Introduction
Renal cell carcinoma (RCC) is a common urological
malignancy (1). In the USA, an
estimated 64,000 new cases of kidney cancer were expected in 2017
(1). Clear cell RCC (CCRCC)
represents 70–80% of RCC cases. In recent years, a number of
molecular targeted therapies have been used in the clinical
treatment of RCC, but the prognosis remains unfavourable (2). Additionally, predictive biomarkers of
response have not been identified. Thus, the identification of
effective biomarkers is required for predicting the progression and
prognosis of RCC, and to advance the development of novel
treatments.
Amyloid β precursor-like protein 2 (APLP2) is a type
I transmembrane protein belonging to the evolutionary conserved
amyloid precursor protein gene family, which has been associated
with Alzheimer's disease pathogenesis (3). The human APLP2 gene is located at 11q24
and is expressed in the majority of tissues (4). It has been demonstrated that APLP2
expression is involved in the development of a number of cancer
types, including pancreatic, colon, breast and prostate cancer
(5). APLP2 has been associated with
certain characteristics of cancer cells, including abnormal growth,
migration and invasion, which indicates that APLP2 may
significantly affect the development and progression of cancer
(6); however, the expression and
function of APLP2 in RCC remains undefined. In the present study,
the expression of APLP2 in CCRCC tissues was measured and the
association between APLP2 expression and the clinicopathological
features of CCRCC was evaluated, in order to determine whether
APLP2 may potentially be used as a novel biomarker and therapeutic
target for CCRCC.
Materials and methods
Patients and tumor specimens
All samples were obtained from Qilu Hospital of
Shandong University (Jinan, China). The clinical and pathological
data of patients (mean, 63; range, 40–81 years of age) who were
diagnosed with CCRCC by computed tomography, magnetic resonance
imaging and pathological examination, and who underwent surgery
between January 2011 and December 2012, were collected upon
receiving ethical approval from the Medical Ethics Committee of
Qilu Hospital of Shandong University. A total of 90 pairs of tumor
and adjacent normal tissues were collected from patients with
primary CCRCC. Patients were excluded from the present study if
they had missing information. Patients with autoimmune disease or
cancer in other systems, and had received neoadjuvant chemotherapy
or radiotherapy were also excluded from the present study. Paired
CCRCC and adjacent non-tumor tissues were obtained, snap-frozen in
liquid nitrogen, and maintained at −80°C until further use. Written
informed consent was obtained from all subjects. Resected tissues
were immediately snap-frozen in liquid nitrogen and stored at −80°C
prior to protein and mRNA extraction. Additionally, tissues were
fixed for immunohistochemical staining. The present study was
approved by the Medical Ethics Committee of Qilu Hospital of
Shandong University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA from 50 mg frozen samples (n=8) was
extracted using a TRIzol™ Plus RNA Purification kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocols. Subsequently, 300 ng total RNA was used
for further complementary DNA synthesis using a Vestar qPCR RT kit
(cat. no. 2220; Deutsche Biotech Innovativ AG, Hennigsdorf,
Germany), according to the manufacturer's protocols. qPCR was
conducted in 3 wells for one sample using a 20-µl reaction system
(SYBR® Green Realtime PCR Master Mix; cat.no. QPK-201;
Toyobo Life Science, Osaka, Japan). The reaction was performed at
95°C for 15 min, followed by 40 cycles at 95°C for 15 sec and 60°C
for 60 sec. The experiment was repeated three times. Relative mRNA
expression was calculated with the comparative 2−ΔΔCq
method (7) and GAPDH was used as the
control. All primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The primer sequences used in
the qPCR were as follows: APLP2 forward,
5′-ACAGGATTTGCTGTTGCTGAG-3′, and reverse,
5′-AGTGCCATGGAACTGGTCTAC-3′; and GAPDH forward,
5′-CGGATTTGGTCGTATTGGG-3′, and reverse,
5′-CTGGAAGATGGTGATGGGATT-3′.
