Introduction
Radiation therapy serves an important role in the
treatment of cancer, with almost half of all patients with cancer
receiving radiotherapy in different stages of their disease. Since
the 1930s, conventionally fractionated radiotherapy (CFRT) has been
used for clinical therapy, with the implemented scheme being
1.8–2.0 Gy/fraction. CFRT exploits inherent differences between
normal and tumor tissues. Tumor cells require increased energy for
proliferation, while normal cells retain the ability to repair DNA
injuries. The therapeutic benefit of CFRT was first explained by
Bergonie and Tribondeau (1).
Administering small daily doses of irradiation is key in CFRT, even
if it damages tumor and normal tissues. Compared with normal
tissues, tumor tissues have a reduced capability of repairing DNA
damage caused by radiation. Over the course of a number of
treatments, tumor tissues sustain increased cumulative damage,
compared with normal tissues (2).
Toxicity of radiotherapy to normal cells limits the
dose of radiation used. Over the past decades, there have been
technical improvements to radiation therapy, in order to reduce the
toxic effects of radiotherapy (2).
Stereotactic Body Radiation Therapy (SBRT), as a novel cancer
treatment strategy, accurately provides high doses of radiation to
tumor tissues, while reducing the exposure of surrounding tissues
to toxic levels of radiation (2,3). Thus, a
single high dose of radiation may be used in radiotherapy (4,5). SBRT, as
an emerging cancer treatment strategy, has evolved in the late
twentieth century. Unlike CFRT, SBRT utilizes ultra-high doses per
fraction in treatment, generally in the range of 10–20 Gy/fraction,
potentially exposing tumor and normal structures to the high-dose
radiation (6–9). SBRT may have risk of
treatment-associated complications while achieving increased rates
of local control. Exposure to the high-dose radiation can
irreversibly damage tumor and normal tissues and irreversible
damage to normal tissue can seriously affect the body's function
(2,10). Thus, not all patients who need
radiation are suitable for this technique.
The present study investigated the cellular changes
in cancer cells following irradiation at different doses. The aim
was to observe the biological effects of different radiation doses
on tumor cells, and this may provide theoretical support for
selecting radiation fractional doses with superior biological
effects. To determine the radiobiological responses of cancer cells
following different radiation fractional doses and the optimal
fractional radiation dose, radiobiological studies were performed
at molecular and cellular levels to provide insights into DNA
damage and repair, and the apoptosis mechanism of cells irradiated
at doses between 0 and 20 Gy. Further elucidation of the cellular
effects of different doses of radiation may contribute to the
rationale for selecting the optimal fractional dose of radiation
for cancer radiotherapy.
Materials and methods
Cell culture
HeLa cells obtained from the American Type Culture
Collection (Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium containing 10% fetal bovine serum (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cell cultures
were stored at 37°C in an atmosphere containing 5% CO2
in a humidified incubator. Cells used for each experiment were
grouped according to the dose of irradiation (0, 2, 4, 6, 8, 10,
12.5, 15 and 20 Gy).
X-irradiation
X-irradiation experiments were performed at the
Irradiation Facility of Zhongnan Hospital of Wuhan University
(Wuhan, China). The medium was replaced prior to irradiation in a
horizontal position. Cells were divided into different groups and
exposed under (0, 2, 4, 6, 8, 10, 12.5, 15 and 20 Gy) X-rays
separately with a Siemens Primus Accelerator machine (6 Mv; Siemens
AG, Munich, Germany).
Cell Counting Kit-8 (CCK-8) assay for
cell proliferation
CCK-8 reagent (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was used to determine the proliferation rate of
cells, the cells was grouped according to the dose of irradiation
(0, 2, 4, 6, 8, 10, 12.5, 15 and 20 Gy). The seeded
(1×104 cells/well in 96-well plates) serum-starved cells
were exposed to different doses of radiation (0, 2, 4, 6, 8, 10,
12.5, 15 and 20 Gy). Cells were washed using PBS prior to
detection, then CCK-8 solution was added (10 µl CCK-8 and 90 µl
Dulbecco's modified Eagle's medium without any supplements). At 3 h
following the addition of CCK-8, the absorbance at 450 nm was
measured at 24, 48, 72, 96 and 120 h followimg irradiation of cells
in each group (0, 2, 4, 6, 8, 10, 12.5, 15 and 20 Gy) by
micro-tablet reader (Microplate Reader, Sunrise, Tecan). The cell
proliferation rates were calculated and the optical density value
from the first day was used for normalization.
Immunocytochemistry for phosphorylated
histone H2AX (γ-H2AX)
Cells were plated and grown on coverslips
(5×104 cells/well), 12 h after cells were seeded, a
series of dose irradiation was performed as aforementioned (0, 2,
4, 6, 8, 10, 12.5, 15 and 20 Gy). At 20 min following irradiation,
cells were fixed in 5% paraformaldehyde (Merck KGaA, Darmstadt,
Germany) for 30 min at room temperature, then washed with PBS and
permeabilized in 0.5% Triton (Sigma-Aldrich; Merck KGaA) for 10 min
at room temperature. The cells were treated with the blocking fluid
(5% bovine serum albumin, cat. no. SBJ-1239; NanJing SenBeiJia
Biotechnology Co. Ltd., Nanjing, China) for 1 h at room
temperature, then washed with PBS. The cells were subsequently
probed with mouse anti-γ-H2AX antibody (ser139) (1:200 dilution;
cat. no. 05-636-AF488; EMD Millipore, Billerica, MA, USA) and
incubated overnight at 4°C. The cells were then washed with PBS,
stained with fluorescein isothiocyanate (FITC)-conjugated goat
anti-rabbit secondary antibody (1:500 dilution; cat. no. 31635;
Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at room
temperature. All antibody dilutions were prepared in 5% bovine
serum albumin (cat. no. SBJ-1239; NanJing SenBeiJia Biotechnology
Co. Ltd.). Subsequently, three washing steps were performed with
PBS, following which a cover glass was incubated with propidium
iodide (PI) for 20 min at room temperature. Finally, images were
captured with a fluorescence microscope (Nikon E-600; Nikon
Corporation, Tokyo Japan) using a 1.3 aperture plan fluor ×40
numerical aperture oil objective.
Cell cycle analysis
Cells were collected at 0, 6, 12, 18, 24, 30, 36, 42
and 48 h after irradiation by trypsinization purification at room
temperature. Considering that there may be adherent cells, the
supernatants and PBS used in washing steps were retained. Samples
were fixed in cold 70% EtOH solution at 4°C for 24 h. Cells were
then washed with PBS two times, then stained with 500 µl PI
solution (50 µg/ml PI and 1% RNase A; Sigma-Aldrich; Merck KGaA)
for 50 min at 37°C. Samples were measured immediately by flow
cytometry (Accuri C6, BD Biosciences, San Jose, CA, USA). PI
fluorescence of a minimum of 1×104 cells was measured.
Cells in the G0/G1, S and G2/M phases were determined followed
filtering for doublets and aggregates. Doublets were filtered based
on a FSC-A vs. FSC-H dot plot with BD Accuri C6 software version
1.0.202.1 (BD Biosciences).
Detection of apoptosis by Annexin
V
During apoptotic cell detection, 1×105
cells were inoculated in the 6-well culture plate and cultured for
12 h prior to irradiation, at 37°C in an atmosphere containing 5%
CO2 in a humidified incubator. Cells were harvested at
48 and 72 h after irradiation at room temperature, then the cells
were re-suspended in 100 µl staining buffer (PBS, cat. no.
SH30256.01B; GE Healthcare Life Sciences, Hyclone, Logan, UT, USA)
with 5 µl Annexin V-FITC (BD Pharmingen; BD Biosciences) and
stained at room temperature for 15 min, followed by a quick
staining with 1 µg/ml PI (cat. no. P4864; Sigma-Aldrich; Merck
KGaA) at room temperature for 10 min. The samples were analyzed on
a LSRII flow cytometer (BD Biosciences). The data was then analyzed
using Flow Jo software version 9.6.2 (FlowJo LLC, Ashland, OR,
USA).
