (16.7) | 0 (0.0) |
|
Non-hematological toxicities
(grade ≥3) | 43 (61.4) | 17 (63.0) | 20 (66.7) | 6 (46.2) |
|
Fatigue | 22 (31.4) | 7 (25.9) | 13 (43.3) | 2 (15.4) |
|
Pneumonia | 6 (8.6) | 3 (16.7) | 1 (3.3) | 2 (15.4) |
|
Esophagitis | 7 (10.0) | 2 (11.1) | 2 (6.7) | 3 (23.1) |
|
Pain | 19 (27.1) | 7 (25.9) | 9 (30.0) | 3 (23.1) |
|
Nausea | 6 (8.6) | 2 (7.4) | 4 (13.3) | 0 (0.0) |
|
Vomiting | 8 (11.4) | 2 (7.4) | 6 (20.0) | 0 (0.0) |
|
Diarrhea | 7 (10.0) | 5 (18.5) | 2 (6.7) | 0 (0.0) |
|
GPT/GOT
elevation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Weight loss | 16 (22.9) | 5 (18.5) | 8 (26.7) | 3 (23.1) |
| Late toxicity | 6 (8.6) | 2 (7.4) | 2 (6.7) | 2 (1.5) |
|
Pneumonitis | 5 (7.1) | 2 (7.4) | 2 (6.7) | 1 (7.7) |
| Heart
disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Esophageal ulcer | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Esophageal stricture | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (7.7) |
|
Esophagitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
The most common non-hematological AEs were fatigue,
chest pain and weight loss (Table
IV). Fatigue and pain were most common in the DNR and SNR
groups, while radiation esophagitis, pain and weight loss were most
common in the NR group (Table IV).
Drug-induced hepatitis occurred mainly as mild elevations of
glutamic-pyruvic transaminase (GPT) or glutamic-oxaloacetic
transaminase (GOT); grade ≥2 GPT/GOT elevation was not observed in
any patients. The only treatment-associated mortality occurred in
the DNR group (radiation-induced pneumonia). No significant
differences were observed in overall grade 3 and 4
non-hematological toxicity incidence rates between the DNR group
and the SNR plus NR group (P=0.468).
Late toxicities
Grade 3 radiation-associated late lung toxicities
were observed in five patients (7.1%): Two in the SNR group, two in
the DNR group and one in the NR group. One case of grade 3
esophageal structure was seen in the NR group. There was only one
mortality caused by late radiation pneumonitis, at 5 months
post-radiotherapy initiation. No other grade ≥3 late toxicities
were observed.
Discussion
A notable finding from the present study was that
three different NDP-based CCRT regimens displayed similar effects
on patient survival and tumor response when used to treat stage
II–III esophageal SCC (ESCC). Notably, the NDP/S-1 and single-NDP
regimens were associated with lower toxicities compared with the
NDP/DOC regimen, suggesting that it may be more appropriate in
these patients.
The majority of patients who are newly diagnosed
with EC in China have advanced disease, which is incurable by
surgery. CCRT may improve clinical outcomes, including survival,
disease response and local control in patients with inoperable EC.
NDP, an alternative to DDP with lower renal and gastrointestinal
toxicities, has demonstrated efficacy in numerous solid tumors,
including esophageal, head/neck, ovarian and lung cancers. A study
of nedaplatin/vindesine for relapsed or refractory non-small-cell
lung cancer identified no complete drug cross-resistance between
DDP and NDP (25). NDP is considered
to have relatively low toxicity (26). A recent meta-analysis indicated that,
in patients with metastatic/recurrent or advanced ESCC, NDP-based
regimens had comparable efficacy, less toxicity and improved
tolerability compared with DDP-based regimens (17). A phase I/II study of patients with EC
established a recommended NDP dosage of 50 mg/m2
repeated twice every 3 weeks, when administered with 5-FU and
concurrent radiotherapy (18). This
regimen achieved an ORR of 85.5% and was generally well tolerated
(18).