Immunohistochemical staining
The procedures for the immunohistochemical staining
of tissue samples have been previously described (4). In brief, tissues were fixed in 4%
polyformaldehyde at room temperature for 24 h, and then embedded in
paraffin wax and cut into sections of 3 µm in thickness. The
paraffin-embedded sections of cancer tissues were subsequently
deparaffinized and heated in a pressure pot (95°C) for 3 min to
retrieve the antigens, were washed three times with xylene for 5
min and were rehydrated in a descending alcohol series (absolute
ethanol, 95, 85 and 75%). Subsequently, the sections were incubated
in a 1:500 dilution of rabbit anti-APLP2 antibody (cat. no.
ab128603; Abcam, Cambridge, UK) overnight at 4°C. Antibody binding
was detected using a peroxidase-conjugated secondary antibody
(SPN-9001; goat anti-rabbit, ZSJQB Co., Ltd. Beijing, China) in a
1:1,000 dilution at 37°C for 30 min. The sections were then stained
with DAB Substrate kit (cat. no. ZLI9017; ZSJQB Co., Ltd.),
according to the manufacturer's protocols, for 2 min, and with
hematoxylin for 2 min at room temperature. The expression of APLP2
in the tissues was observed under an optical microscope (DP72;
Olympus Corporation, Tokyo, Japan) at ×200 magnification.
The protein expression of APLP2 in the tissues was
quantified by the intensity and extent of staining. The intensity
of the staining was evaluated using the following criteria: 0,
negative; 1, low; 2, medium; and 3, high. The extent of staining
was scored as 0, 0% stained; 1, 1–25% stained; 2, 26–50% stained;
and 3, 51–100% stained. The total expression of APLP2 protein was
calculated by multiplying the staining intensity score with the
extent score. APLP2 was considered to be underexpressed when the
result of this computation was <6.
Western blot analysis
The paired specimens (n=10) were homogenized in an
ice-cold radioimmunoprecipitation assay buffer (cat. no. AR0105;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) containing
1 mM phenylmethylsulfonyl fluoride to extract the tissue proteins
in 4°C for 30 min. A bicinchoninic acid assay kit (cat. no.
BPT0001; BioRike; Changsha Dalfeng Biotechnology Co., Ltd.,
Changsha, China) was used to measure total protein concentrations,
followed by western blot analysis as previously described (4). Briefly, denaturing 10% SDS-PAGE was used
to separate 60 mg protein, which was then transferred onto a
polyvinylidene fluoride (PVDF) membrane electrophoretically. The
PVDF membrane was blocked for 1 h at room temperature with 5%
non-fat milk with Tris-buffered saline Tween-20 (TBST) dilution and
then incubated with the aforementioned rabbit anti-APLP2 antibody
(1:1,000 dilution) or β-actin antibody (1:1,000 dilution; cat. no.
BM0627; Wuhan Boster Biological Technology, Ltd.) overnight at 4°C.
The membrane was then washed with TBST three times and incubated
with horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies at a dilution of 1:2,000 at room temperature (cat. no.
BA1054; Wuhan Boster Biological Technology, Ltd.) for 2 h. Finally,
the protein bands were detected using a super enhanced
chemiluminescence plus detection reagent (cat. no. EK1002; Wuhan
Boster Biological Technology, Ltd.).
Statistical analysis
The association between the expression of APLP2 and
the survival time of patients with RCC was analyzed by the
Kaplan-Meier method with the log-rank test. Each experiment was
performed in triplicate, and the values were presented as the mean
± standard deviation, unless otherwise stated. The two-tailed
Pearson's χ2 and Fisher's exact tests were used to
compare categorical variables. A multivariate analysis was
performed using the multivariate Cox proportional hazards model.