Mitochondrial membrane potential
analysis
The JC-1 kit (Beyotime Institute of Biotechnology,
Haimen, China) was used to detect the mitochondrial membrane
potentials. Cell suspensions at 1×105 cells/ml were
cultured on glass cover-slips for 12 h at 37°C in an atmosphere
containing 5% CO2 in a humidified incubator. At 6 h
after irradiation, the cells were incubated with Dulbecco's PBS
(DPBS) which was provided in the JC-1 kit and 1 µl JC-1 reagent at
37°C for 15 min. Subsequently, the cells were washed with DPBS
three times. The mitochondrial membrane potential was detected
under a fluorescence microscope (×100 magnifications, Nikon E-600).
The reduction in red fluorescence and the increase in green
fluorescence indicated a decrease in the mitochondrial membrane
potential.
Microscopic analysis of
dihydroethidium (DHE) oxidation [reactive oxygen species (ROS)
production] in cells
With the use of a 12-well plate, cells were plated
at a density of 5×104 cells/ml were incubated in
Dulbecco's modified Eagle's medium containing 20 µM DHE (Vigorous
Biotechnology Beijing Co., Ltd., Beijing, China; http://www.bioon.com.cn/show/reagent_88470_c0_p2.html.)
at 37°C for 10 min. Following loading DHE into the cells, the plate
was mounted on a fluorescence microscope (×100 magnification, Nikon
E-600), and fluorescence images were captured with a digital
charge-coupled device camera (Roper Scientific RTE/CCD-1300-Y/HS)
controlled by MetaMorph Image-analysis software version 4.01
(Universal Imaging, Inc., Bedford Hills, NY, USA). The Eth-DNA
fluorescence was monitored at 490 nm excitation and 610 nm
emission.
Western blot analysis
Cells were washed with ice-cold PBS twice. Lysis
buffer (25 Mm Tris-HCl, Ph 7.4, 25 Mm NaCl, 0.5 Mm EDTA, 1 Mm
sodium orthovanadate, 10 Mm NaF, 25 Mm β-glycerophosphate, 10 Mm
sodium pyrophosphate, 0.2 Mm sodium molybdate, 10 mg/ml aprotinin,
2 Mm phenylmethylsulfonyl fluoride and 1% Triton X-100) was added
to the cells, which were subjected to lysis by sonication for 60
sec on ice. The lysates were treated by centrifugation for 10 min
at 12,000 × g at 4°C, and the protein concentration was determined
with a BCA protein concentration assay (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of protein (30 µg) were separated
on 10–12.5% SDS-PAGE gels, electrophoretically transferred to
polyvinylidene difluoride membranes with transfer buffer (25 mM
Tris, 250 mM glycine and 10% methanol), the membranes were blocked
with Western Blocking Buffer (cat. no. SW3010; Solarbio Life
Sciences, China) at room temperature for 1 h. Probed with primary
antibodies at 4°C for 8 h, then horseradish peroxidase-conjugated
secondary antibodies (Goat anti-rabbit IgG, HRP-linked Antibody,
1:1,000 dilution; cat. no. 7074; Cell Signaling Technology Inc.,
Danvers, MA, USA) at room temperature for 1 h. Immunoblots were
detected using an ECL Prime Western Blotting Detection Reagent (GE
Healthcare Life Sciences, Little Chalfont, UK), according to the
manufacturer's protocol, autoradiography and recorded on film.
Quantification was performed using ImageJ software version 1.45S
(National institutes of Health, Bethesda, MD, USA). The primary
antibodies used included: Rabbit anti-β-actin (1:5,000 dilution;
cat. no. A2066; Sigma-Aldrich; Merck KGaA), γ-H2AX (ser139) (cat.
no. 05-636-AF488; EMD Millipore) (1:2,000 dilution), cyclin
dependent kinase 1 (CDK1; 1:5,000 dilution; cat. no. PLA0287;
Sigma-Aldrich; Merck KGaA), checkpoint kinase 1 (CHK1; 1:500; cat.
no. SAB4500207; Sigma-Aldrich; Merck KGaA), RAD51 recombinase
(Rad51; dilution 1:500; cat. no. SAB2101936; Sigma-Aldrich; Merck
KGaA), Ku70 (1:1,000 dilution; cat. no. 4104; Cell Signaling
Technology Inc., Danvers, MA, USA) and Ku80 (1:1,000 dilution; cat.
no. 2753; Cell Signaling Technology Inc.), B-cell lymphoma (Bcl-2;
1:5,000 dilution; cat. no. SAB4500003; Sigma-Aldrich; Merck KGaA),
Bcl-2-associated X (Bax; 1:500 dilution; cat. no. SAB4502546;
Sigma-Aldrich; Merck KGaA), BH3 interacting domain death agonist
(Bid; 1:500 dilution; cat. no. SAB3500353; Sigma-Aldrich; Merck
KGaA) and Caspase-9 (1:500 dilution; cat. no. 9502; Cell Signaling
Technology Inc.), hypoxia inducible factor-1α (HIF-1α; 1:2,000
dilution; cat. no. SAB1405933; Sigma-Aldrich; Merck KGaA), glucose
transporter 1 (GLUT1; 1:1,000 dilution; cat. no. SAB4502803;
Sigma-Aldrich; Merck KGaA), c-Myc (1:1,000 dilution; cat. no.
13987; Cell Signaling Technology Inc.) and pyruvate kinase M2
(PKM2; 1:1,000; cat. no. 4053; Cell Signaling Technology Inc.).
Statistical analysis
All the experiments were repeated ≥3 times.
Statistical analysis was performed with GraphPad Prism 5.0 Software
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
the mean ± SEM. P≤0.05 was considered to indicate a statistically
significant difference. Cell cycle data were analyzed by two-way
analysis of variance with dose and time point as independent
variables, with Tukey's honestly significant difference (Tukey's
HSD) as post-hoc test.
Results
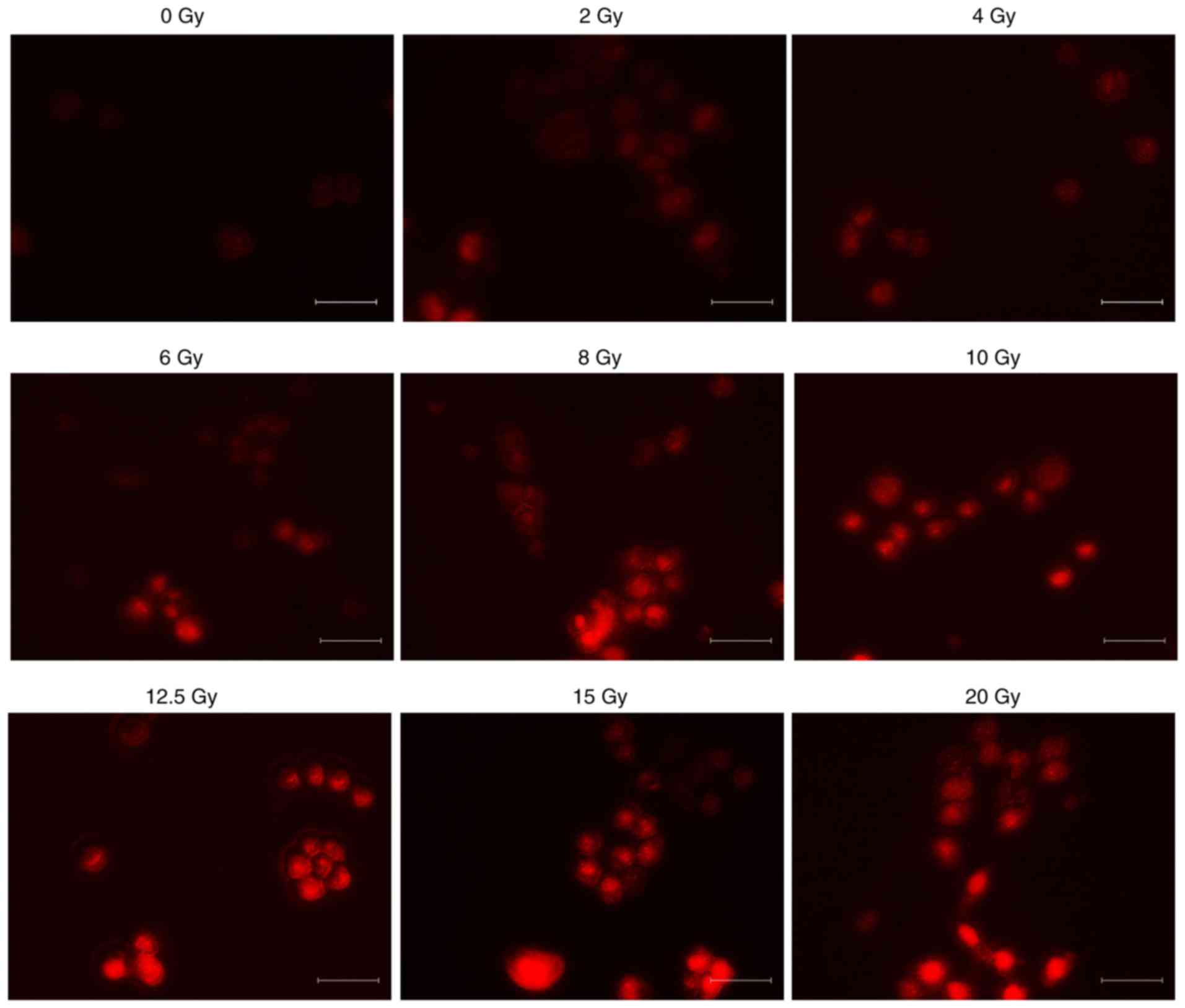
ROS production in the cells following
irradiation
Cells were incubated with DHE for 10 min after
incremental doses of irradiation were administered. Red
fluorescence produced by the formation and binding of ethidium
(Eth) from DHE represents ROS production. The typical fluorescence
microscopic images recorded at 10 min after incubation of DHE with
different irradiation doses present maximal ROS-induced Eth-DNA red
fluorescence. Red fluorescence of cells following irradiation,
indicating production of ROS, was increased with a greater X-ray
irradiation dose. As illustrated in Fig.