DDP plus 5-FU is conventionally employed in CCRT for
EC, yet oncologists avoid using this regimen in patients who are
elderly or have poor renal or cardiac function. Numerous studies
have reported promising outcomes for S-1 in the treatment of
gastrointestinal tumors (27–30). Additionally, two studies observed good
anti-tumor effects and sensitization of radiotherapy in patients
with EC when S-1 was used in multidrug chemoradiotherapy (19,20).
Regimens combining DOC with platinum-based drugs are extensively
used for numerous types of solid malignant tumors. A regimen
combining DOC, NDP and 5-FU was identified to be effective for EC
(15,31,32). These
previous studies suggested that NDP plus S-1 or DOC may be a
feasible regimen in CCRT for EC. In the present study, comparisons
of OS, PFS and disease response rates between the SNR, DNR and NR
groups revealed similar treatment effects.
The optimal radiation dose and volume remain
controversial, particularly when considering the elective lymph
node region. Although one study revealed that reducing the dose to
50.4 Gy improved tolerance without decreasing survival (6), another argued that high-dose
radiotherapy (≥60 Gy) with concurrent chemotherapy for stage II–III
EC improved locoregional control and PFS, without increasing
toxicity (33). ENI appears effective
at preventing regional nodal failure in patients with ESCC treated
with CCRT (34) and reducing
locoregional and distant failure rates (35,36).
Locoregional control may be key to improving treatment outcomes in
patients with ESCC undergoing CCRT (37). ENI remains commonly used in China. The
present study was conducted using ENI, and obtained an overall
local recurrence rate of 59.3%, a regional node recurrence rate of
37.2% and a distant metastasis rate of 27.1%, with no difference in
overall treatment failure between groups. Although the outcomes
appeared to be slightly worse compared with those reported
previously, the cohort contained an increased number of patients
with stage T4 and N1 disease.
A single-drug arm was included to allow for
comparison of outcomes with the multidrug groups and to investigate
differences in treatment failures, particularly distant metastasis.
Concurrent chemotherapy was administered triweekly instead of
weekly, in order to improve the control of distant metastasis. The
distant metastasis rates were 22.7, 24 and 41.7% in the SNR, DNR
and NR groups, respectively. Distant metastasis occurred more
frequently in the NR group compared with the other two groups.
However, the present study may have been underpowered to detect
real differences between the three treatment groups. Additional
studies with larger cohorts are merited to study this further.
Nonetheless, the SNR and DNR groups exhibited comparable control of
distant metastasis.
Grade ≥3 hematological AEs occurred most frequently
in the DNR group, with a high incidence rate of 66.7%, that
indicated a significantly reduced safety of the treatment for
patients in this group compared with those in the other two groups.
There was no significant difference in grade ≥3 hematological AEs
between the SNR and NR groups. The principal radiation-associated
non-hematological AEs were acute esophagitis and pneumonitis, which
are intractable and lethal once they occur. It was reported that
grade 3–4 acute and sub-acute esophagitis occurred in 25% of
patients treated with ENI and 10% of patients treated with IFRT
(38). Grade 1–2 esophageal and lung
toxicities were quite common in the present study; however, grade
≥3 acute esophagitis and pneumonia occurred in only seven (10%) and
six (8.6%) patients, respectively. Grade ≥3 fatigue, pain, nausea,
vomiting and weight loss were more common in the DNR group compared
with the other groups. The DNR group had the highest rates of grade
≥3 non-hematological toxicities (66.7%). Additionally, 20% of
patients in the DNR group experienced grade 1–2 GPT/GOT elevation,
although more severe liver injury was not observed. However, no
patient dropped out during the treatment due to serious
hematological toxicities. Overall, although the AEs in this study
were of an acceptable level, NDP plus DOC had a higher incidence of
toxicities compared with NDP plus S-1 and NDP monotherapy when used
in CCRT.