Additionally, a paired Student's t-test was performed for
investigating differences between two groups. Data analyses were
performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Downregulation of APLP2 in human CCRCC
tissues
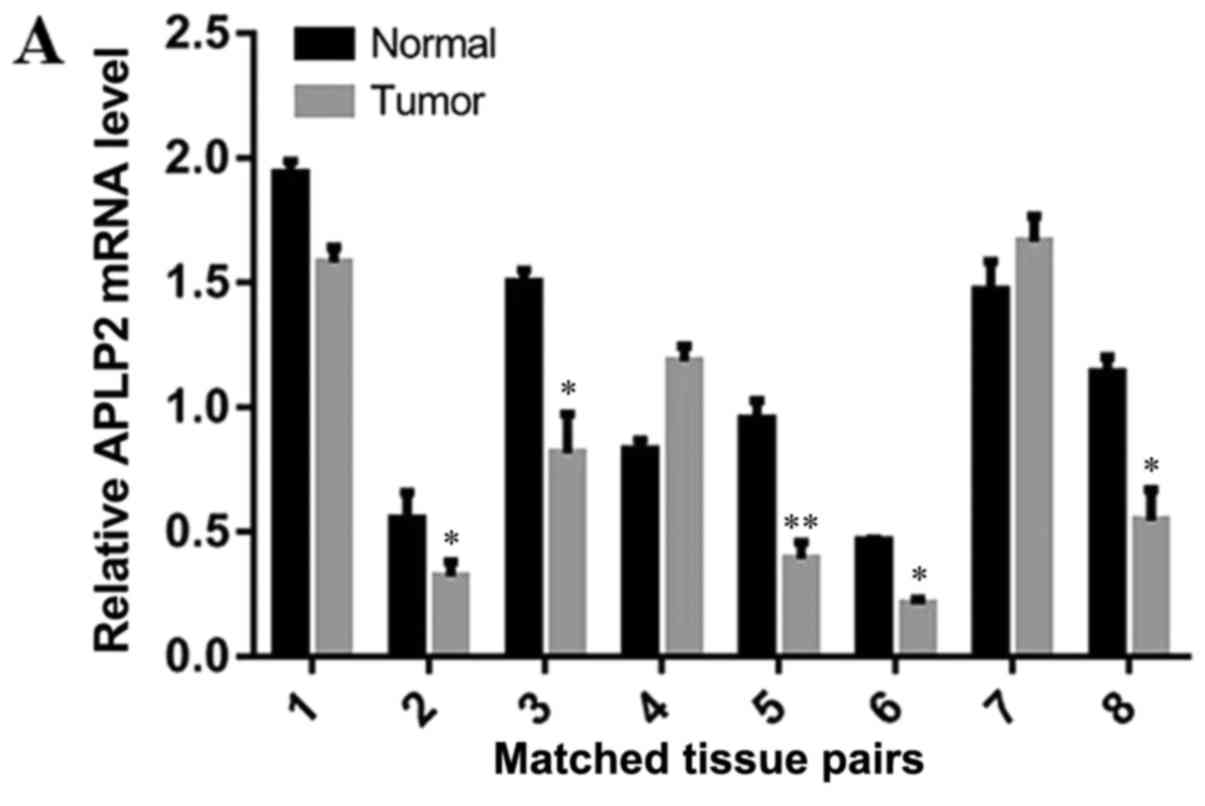
The mRNA levels of APLP2 in 8 human CCRCC and
adjacent tissues were measured by RT-qPCR. In 5/8 (62.5%) tissue
pairs, the mRNA level of APLP2 in the RCC tissues was reduced
compared with that of the adjacent normal tissues (Fig. 1A). The paired Student's t-test data
analysis demonstrated that the mRNA level of APLP2 was
significantly reduced in the CCRCC tissues compared with that in
the adjacent normal tissues (Fig. 1B;
P=0.02).
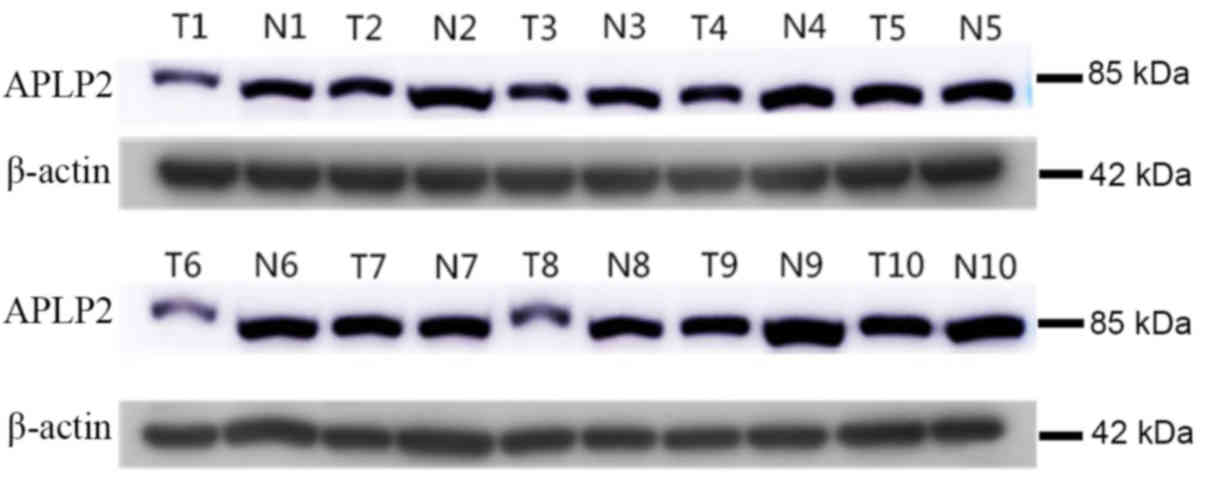
Downregulation of APLP2 in human RCC
tissues
The levels of APLP2 protein in 10 human CCRCC and
adjacent tissues were measured by western blot analysis. In 8/10
pairs of samples, the protein level of APLP2 was observed to be
reduced in the CCRCC tissues compared with that in the adjacent
normal tissues (Fig. 2).