1, the ROS production in cells following irradiation was
increased, and increased at increased doses. However, the
fluorescence intensity demonstrated that there was no significant
difference in ROS production between the 2 and 4 Gy groups.
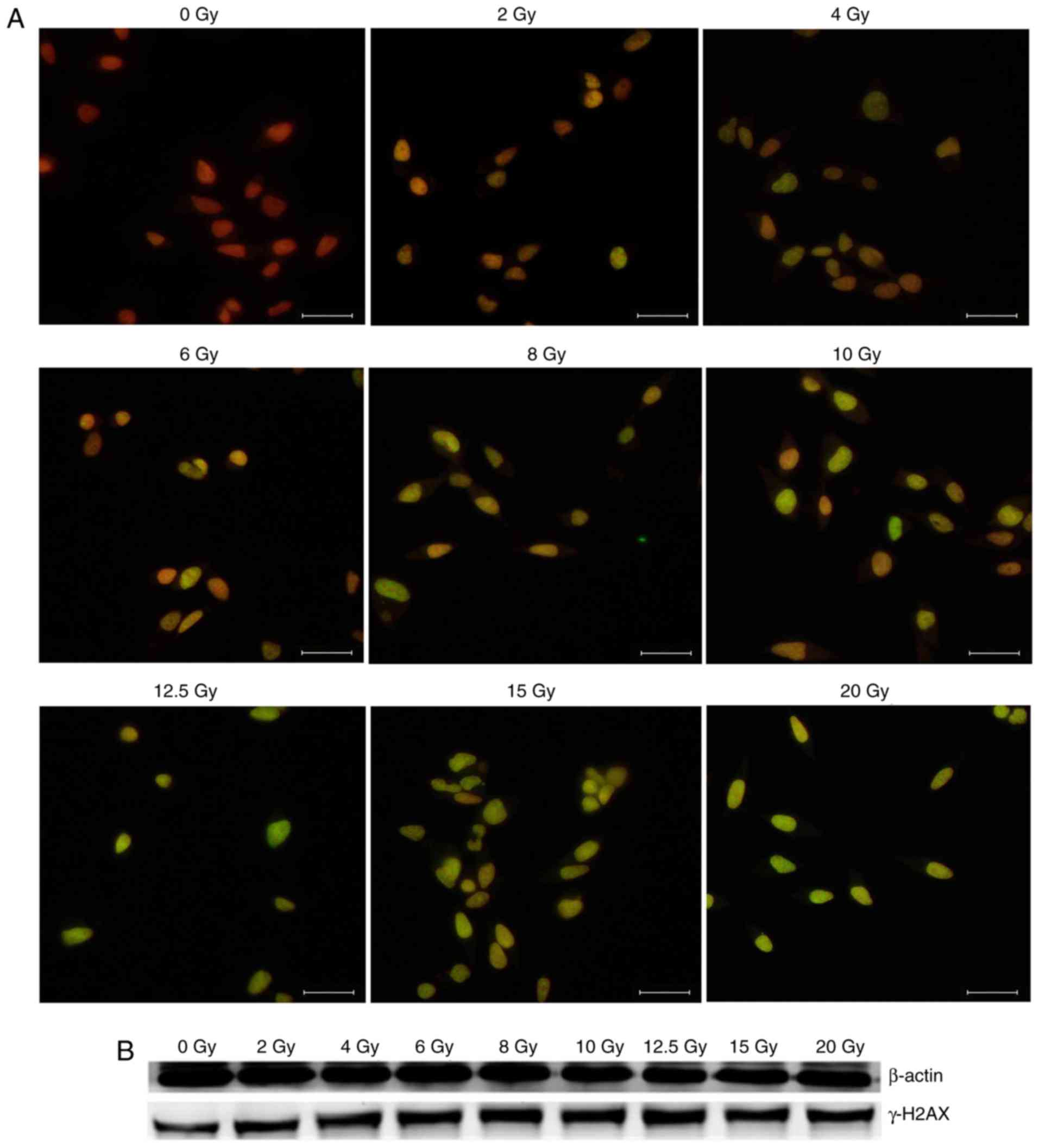
Radiation-induced DNA damage
DNA double-strand breaks (DSBs) are induced directly
by ionizing energy or indirectly by secondary radicals mediated by
ROS (11). Phosphorylation of H2AX at
Ser 139 (γ-H2AX) is the most sensitive marker used to detect DNA
damage (12,13). In the present experiment, γ-H2AX was
used as a marker of radiation-induced DNA DSBs.
To examine the dose-effect of radiation, DNA DSBs
were visualized using immunofluorescent staining for γ-H2AX foci at
15 min after irradiation. Representative images of the γ-H2AX foci
in HeLa cells were depicted in Fig.
2A. γ-H2AX was stained with green fluorescence (FITC),
indicating that DSBs merged with the nucleus stained with red
fluorescence (PI). A clear dose-dependent induction of the γ-H2AX
foci was observed and was associated with the presence of DSBs. The
expression of γ-H2AX at the protein level was detected by western
blot analysis. The results of western blot analysis demonstrated
that the expression of γ-H2AX increased following irradiation
(Fig. 2B).
Cell cycle and cell proliferation
following irradiation
Radiation-induced cell cycle changes were analyzed
by flow cytometry at 0, 6, 12, 18, 24, 30, 36, 42 and 48 h after
X-irradiation using PI staining. The primary results are depicted
in Fig. 3A. The change trend of
S-phase under different doses of irradiation was similar, within
the 24 h following irradiation, the S-phase of cells with different
dose were decreased gradually; and 30 h following irradiation, the
change of S-phase exhibited a gradual increase. Following
irradiation, a decrease trend of G1-phase was exhibited in HeLa
cells, the higher the irradiation dose received, the greater the
tendency of G1 phase reduction. Correspondingly, a significant
increase in the number of HeLa cells in the G2/M-phase was observed
following irradiation, indicative of DNA damage repair following
irradiation (14,15). An increased dose of irradiation
resulted in a greater G2/M delay. Cell cycling distribution
returned to normal within 24 h after 2 Gy irradiation. The cells
exposed to 4, 6 and 8 Gy returned to a normal cell cycle within 36
h, and cells exposed to 10 Gy returned to a normal cell cycle
within 48 h. The cell cycle did not return to normal levels within
48 h when the radiation dose was >10 Gy (Fig. 3A). The expression of cell
cycle-associated protein CDK1 markedly decreased following various
doses of irradiation and the expression of CHK1 increased following
irradiation. Variations in CDK1 and CHK1 were consistent with the
accumulation of G2/M-phase cells (Fig.