One limitation of this study is that it may have
been underpowered to detect significant differences in outcomes due
to the small sample size. Additionally, node-positive cases require
further study in order to compare between N1, N2 and N3 disease, as
prognosis is closely associated with nodal metastasis.
In conclusion, patients with stage II–III thoracic
ESCC displayed good clinical outcomes following CCRT. NDP/S-1 and
NDP single-agent regimens may be preferable to NDP plus DOC due to
similar survival rates and disease responses, yet fewer
hematological toxicities. The NDP/S-1 regimen may have the
advantage of decreasing distant metastasis compared with the NDP
regimen. Further studies are required in order to investigate the
use of NDP-based CCRT regimens in the treatment of cervical EC.
Acknowledgements
The authors would like to thank Professor Yun Zuo
for constructive comments on the revised manuscript.
Funding
This study was supported by grants from the National
Science Foundation of China (grant nos. 81472809, 81502653,
81672983 and 81703028), ‘333’ Project of Jiangsu Province (grant
no. BRA2012210), the Priority Academic Program Development of
Jiangsu Higher Education Institutions (grant no. JX10231801), and
the Six Major Talent Peak Project of Jiangsu Province (grant no.
2013-WSN-040).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
MY and XS designed this study. YZ and YL evaluated
the characteristics of patients and the side effects of treatment.
XG and HZ performed radiotherapy and chemotherapy with all other
authors assisting with data collection. QQ was responsible for data
analysis of survival, response and failure patterns. HZ, XG and YL
wrote the manuscript, and all authors provided feedback on the
manuscript.
Ethics approval and consent to
participate
Patients were enrolled in this study with informed
consent in accordance with the Declaration of Helsinki. Approval
was obtained from the Ethics Committees of The First Hospital
Affiliated to Nanjing Medical University (Jiangsu, China).
Patient consent for publication
All patients participated in this study provided
their consent for the publication of any data and associated images
and all identifying patient data was removed.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Pardo BJ, Bronson NW, Diggs BS, Thomas
CR Jr, Hunter JG and Dolan JP: The global burden of esophageal
cancer: A disability-adjusted life-year approach. World J Surg.
40:395–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W: Cancer statistics: Updated cancer
burden in China. Chin J Cancer Res. 27:12015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen DJ and Ajani J: An expert opinion on
esophageal cancer therapy. Expert Opin Pharmacother. 12:225–239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation therapy oncology group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji K, Zhao L, Yang C, Meng M and Wang P:
Three-dimensional conformal radiation for esophageal squamous cell
carcinoma with involved-field irradiation may deliver considerable
doses of incidental nodal irradiation. Radiat Oncol. 7:2002012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Li M, Meng X, Kong L, Zhang Y,
Wei G, Zhang X, Shi F, Hu M, Zhang G and Yu J: Involved-field
irradiation in definitive chemoradiotherapy for locally advanced
esophageal squamous cell carcinoma. Radiat Oncol. 9:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HJ, Suh YG, Lee YC, Lee SK, Shin SK,
Cho BC and Lee CG: Dose-response relationship between radiation
dose and loco-regional control in patients with stage II-III
esophageal cancer treated with definitive chemoradiotherapy. Cancer
Res Treat. 49:669–677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herskovic A, Martz K, al-Sarraf M,
Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L
and Emami B: Combined chemotherapy and radiotherapy compared with
radiotherapy alone in patients with cancer of the esophagus. N Engl
J Med. 326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suntharalingam M, Moughan J, Coia LR,
Krasna MJ, Kachnic L, Haller DG, Willett CG, John MJ, Minsky BD and
Owen JB: 1996-1999 Patterns of CareStudy: The national practice for
patients receiving radiation therapy for carcinoma of the
esophagus: Results of the 1996-1999 Patterns of Care Study. Int J
Radiat Oncol Biol Phys. 56:981–987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu CH, Yeh KH, Lui LT, Lee YC, Bu CF,
Wang HP, Lin JT and Cheng AL: Concurrent chemoradiotherapy for
locally advanced esophageal cancer-a pilot study by using daily
low-dose cisplatin and continuous infusion of 5-fluorouracil.