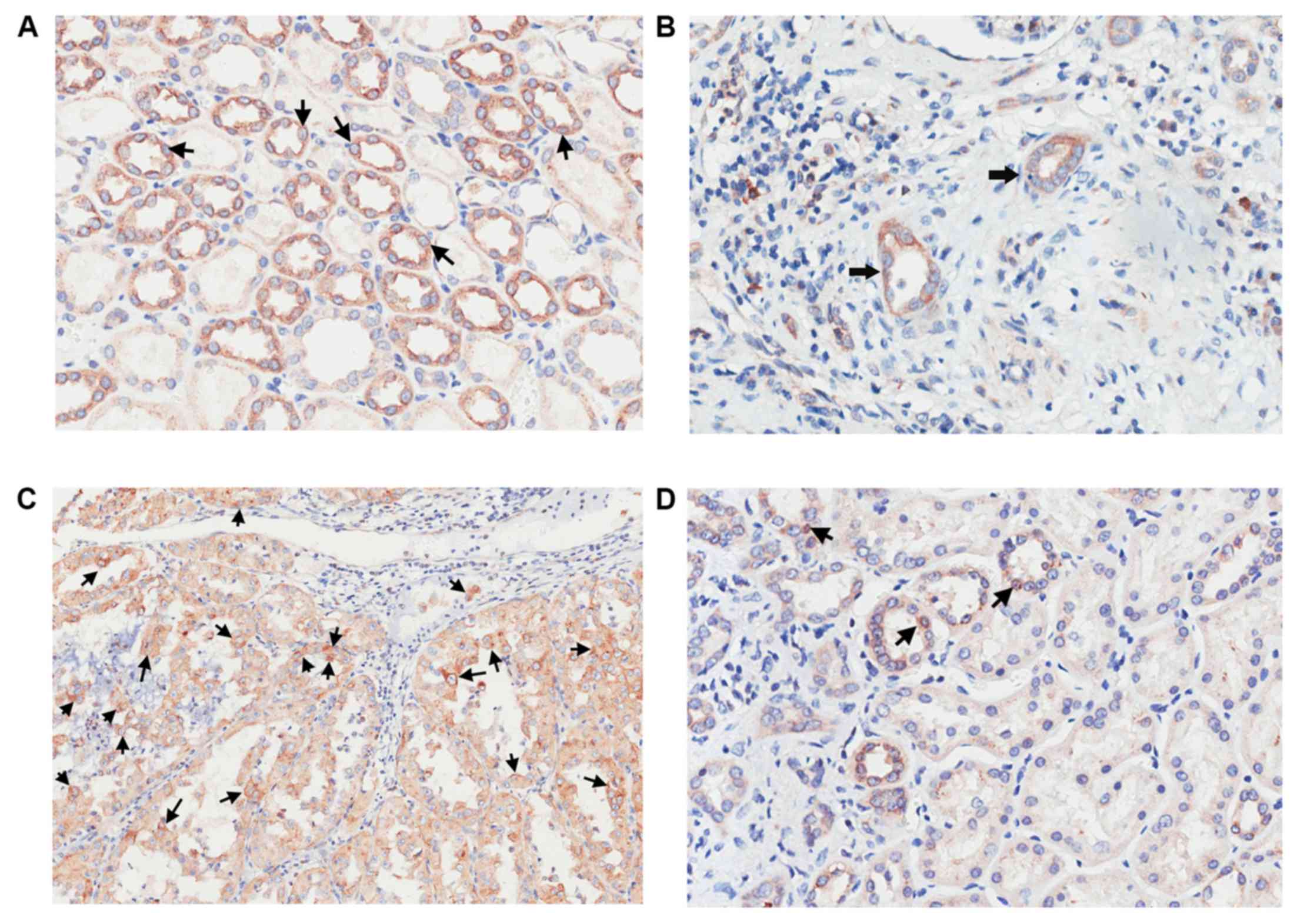
Clinicopathological features and APLP2
expression
Randomly selected expression profiles of APLP2 in
RCC and adjacent normal renal cortical tissues are depicted in
Fig. 3. The expression of APLP2
protein in normal kidney tissues indicated a score >6 (Fig. 3A), CCRCC tissues indicated a score
<6 (Fig. 3B), CCRCC tissues a
score >6 (Fig. 3C) and in normal
kidney tissues a score <6 Fig.