3B).
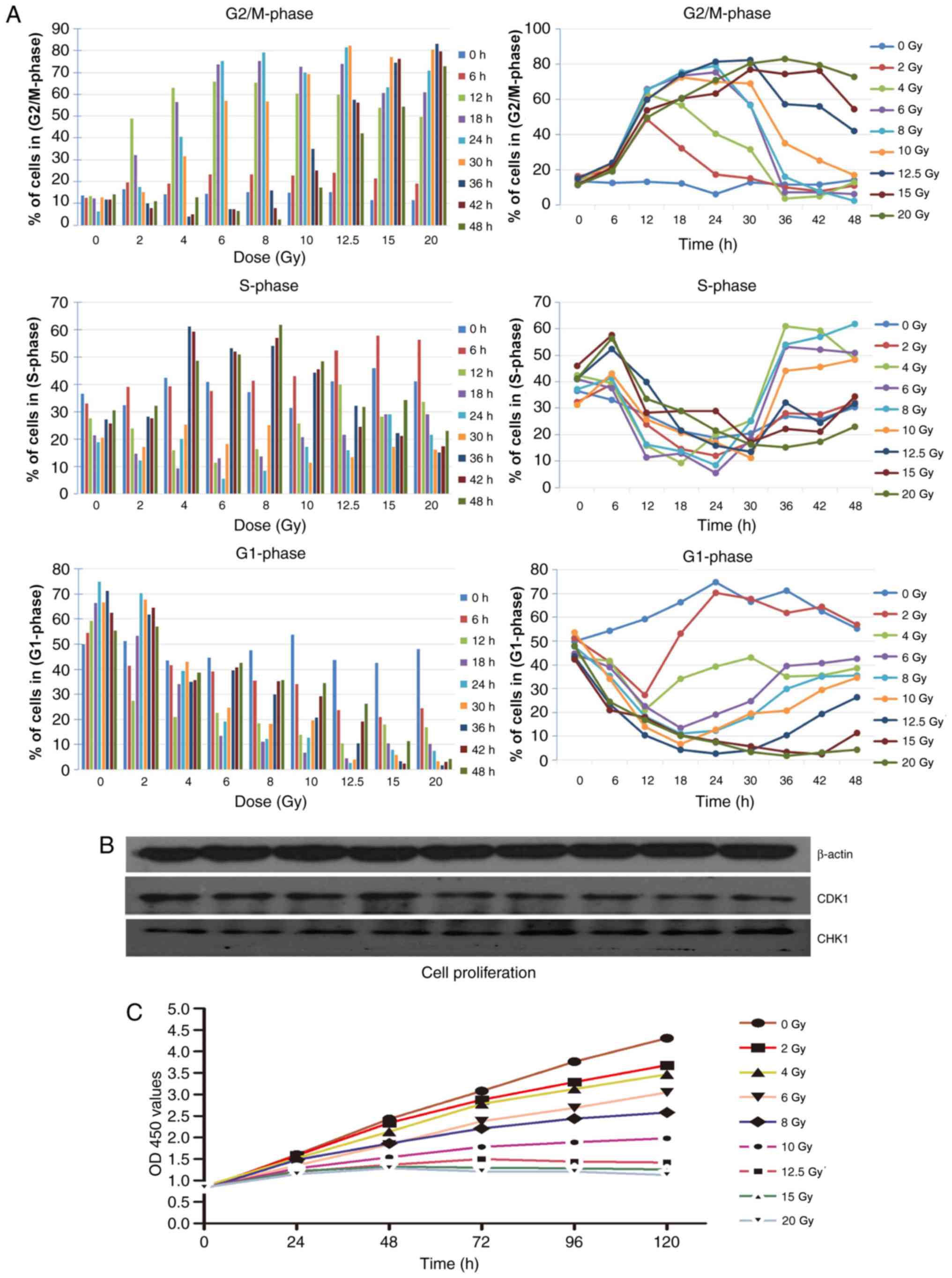 | Figure 3.Cell cycle following irradiation. (A)
Cell cycle (G1, S and G2/M) changes were analyzed by flow cytometry
at 0, 6, 12, 18, 24, 30, 36, 42 and 48 h after X-ray irradiation
using propidium iodide staining. Irradiation of HeLa cells resulted
in a notable increase of cells in the G2/M-phase. (B) Results of
western blot analysis demonstrated the level of CDK1 and CHK1 in
cells following irradiation, the expression of CDK1 markedly
decreased following various doses of irradiation and the expression
of CHK1 increased following irradiation. (C) Cell proliferation was
detected using Cell Counting Kit-8 assays in cells following
irradiation. CDK1, cyclin dependent kinase 1; CHK1, checkpoint
kinase 1; OD, optical density. |
Cell proliferation was detected by CCK-8 assays. The
results represent the survival curves of HeLa cells exposed to the
different doses of X-irradiation (Fig.
3C). The proliferation ability of cells irradiated with X-rays
decreased with increasing doses.
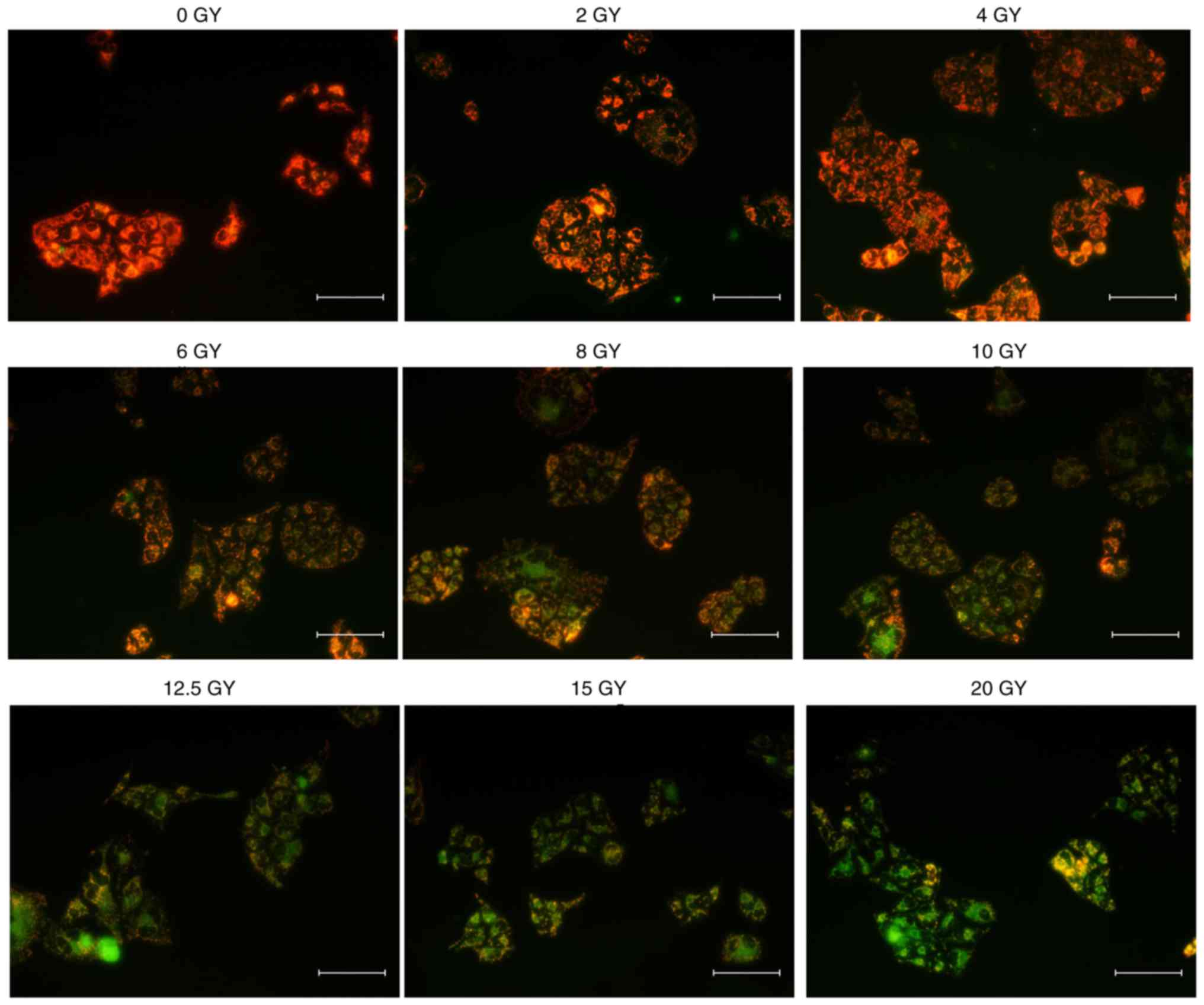
Mitochondrial membrane potential and
apoptosis following irradiation
Decrease in the mitochondrial membrane potential is
an important signal of apoptosis in its early stage (16). The decrease in red fluorescence
combined with the increase in green fluorescence indicated a loss
of mitochondrial membrane potential, as depicted in Fig. 4. Compared with the cells without
irradiation (0 Gy), the mitochondrial membrane potential of HeLa
cells decreased following irradiation, and decreased at increased
doses. The change of fluorescence also demonstrated that there was
similar change of mitochondrial membrane potential between the 4
and 2 Gy groups. A dose-dependent decrease in mitochondrial
membrane potential (MMP) was observed in cells irradiated at doses
>4 Gy.