Anticancer Res. 19:4463–4467. 1999.PubMed/NCBI
|
|
13
|
Tu L, Sun L, Xu Y, Wang Y, Zhou L, Liu Y,
Zhu J, Peng F, Wei Y and Gong Y: Paclitaxel and cisplatin combined
with intensity-modulated radiotherapy for upper esophageal
carcinoma. Radiat Oncol. 8:752013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu HT, Ai DS, Tang HR, Badakhshi H, Fan
JH, Deng JY, Zhang JH, Chen Y, Zhang Z, Xia Y, et al: Long-term
results of paclitaxel plus cisplatin with concurrent radiotherapy
for loco-regional esophageal squamous cell carcinoma. World J
Gastroenterol. 23:540–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo JF, Zhang B, Wu F, Wang B, Xing H, Zhu
GY, Nie XY and Peng J: A phase II trial of docetaxel plus
nedaplatin and 5-fluorouracil in treating advanced esophageal
carcinoma. Chin J Cancer. 29:321–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao T, Chen H and Zhang T: Docetaxel and
cisplatin concurrent with radiotherapy versus 5-fluorouracil and
cisplatin concurrent with radiotherapy in treatment for locally
advanced oesophageal squamous cell carcinoma: A randomized clinical
study. Med Oncol. 29:3017–3023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang F, Wang Y, Wang ZQ, Sun P, Wang DS,
Jiang YX, Zhang DS, Wang FH, Xu RH and Li YH: Efficacy and safety
of cisplatin-based versus nedaplatin-based regimens for the
treatment of metastatic/recurrent and advanced esophageal squamous
cell carcinoma: A systematic review and meta-analysis. Dis
Esophagus. 30:1–8. 2017. View Article : Google Scholar
|
|
18
|
Sato Y, Takayama T, Sagawa T, Okamoto T,
Miyanishi K, Sato T, Araki H, Iyama S, Abe S, Murase K, et al: A
phase I/II study of nedaplatin and 5-fluorouracil with concurrent
radiotherapy in patients with esophageal cancer. Cancer Chemother
Pharmaco. 58:570–576. 2006. View Article : Google Scholar
|
|
19
|
Tanaka Y, Yoshida K, Tanahashi T, Okumura
N, Matsuhashi N and Yamaguchi K: Phase II trial of neoadjuvant
chemotherapy with docetaxel, nedaplatin, and S1 for advanced
esophageal squamous cell carcinoma. Cancer Sci. 107:764–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka Y, Yoshida K, Osada S, Yamaguchi K
and Takahashi T: Docetaxel, nedaplatin, and S-1 (DGS) chemotherapy
for advanced esophageal carcinoma: A phase I dose-escalation study.
Anticancer Res. 31:4589–4597. 2011.PubMed/NCBI
|
|
21
|
Ohba A, Kato K, Ito Y, Katada C, Ishiyama
H, Yamamoto S, Ura T, Kodaira T, Kudo S and Tamaki Y:
Chemoradiation therapy with docetaxel in elderly patients with
stage II/III esophageal cancer: A phase 2 trial. Adv Radiat Oncol.
1:230–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ajani JA, Buyse M, Lichinitser M,
Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha
R, Carrato A, et al: Combination of cisplatin/S-1 in the treatment
of patients with advanced gastric or gastroesophageal
adenocarcinoma: Results of noninferiority and safety analyses
compared with cisplatin/5-fluorouracil in the First-Line Advanced
Gastric Cancer Study. Eur J Cancer. 49:3616–3624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang H, Shin SK, Cho BC, Lee CG, Kim CB,
Kim DJ, Lee JG, Hur J, Lee CY, Bae MK, et al: A prospective phase
II trial of S-1 and cisplatin-based chemoradiotherapy for
locoregionally advanced esophageal cancer. Cancer Chemother
Pharmacol. 73:665–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takigawa N, Segawa Y, Ueoka H, Kiura K,
Tabata M, Shibayama T, Takata I, Miyamoto H, Eguchi K and Harada M:
Combination of nedaplatin and vindesine for treatment of relapsed
or refractory non-small-cell lung cancer. Cancer Chemother
Pharmacol. 46:272–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hartmann JT and Lipp HP: Toxicity of
platinum compounds. Expert Opin Pharmacother. 4:889–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takechi T, Nakano K, Uchida J, Mita A,
Toko K, Takeda S, Unemi N and Shirasaka T: Antitumor activity and
low intestinal toxicity of S-1, a new formulation of oral tegafur,
in experimental tumor models in rats. Cancer Chemother Pharmacol.