3D). The clinicopathologic characteristics of 90 patients are
indicated in Table I. In detail, the
expression of APLP2 was significantly associated with Fuhrman grade
(P=0.018), pT stage (P=0.033), and the presence of distant
metastasis (P=0.018) and lymph node metastasis (P=0.037).
Pathological T stages was evaluated in accordance with the World
Health Organization 1973 guidelines (8), and tumor grade according to Fuhrman
et al (9). Additionally, there
was no significant association between APLP2 expression and the sex
or age of the patients, and the size of the renal tumor.
 | Table I.Clinicopathological features and their
associations with APLP2 expression. |
Table I.
Clinicopathological features and their
associations with APLP2 expression.
|
|
| APLP2 expression
level |
|
|---|
|
|
|
|
|
|---|
| Variables | Total cases
(n=90) | <6 | ≥6 | P-value |
|---|
| Tissues |
|
|
| 0.003a |
| Tumor
tissues | 90 | 37 | 53 |
|
| Normal
tissues | 90 | 57 | 33 |
|
| Age (years) |
|
|
| 0.866 |
| ≤65 | 59 | 37 | 22 |
|
|
>65 | 31 | 20 | 11 |
|
| Sex |
|
|
| 0.65 |
| Male | 49 | 30 | 19 |
|
|
Female | 41 | 27 | 14 |
|
| Fuhrman grade |
|
|
| 0.018a |
| I–II | 48 | 25 | 23 |
|
|
III–IV | 42 | 32 | 10 |
|
| Tumor size (longest
diameter, cm) |
|
|
| 0.31 |
| ≤7 | 51 | 30 | 21 |
|
|
>7 | 39 | 27 | 12 |
|
| Lymph node
metastasis |
|
|
| 0.037a |
|
Absence | 73 | 42 | 31 |
|
|
Presence | 17 | 15 | 2 |