Further investigation of the dose response of
apoptosis was performed by harvesting cells at 48 and 72 h after
irradiation, staining with Annexin V-fluorescein isothiocyanate and
PI, and analysis using a flow cytometer. It was determined that the
proportion of apoptotic cells at 72 h after irradiation was
increased, compared with the same dose at 48 h following
irradiation (Fig. 5). Additionally,
48 and 72 h after irradiation have the identical change tendencies
of apoptotic cells with different irradiation doses, a
dose-dependent increase in the percentage of apoptotic cells was
observed 48 and 72 h following irradiation. The results of the
apoptosis assay indicated an increase in the percentage of
apoptotic cells following irradiation (Fig. 5) The radio of apoptotic cells of two
groups with adjacent doses were compared with each other. Except 2
and 4 Gy groups, the difference in the percentage of apoptotic
cells between other adjacent two groups were significant; however,
the difference in the percentage of apoptotic cells between the 2
and 4 Gy groups was not significant.
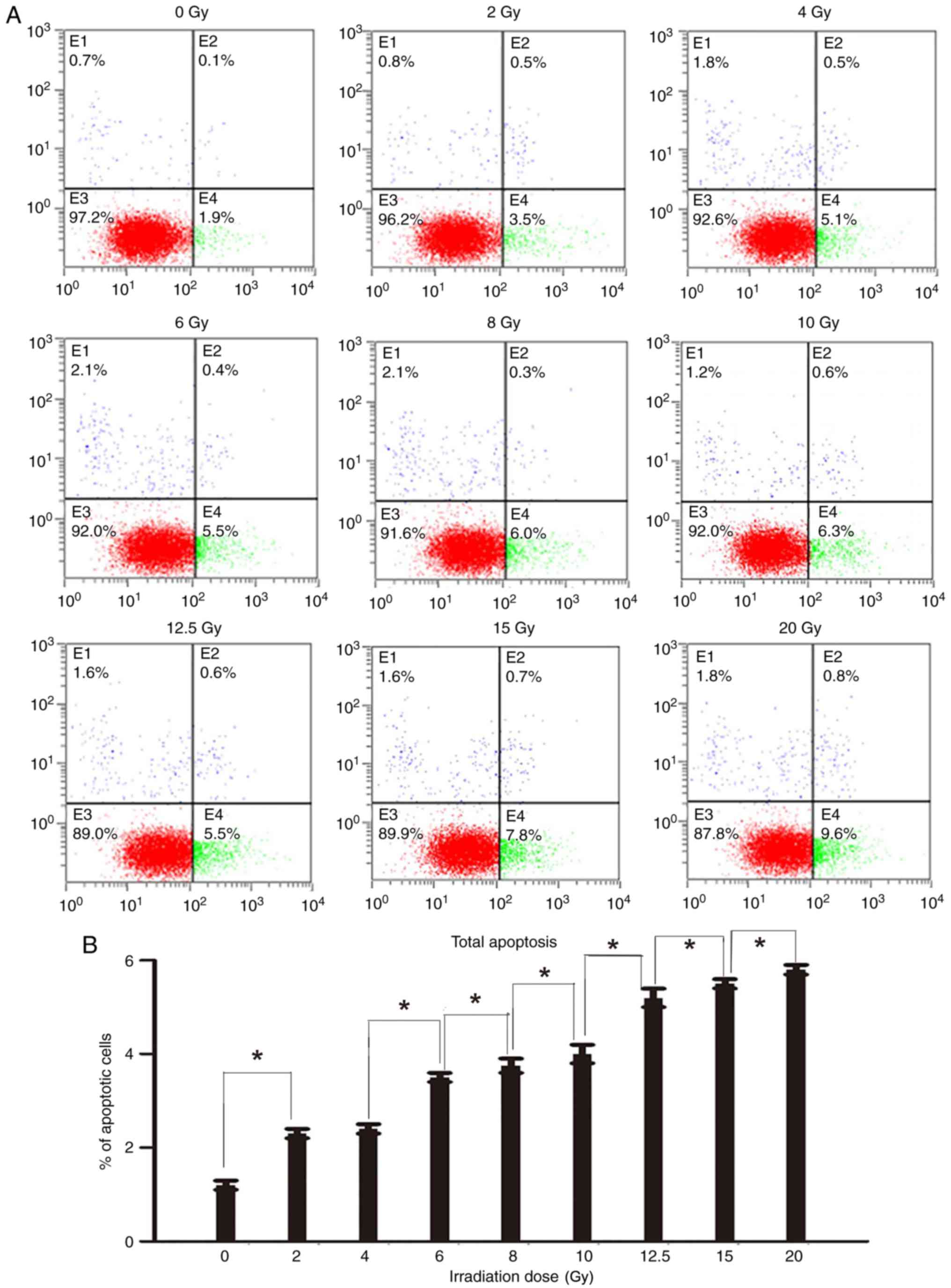 | Figure 5.Apoptosis of cells following
irradiation. (A) The apoptotic cells at 48 h after irradiation,
stained with Annexin V-FITC and PI, were analyzed using a flow
cytometer. Cells were classified into 4 subpopulations as follows:
Viable cells (lower left), early apoptotic cells (lower right),
cell fragments and damaged cells (upper left), and late apoptotic
cells (upper right). (B) The percentage of apoptotic cells at 48 h
after irradiation with different irradiation doses (lower right and
upper right). The radio of apoptotic cells of two groups with
adjacent doses were compared with each other. Except 2 and 4 Gy
groups (difference between the 2 and 4 Gy groups was not
significant), the difference in the percentage of apoptotic cells
between other adjacent two groups were significant *P<0.05. (C)
The apoptotic cells at 72 h after irradiation, stained with Annexin
V-FITC and PI, were analyzed using a flow cytometer. Cells were
classified into 4 subpopulations as follows: Viable cells (lower
left), early apoptotic cells (lower right), cell fragments and
damaged cells (upper left), and late apoptotic cells (upper right).