39:205–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakata B, Mitachi Y, Tsuji A, Yamamitsu S,
Hirata K, Shirasaka T and Hirakawa K: Combination Phase I trial of
a novel oral fluorouracil derivative S-1 with low-dose cisplatin
for unresectable and recurrent gastric cancer (JFMC27-9902). Clin
Cancer Res. 10:1664–1669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akutsu Y, Shuto K, Kono T, Uesato M,
Hoshino I, Shiratori T, Miyazawa Y, Isozaki Y, Akanuma N and
Matsubara H: A phase 1/11 study of second-line chemotherapy with
fractionated docetaxel and nedaplatin for 5-FU/cisplatin-resistant
esophageal squamous cell carcinoma. Hepatogastroenterology.
59:2095–2098. 2012.PubMed/NCBI
|
|
32
|
Miyazaki T, Ojima H, Fukuchi M, Sakai M,
Sohda M, Tanaka N, Suzuki S, Ieta K, Saito K, Sano A, et al: Phase
II study of docetaxel, nedaplatin, and 5-fluorouracil combined
chemotherapy for advanced esophageal cancer. Ann Surg Oncol.
22:3653–3658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suh YG, Lee IJ, Koom WS, Cha J, Lee JY,
Kim SK and Lee CG: High-dose versus standard-dose radiotherapy with
concurrent chemotherapy in stages II-III esophageal cancer. Jpn J
Clin Oncol. 44:534–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamashita H, Okuma K, Wakui R,
Kobayashi-Shibata S, Ohtomo K and Nakagawa K: Details of recurrence
sites after elective nodal irradiation (ENI) using 3D-conformal
radiotherapy (3D-CRT) combined with chemotherapy for thoracic
esophageal squamous cell carcinoma-a retrospective analysis.
Radiother Oncol. 98:255–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu FM, Lee JM, Huang PM, Lin CC, Hsu CH,
Tsai YC, Lee YC and Chia-Hsien Cheng J: Retrospective analysis of
outcome differences in preoperative concurrent chemoradiation with
or without elective nodal irradiation for esophageal squamous cell
carcinoma. Int J Radiat Oncol Biol Phys. 81:593–599. 2011.
View Article : Google Scholar
|
|
36
|
Onozawa M, Nihei K, Ishikura S, Minashi K,
Yano T, Muto M, Ohtsu A and Ogino T: Elective nodal irradiation
(ENI) in definitive chemoradiotherapy (CRT) for squamous cell
carcinoma of the thoracic esophagus. Radiother Oncol. 92:266–269.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim HW, Kim JH, Lee IJ, Kim JW, Lee YC,
Lee CG, Park JJ, Youn YH and Park H: Local control may be the key
in improving treatment outcomes of esophageal squamous cell
carcinoma undergoing concurrent chemoradiation. Digestion.
90:254–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamashita H, Takenaka R, Omori M, Imae T,
Okuma K, Ohtomo K and Nakagawa K: Involved-field radiotherapy
(IFRT) versus elective nodal irradiation (ENI) in combination with
concurrent chemotherapy for 239 esophageal cancers: A single
institutional retrospective study. Radiat Oncol. 10:1712015.
View Article : Google Scholar : PubMed/NCBI
|