|
| Distant
metastasis |
|
|
| 0.018a |
|
Absence | 79 | 46 | 33 |
|
|
Presence | 11 | 11 | 0 |
|
| pT stage |
|
|
| 0.033a |
|
T1-T2 | 71 | 41 | 30 |
|
|
T3-T4 | 19 | 16 | 3 |
|
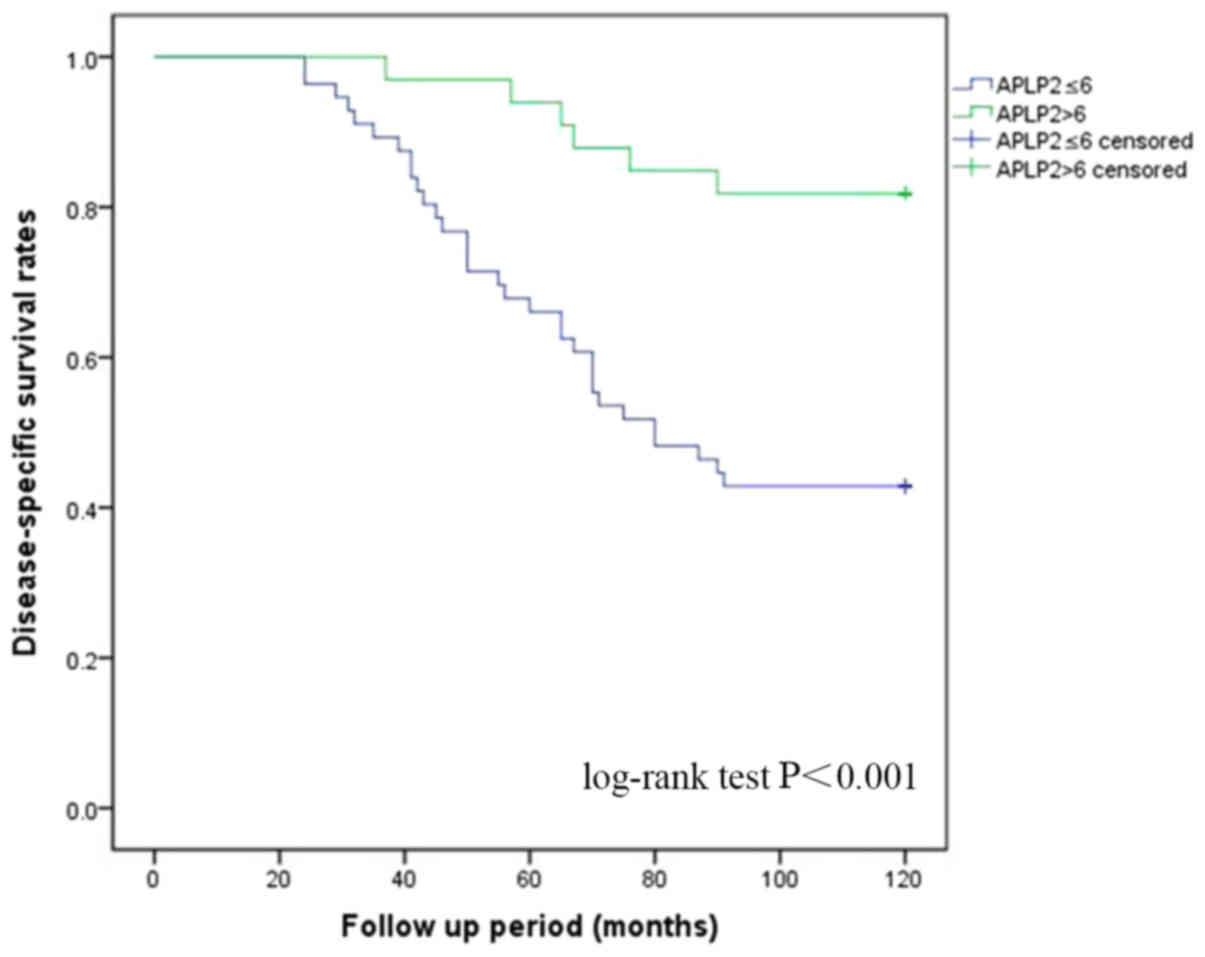
Survival analyses
Survival analyses were performed using the tissues
and the follow-up information from well-documented patients. The
Kaplan-Meier disease-specific survival curves were constructed
using the scores of APLP2 expression. According to the results from
the log-rank test, the expression of APLP2 was signicantly
associated with disease-specific survival (P<0.001; Fig. 4). Additionally, according to the
results from the univariate analysis, age (>65 years), high
Fuhrman grade (P=0.018), high pT stage (P=0.033), and the presence
of lymph node (P=0.037) and distant metastasis (P=0.018) were also
significant predictors of survival. However, sex and tumor size
(>7 cm) were not significant predictors of survival (Table II). The subsequent multivariate Cox
analysis demonstrated that the increased expression of APLP2 was an
independent predictor of disease-specific survival (P=0.026).
Additionally, age (>65 years), high pT stage, and presence of
distant and lymph node metastasis were independent predictors.
However, high Fuhrman grade was not a significant independent
predictor (Table II).
 | Table II.Cox regression analyses for
disease-specific survival. |
Table II.