(D) The percentage of apoptotic cells at 72 h after irradiation
with different irradiation doses (lower right and upper right). The
radio of apoptotic cells of two groups with adjacent doses were
compared with each other. Except 2 and 4 Gy groups (difference
between the 2 and 4 Gy groups was not significant), the difference
in the percentage of apoptotic cells between other adjacent two
groups were significant *P<0.05. FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
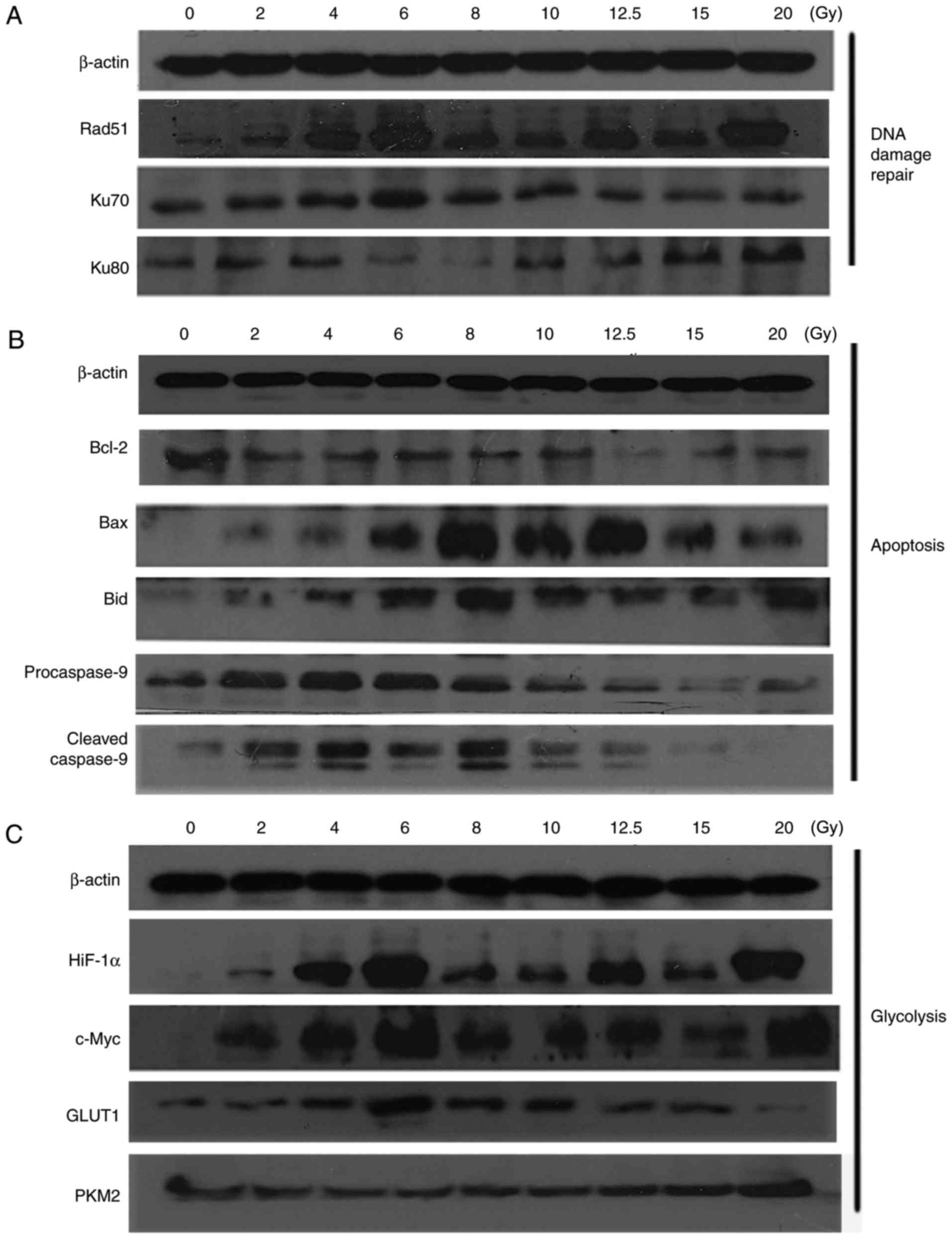
DNA damage repair- and
apoptosis-associated proteins following irradiation
The expression of DNA damage-associated proteins was
detected by western blot analysis (Fig.
6A). The expression of Rad51, which participates in homologous
recombination (HR) repair (17), was
markedly increased following irradiation, and the expression in the
2, 8 and 10 Gy groups was reduced, compared with the other groups.
Generally, the expression of Ku70 and Ku80, which serve a notable
role in Non-Homologous Endjoining (NHEJ) (18), were also increased following
irradiation. Furthermore, levels of Ku70 in the 2, 8, 10, 12.5, 15
and 20 Gy groups, and Ku80 in the 6 and 8 Gy groups were markedly
reduced, compared with the other groups. As reported, HR repair
occurs in the S and G2-phases; however, NHEJ occurs throughout all
phases of the cell cycle (19,20).
Combined with the results of these two primary repair pathways, it
may be concluded that for HeLa cells cultured in vitro, the
DNA damage repair capability in the groups of 2, 6, 8 and 10 Gy
were reduced, compared with the other groups, following
irradiation.
Expression of apoptosis-associated proteins was
detected by western blot analysis (Fig.
6B). Compared with the 0 Gy group, the expression of the Bcl-2
apoptosis suppression protein was markedly decreased following
different doses of irradiation. However, pro-apoptosis protein Bax
increased following irradiation, but the expression of Bax in the
6, 8, 10 and 12.5 Gy groups was notably increased, compared with
the other groups. Additionally, the apoptosis-associated proteins
Bid was elevated following irradiation, compared with the control
group (0 Gy). The expression of Bid in the 6, 8 and 10 Gy groups
were increased, compared with the other groups. The expression of
procaspase-9 and cleaved Caspase-9 was increased in the 2, 4, 6 and
8 groups, however, a downward trend was observed following 6 Gy.
Generally, increased expression levels of apoptosis-associated
proteins were determined in the 2, 6, 8 and 10 Gy groups.
Aerobic glycolysis metabolic following
irradiation
Given that radiation may alter tumor glucose
metabolism through the aerobic glycolysis metabolic pathway
(21,22), the present study detected changes
associated with aerobic glycolysis following irradiation that may
alter the energy of cancer cells.
Compared with 0 Gy, the results of the western blot
analysis (Fig. 6C) demonstrated an
increasing trend in the expression of HIF-1α following irradiation
in a dose-dependent manner, and a similar trend was observed in the
expression of c-Myc; however, the expression of HIF-1α and c-Myc in
the 4, 6, 12.5 and 20 Gy were higher than the other groups. GLUT1
is one of most important glucose transporters in cancer cells
(23). The expression level of GLUT1
was markedly increased following irradiation, but was reduced in
the 12.5, 15 and 20 Gy groups. HIF-1α, c-Myc and GLUT1 serve an
important role in promoting glycolysis and glucose uptake (24). The expression of PKM2 in the groups
with irradiation doses from 2–10 Gy were reduced, compared with the
other groups, indicating that the levels of glycolysis in these
groups may be reduced, compared with the other groups.
Discussion
In recent years, SBRT and stereotactic radiation
surgery have been increasingly used to treat cancer (25). SBRT is a novel radiotherapy technique,
which has high locoregional control rates (2). The rapid development of SBRT was
primarily due to the advantage that one or a few fractions of
high-dose ionizing radiation may be administered with high-target
accuracy and rapid dose-falloff gradients (8,26). A
notable cancer control result with limited complications can be
obtained.
Ionizing radiation is one of the most important
inducers of cellular damage. Indicators of cellular damage
detection include identification of DNA damage, such as through the
detection of γ-H2AX foci, DNA damage repair and cell survival.
Cellular damage indicators may also include other effects,
including cell cycle alterations, glycolysis effect and cell
apoptosis (11,12). Direct and indirect effects may be
induced by cellular exposure to X-ray radiation (27). The DNA damage induced by irradiation
could result in direct effects, whereas indirect biological effects
are associated with ROS generated by radiolysis and subsequent
reactions (28). DNA is more
susceptible to being attacked by ROS generated by low LET radiation
(28). The ROS produced during
irradiation can oxidize bases, and induce single-strand breaks
(SSBs) and DNA double-strand breaks (DSBs) (29). In the present experiment, the
production of ROS and γ-H2AX foci exhibited identical variation
tendencies in HeLa cells following different X-ray irradiation
doses, the production of ROS and γ-H2AX foci in cells following
irradiation were all increased, and increased at increased doses,
this suggests that ROS production has a critical relationship with
DNA damage.