Cox regression analyses for
disease-specific survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>65
years) | 0.281
(0.146–0.540) |
<0.001a | 6.454
(2.887–14.427) |
<0.001a |
| Sex female | 1.072
(0.146–2.025) | 0.830 | 0.819
(0.356–1.887) | 0.640 |
| Grade high
(III–IV) | 0.490
(0.255–0.945) | 0.033a | 0.845
(0.372–1.921) | 0.687 |
| Tumor size (>7
cm) | 1.227
(0.648–2.323) | 0.530 | 0.452
(0.200–1.018) | 0.055 |
| pT high
(T3-T4) | 0.112
(0.057–0.222) |
<0.001a | 3.380
(1.375–8.307) | 0.008a |
| Lymph node
metastasis | 0.122
(0.061–0.244) |
<0.001a | 2.584
(1.126–5.929) | 0.025a |
| Distant
metastasis | 0.140
(0.067–0.292) |
<0.001a | 4.131
(1.431–11.928) | 0.009a |
| APLP2 score ≤6 | 4.401
(1.837–10.545) | 0.001a | 3.138
(1.148–8.581) | 0.026a |
Discussion
A previous study demonstrated that APLP2 expression
is aberrantly altered in a number of cancer types (6). However, there is limited knowledge
regarding the expression of APLP2 in RCC, including the association
between the expression of APLP2 and the prognosis of the disease.
In the present study, the association between APLP2 and the
pathological and prognostic features of CCRCC was examined. It was
determined that the mRNA and protein levels of APLP2 were
significantly decreased in RCC tissues compared with those in
matched normal tissues. The expression of APLP2 was associated with
the Fuhrman grade and pT stage of CCRCC. Subsequently, the survival
analysis demonstrated that low expression of APLP2 was an
independent predictor of disease-specific survival.
Data from the present study indicated that APLP2 may
be a cancer suppressor gene in CCRCC. A previous study also
reported that APLP2 was notably downregulated in neuroendocrine
lung tumor cases (10). However, a
number of studies demonstrated that the expression of APLP2 was
significantly increased in other cancer types, including breast
cancer (11), pancreatic cancer
(12), myeloid leukaemia (13) and testicular germ cell tumors
(14). Furthermore, the reason for
the different expression of APLP2 in different cancer types remains
unclear. This difference may be due to different pathogeneses in
different cancer types. Additionally, this phenomenon may account
for the different mechanisms of carcinogenesis in different cancer
types (15). In addition, a previous
study indicated that splicing may result in its gene diversity, and
the different expression profiles and functions of APLP2 in
different tissues (16). The results
of the present study also demonstrated that APLP2 may serve
different roles in different tissues.
APLP2 has been associated with certain
characteristics of cancer cells, including pro-growth and
pro-invasion functions (5); however,
the specific role of APLP2 in cancer cell development and
progression remains unclear. At present, the signalling pathways by
which APLP2 wields its effects in cancer cells are poorly
understood. Pandey et al (5)
determined that loss of APLP2 decreased cortical actin and
increased intracellular actin filaments in pancreatic cancer cells,
reducing the ability of these cells to migrate and invade. Other
studies demonstrated that APLP2 can regulate major
histocompatibility complex class I molecule expression in cancer
cells and increase cancer immune evasion (17,18). There
is further research required in order to determine the biological
activity of APLP2 in cancer cells. In the present study, APLP2
exhibited significantly reduced expression in tissues from patients
with CCRCC, and this information indicated that APLP2 may serve as
a suppressor in CCRCC. However, further studies are required to
investigate the specific role of APLP2 in RCC.
There are a number of limitations in the present
study. Firstly, all tissue samples were collected from one region,
which may result in bias. Secondly, the sample size in the present
study was relatively small and further larger studies on APLP2 in
CCRCC are required in the future. Finally, APLP2 was determined to
serve as a potential suppressor of RCC, but its underlying
mechanism has not been investigated. In particular, since mutations
of the von Hippel-Lindau (VHL) tumor suppressor gene are considered
as drivers of CCRCC (19), whether
APLP2 is associated with VHL remains unknown.
In conclusion, the present study provided initial
evidence that APLP2 is downregulated in CCRCC and is associated
with certain pathological features of CCRCC. The results also
demonstrated that APLP2 expression is significantly and
independently associated with the disease-specific survival of
patients with RCC. The measurement of APLP2 levels may therefore
assist in improving the accuracy of RCC diagnosis, particularly
CCRCC, to evaluate the therapeutic efficacy of treatment, and to
predict prognosis. Further efforts are required to clarify the
precise molecular mechanism of APLP2 in the occurrence, progression
and metastasis of CCRCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
and Development Grants of Shandong Province, China (grant no.,
2016GSF201011).