There are two molecularly distinct G2/M checkpoints
that may be identified. The first one occurs early, is very
transient, and is dose independent; in contrast, the second one is
‘late’ G2/M accumulation, typically assessed by propidium iodide
staining, the late G2/M accumulation has an important character
which is ATM independent and dose-dependent. The late one can be
used to represent the accumulation of cells in early stages of the
cell cycle following exposure to radiation (30). The G2/M arrest detected in the present
study indicates late G2/M accumulation, which is dose-dependent,
and this character of late G2/M accumulation which is
dose-dependent combined with the present results, the G2/M delay in
cells following irradiation was increased at increased irradiation
doses. In contrast, early G2/M arrest is transient, ATM dependent
and dose-independent. Additionally, it can be induced in the early
period following exposure to ionizing radiation (31,32). G2/M
delay in tumor cells following irradiation may provide sufficient
time for repair processes and is important for ensuring cell
survival following sub-lethal DNA damage in cells (32). Considering HR repair occurs in the S
and G2-phases and G2/M delay following irradiation may provide
sufficient time for repair (17,32), the
G2/M-phase arrest is important for DNA damage repair following
irradiation (14,15). The present results demonstrated that
the cell cycle returned to the normal range within 48 h after
irradiation with doses <10 Gy, while the cell cycle could not
return to normal level within 48 h with doses ≥10 Gy. This
phenomenon may indicate that the DNA repair ability was not
sufficient to repair DNA damage induced by irradiation when the
dose exceeded 10 Gy. Apoptotic cells were detected at 48 and 72 h
after irradiation for each radiation dose. The proportion of
apoptotic cells at 72 h after irradiation was increased, compared
with at 48 h. Taken together these results suggest that DNA damage
that cannot be repaired within 48 h after irradiation may have
resulted in the number of apoptotic cells at 72 h after irradiation
being increased, compared with that at 48 h.
DSBs are detected by detecting γ-H2AX activation in
cells (33). Repair enzymes are
attracted and gathered to the damaged points of DNA as cells go
into cell cycle arrest, producing sufficient time for cellular
repair (13). There are two pathways
for DSB repair that have been identified in mammalian cells: HR and
NHEJ, NHEJ repair occurs in all phases of the cell cycle, but
primarily in the G1 phase, whereas HR generally occurs during the
S/G2 phases (27,34,35).
Additionally, previous studies indicated that while HR has the
capacity to repair damage in G2 phase, NHEJ is the primary repair
pathway in the G2 and G1 phases with 75–85% of irradiation-induced
DSBs undergoing repair via NHEJ in mammalian cells (19,20,36).
The NHEJ pathway is the most important pathway to
repair DSBs induced by radiation (37). The major molecule in this pathway is
the Ku70/80 heterodimer (KU) (18).
To activate the NHEJ pathway, the KU binds to blunt or near-blunt
DNA ends. Subsequently, the recruitment and activation of
DNA-dependent protein kinase catalytic subunit, that depends on
DSB-bound KU, triggers a wide range of downstream signaling
cascades that coordinate the repair processes (18). Rad51 is the key molecule in HR
(17) and interacts with the BRCA2
DNA repair associated molecule in the process of combining the two
homologous DNA strands (38,39).
If DNA damage cannot be adequately repaired, cells
progress towards apoptosis and/or necrosis (36). A decrease in the mitochondrial
membrane potential is an important initiator of apoptosis in its
early stages (15). The change in the
mitochondrial membrane potential of cells exposed to radiation in
the present study indicated that the mitochondrial apoptotic
pathway was activated, but the change in the 2 and 4 Gy groups were
similar, and a clear dose-dependent decrease of MMP was observed in
cells administered with an irradiation dose >4 Gy. Bcl-2 family
proteins, including the pro-apoptosis protein Bax and the
apoptosis-inhibitor Bcl-2, regulate cell apoptosis. Characteristic
morphological changes in cells and DNA that are associated with
apoptosis are primarily associated with the activation of cysteine
proteinases (caspases) (40). Cell
apoptosis through the mitochondrial-dependent pathway is
accompanied by the release of cytochrome c, followed by
activation of the caspase cascade (41). The present results demonstrated that
the 2–10 Gy groups achieved an improved result with a pro-apoptosis
effect.
Toxicity to normal tissue and other cellular
effects, including hypoxia and glycolysis, are limitations on the
efficacy of radiotherapy in solid tumor types (42). Glycolysis is an advantageous metabolic
pathway for cancer cells, including adaptation to hypoxia and
resistance to mitochondria-mediated apoptosis (23,43). A
number of studies have observed an increase in the activity of the
HIF-1α transcription factor following radiation, which regulates
metabolism, invasion and protection against oxidative stress
(24,44). Numerous glycolytic enzymes are
regulated by HIF-1α, including lactate dehydrogenase and PKM2, its
upregulation stimulates glucose uptake and glycolysis in cells
(24). Previous reports have
identified the glycolysis effect to be implicated in resistance to
cytotoxic stress, including that caused by ionizing radiation and
chemotherapy (21,45). The present data indicated that the
expression of HIF-1α and c-Myc markedly increased following
irradiation, serving an important role in promoting glycolysis.
However, the level of GLUT1 in the 12.5, 15 and 20 Gy groups
indicated a diminished ability for glucose uptake in these groups.
These results were consistent with overall cell cycle changes. In
the present study, cell cycle progression did not return to normal
level within 48 h after irradiation with doses ≥10 Gy, which may
indicate that DNA damage exceeded the capacity of repair
mechanisms. Cells may then progress towards apoptosis and necrosis.
Aerobic glycolysis can be induced with the expression of PKM2,
which is a regulator of glycolysis (46,47). The
glycolysis effect was reduced in the 2–10 Gy dose groups. Since
these results were gathered from cancer cells cultured in
vitro, further corroboration is required using in vivo
experiments.
In conclusion, in tumor cells, X-ray irradiation is
known to induce DNA DSBs, ROS, cellular apoptosis and G2/M phase
arrest. Compared with 2 Gy of radiation/fraction, a 4 Gy/fraction
dose did not demonstrate evident advantages in common biological
effects. Considering DNA damage repair and the apoptotic mechanism
of cells at the molecular and cellular levels following different
dose of irradiation, it may be concluded that 2, 6, 8 and 10 Gy may
be the optimal fractional doses with notable biological
responses.
Acknowledgements
The authors would like to thank Dr. Ji Chen who
worked in Department of Radiation and Medical Oncology, Zhongnan
Hospital Affiliated to Wuhan University for guiding them in the use
of the irradiation facility.
Funding
This research was supported by National Natural
Science Foundation of China (grant no. 81472799).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YunZ and FZ designed the experiments. ZM, HZ and
YafZ performed the experiments. RL, YL and ZH analyzed the data. HZ
wrote the paper. YunZ edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bergonié J and Tribondeau L:
Interpretation of some results from radiotherapy and an attempt to
determine a rational treatment technique. 1906. Yale J Biol Med.