Availability of data and materials
The datasets used or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BS designed the project and performed study
supervision. LG and HZ designed the project and performed the
development of the methodology. DZ, CZ, YL, HW, CR and YX
collected, analyzed and interpreted the data. YL and YX contributed
to drafting the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Qilu Hospital of Shandong University. Written informed
consent was obtained from all patients.
Patient consent for publication
The study has obtained consents from all patient for
publication.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muller UC, Deller T and Korte M: Not just
amyloid: Physiological functions of the amyloid precursor protein
family. Nat Rev Neurosci. 18:281–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shariati SA and De Strooper B: Redundancy
and divergence in the amyloid precursor protein family. FEBS Lett.
587:2036–2045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandey P, Rachagani S, Das S,
Seshacharyulu P, Sheinin Y, Naslavsky N, Pan Z, Smith BL, Peters
HL, Radhakrishnan P, et al: Amyloid precursor-like protein 2
(APLP2) affects the actin cytoskeleton and increases pancreatic
cancer growth and metastasis. Oncotarget. 6:2064–2075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pandey P, Sliker B, Peters HL, Tuli A,
Herskovitz J, Smits K, Purohit A, Singh RK, Dong J, Batra SK, et
al: Amyloid precursor protein and amyloid precursor-like protein 2
in cancer. Oncotarget. 7:19430–19444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arvidsson Y, Andersson E, Bergström A,
Andersson MK, Altiparmak G, Illerskog AC, Ahlman H, Lamazhapova D
and Nilsson O: Amyloid precursor-like protein 1 is differentially
upregulated in neuroendocrine tumours of the gastrointestinal
tract. Endocr Relat Cancer. 15:569–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim S, Yoo BK, Kim HS, Gilmore HL, Lee Y,
Lee HP, Kim SJ, Letterio J and Lee HG: Amyloid-beta precursor
protein promotes cell proliferation and motility of advanced breast
cancer. BMC Cancer. 14:9282014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peters HL, Tuli A, Wang X, Liu C, Pan Z,
Ouellette MM, Hollingsworth MA, Macdonald RG and Solheim JC:
Relevance of amyloid precursor-like protein 2 C-terminal fragments
in pancreatic cancer cells. Int J Oncol. 41:1464–1474. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Yu G, Meng W, Wang Z, Meng F and
Ma W: Overexpression of amyloid precursor protein in acute myeloid
leukemia enhances extramedullary infiltration by MMP-2. Tumour
Biol. 34:629–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkataramani V, Thiele K, Behnes CL, Wulf
GG, Thelen P, Opitz L, Salinas-Riester G, Wirths O, Bayer TA and
Schweyer S: Amyloid precursor protein is a biomarker for
transformed human pluripotent stem cells. Am J Pathol.
180:1636–1652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cojocaru E, Lozneanu L, Giuşcă SE, Căruntu
ID and Danciu M: Renal carcinogenesis-insights into signaling
pathways. Rom J Morphol Embryol. 56:15–19. 2015.PubMed/NCBI
|
|
16
|
Sandbrink R, Masters CL and Beyreuther K:
Similar alternative splicing of a non-homologous domain in beta
A4-amyloid protein precursor-like proteins. J Biol Chem.
269:14227–14234. 1994.PubMed/NCBI
|
|
17
|
Meng JY, Kataoka H, Itoh H and Koono M:
Amyloid beta protein precursor is involved in the growth of human
colon carcinoma cell in vitro and in vivo. Int J Cancer. 92:31–39.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peters HL, Tuli A, Sharma M, Naslavsky N,
Caplan S, MacDonald RG and Solheim JC: Regulation of major
histocompatibility complex class I molecule expression on cancer
cells by amyloid precursor-like protein 2. Immunol Res. 51:39–44.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehdi A and Riazalhosseini Y: Epigenome
aberrations: Emerging driving factors of the clear cell renal cell
carcinoma. Int J Mol Sci. 18:E17742017. View Article : Google Scholar : PubMed/NCBI
|