76:181–182. 2003.PubMed/NCBI
|
|
2
|
Timmerman RD and Kavanagh BD: Stereotactic
body radiation therapy. Curr Probl Cancer. 29:120–157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim MS, Kim W, Park IH, Kim HJ, Lee E,
Jung JH, Cho LC and Song CW: Radiobiological mechanisms of
stereotactic body radiation therapy and stereotactic radiation
surgery. Radiat Oncol J. 33:265–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsang MW: Stereotactic body radiotherapy:
Current strategies and future development. J Thorac Dis. 8 Suppl
6:S517–S527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sapkaroski D, Osborne C and Knight KA: A
review of stereotactic body radiotherapy-is volumetric modulated
arc therapy the answer? J Med Radiat Sci. 62:142–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kavanagh BD, Timmerman RD, Benedict SH, Wu
Q, Schefter TE, Stuhr K, McCourt S, Newman F, Cardinale RM and
Gaspar LF: How should we describe the radioblologic effect of
extracranial stereotactic radlosurgery: Equivalent uniform dose or
tumor control probability? Med Phys. 30:321–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hof H, Herfarth KK, Münter M, Hoess A,
Motsch J, Wannenmacher M and Debus JJ: Stereotactic single-dose
radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J
Radiat Oncol Biol Phys. 56:335–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Timmerman R, Papiez L and Suntharalingam
M: Extracranial stereotactic radiation delivery: Expansion of
technology beyond the brain. Technol Cancer Res Treat. 2:153–160.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Potters L, Steinberg M, Rose C, Timmerman
R, Ryu S, Hevezi JM, Welsh J, Mehta M, Larson DA and Janjan NA;
American Society for Therapeutic Radiology and Oncology; American
College of Radiology, . American society for therapeutic radiology
and oncology and american college of radiology practice guideline
for the performance of stereotactic body radiation therapy. Int J
Radiat Oncol Biol Phys. 60:1026–1032. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Timmerman RD, Forster KM and Chinsoo Cho
L: Extracranial stereotactic radiation delivery. Semin Radiat
Oncol. 15:202–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barilla J, Lokajíček M, Pisaková H and
Simr P: Analytical model of chemical phase and formation of DSB in
chromosomes by ionizing radiation. Australas Phys Eng Sci Med.
36:11–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma A, Singh K and Almasan A: Histone
H2AX phosphorylation: A marker for DNA damage. Methods Mol Biol.
920:613–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka T, Halicka D, Traganos F and
Darzynkiewicz Z: Cytometric analysis of DNA damage: Phosphorylation
of histone H2AX as a marker of DNA double-strand breaks (DSBs).
Methods Mol Biol. 523:161–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan Y, Black CP and Cowan KH:
Irradiation-induced G2/M checkpoint response requires ERK1/2
activation. Oncogene. 26:4689–4698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu D and Kipps TJ: Reduction in
mitochondrial membrane potential is an early event in
Fas-independent CTL-mediated apoptosis. Cell Immunol. 195:43–52.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao
C, Feng S, Guo H, Xu B, Yang Q, et al: Berberine radiosensitizes
human esophageal cancer cells by downregulating homologous
recombination repair protein RAD51. PLoS One. 6:e234272011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dynan WS and Yoo S: Interaction of Ku
protein and DNA-dependent protein kinase catalytic subunit with
nucleic acids. Nucleic Acids Res. 26:1551–1559. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orthwein A, Fradet-Turcotte A, Noordermeer
SM, Canny MD, Brun CM, Strecker J, Escribano-Diaz C and Durocher D:
Mitosis inhibits DNA double-strand break repair to guard against
telomere fusions. Science. 344:189–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Symington LS and Gautier J: Double-strand
break end resection and repair pathway choice. Annu Rev Genet.
45:247–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pitroda SP, Wakim BT, Sood RF, Beveridge
MG, Beckett MA, MacDermed DM, Weichselbaum RR and Khodarev NN:
STAT1-dependent expression of energy metabolic pathways links
tumour growth and radioresistance to the Warburg effect. BMC Med.
7:682009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khodarev NN, Beckett M, Labay E, Darga T,
Roizman B and Weichselbaum RR: STAT1 is overexpressed in tumors
selected for radioresistance and confers protection from radiation
in transduced sensitive cells. Proc Natl Acad Sci USA.
101:1714–1719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondoh H, Lleonart ME, Gil J, Wang J,
Degan P, Peters G, Martinez D, Carnero A and Beach D: Glycolytic
enzymes can modulate cellular life span. Cancer Res. 65:177–185.
2005.PubMed/NCBI
|
|
25
|
Lo SS, Slotman BJ, Lock M, Nagata Y,
Guckenberger M, Siva S, Foote M, Tan D, The BS, Mayr NA, et al: The
development of stereotactic body radiotherapy in the past decade: A
global perspective. Future Oncol. 11:2721–2733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Potters L, Kavanagh B, Galvin JM, Hevezi
JM, Janjan NA, Larson DA, Mehta MP, Ryu S, Steinberg M, Timmerman
R, et al: American society for therapeutic radiology and oncology
(ASTRO) and american college of radiology (ACR) practice guideline
for the performance of stereotactic body radiation therapy. Int J
Radiat Oncol Biol Phys. 76:326–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borek C: Antioxidants and radiation
therapy. J Nutr. 134:3207S–3209S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aparicio T, Baer R and Gautier J: DNA
double-strand break repair pathway choice and cancer. DNA Repair
(Amst). 19:169–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Illner D and Scherthan H: Ionizing
irradiation-induced radical stress stalls live meiotic chromosome
movements by altering the actin cytoskeleton. Proc Natl Acad Sci
USA. 110:16027–16032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu B, Kim ST, Lim DS and Kastan MB: Two
molecularly distinct G(2)/M checkpoints are induced by ionizing
irradiation. Mol Cell Biol. 22:1049–1059. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marples B, Wouters BG and Joiner MC: An
association between the radiation-induced arrest of G2-phase cells
and low-dose hyper-radiosensitivity: A plausible underlying
mechanism? Radiat Res. 160:38–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fingert HJ, Chang JD and Pardee AB:
Cytotoxic, cell cycle, and chromosomal effects of methylxanthines
in human tumor cells treated with alkylating agents. Cancer Res.
46:2463–2467. 1986.PubMed/NCBI
|
|
33
|
Kinner A, Wu W, Staudt C and Iliakis G:
Gamma-H2AX in recognition and signaling of DNA double-strand breaks
in the context of chromatin. Nucleic Acids Res. 36:5678–5694. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Helleday T, Lo J, van Gent DC and
Engelward BP: DNA double-strand break repair: From mechanistic
understanding to cancer treatment. DNA Repair (Amst). 6:923–935.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rothkamm K, Krüger I, Thompson LH and
Löbrich M: Pathways of DNA double-strand break repair during the
mammalian cell cycle. Mol Cell Biol. 23:5706–5715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beucher A, Birraux J, Tchouandong L,
Barton O, Shibata A, Conrad S, Goodarzi AA, Krempler A, Jeggo PA
and Löbrich M: ATM and Artemis promote homologous recombination of
radiation-induced DNA double-strand breaks in G2. EMBO J.
28:3413–3427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vandersickel V, Depuydt J, Van Bockstaele
B, Perletti G, Philippe J, Thierens H and Vral A: Early increase of
radiation-induced γH2AX foci in a human Ku70/80 knockdown cell line
characterized by an enhanced radiosensitivity. J Radiat Res.
51:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saydam O, Saydam N, Glauser DL, Pruschy M,
Dinh-Van V, Hilbe M, Jacobs AH, Ackermann M and Fraefel C: HSV-1
amplicon-mediated post-transcriptional inhibition of Rad51
sensitizes human glioma cells to ionizing radiation. Gene Ther.
14:1143–1151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnson RD and Jasin M: Sister chromatid
gene conversion is a prominent double-strand break repair pathway
in mammalian cells. EMBO J. 19:3398–3407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roos WP and Kaina B: DNA damage-induced
cell death: From specific DNA lesions to the DNA damage response
and apoptosis. Cancer Lett. 332:237–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pardo FS, Su M and Borek C: Cyclin D1
induced apoptosis maintains the integrity of the G1/S checkpoint
following ionizing radiation irradiation. Somat Cell Mol Genet.
22:135–144. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown JM, Diehn M and Loo BW Jr:
Stereotactic ablative radiotherapy should be combined with a
hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys.
78:323–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Semenza GL: Oxygen sensing, homeostasis,
and disease. N Engl J Med. 365:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JW and Dang CV: Cancer's molecular
sweet tooth and the warburg effect. Cancer Res. 66:8927–8930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang T, Marquardt C and Foker J: Aerobic
glycolysis during lymphocyte proliferation. Nature. 261:702–705.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|