Introduction
Bladder cancer is the ninth cause of tumor in the
world and the second most common genitourinary malignancy.
Urothelial carcinoma represents 90% of all primary bladder tumors
(1). Half of patients affected by
these tumors, will develop local recurrence or distant metastases
after radical surgery and treatment in this setting remains
exclusively palliative. Lymph nodes, liver, lung and bones
represent the metastatic sites with higher incidence (2). The eye is a rare site for disseminated
malignancies because of the absence of a lymphatic system and
metastases may occur by haematogenous spread (3). Therefore, eye structures with the
highest vascular supply are more likely affected, with an incidence
from 1 to 13% (2). Breast cancer is
the most common primary tumor metastasizing to the eye, followed in
order of frequency by: Lung cancer, gastrointestinal tumors, and
less commonly, thyroid, prostate, kidney, testicles, pancreatic,
ovarian and liver cancer (4). Eye
metastases comprise both orbital (bone, muscle and fat) and ocular
(mainly uveal) localizations (5,6). Majority
of eye metastases in adults are located in the uvea and mainly in
the choroid and orbital metastases are less frequent than uveal
metastases (5). Generally, they onset
as synchronous or metachronous localizations in patients with
multiple metastatic sites and life expectancy is very poor.
Twenty-three cases of urothelial or bladder tumors with eye
metastases have been described in literature so far (2,4–23). Here we report the first documented
case, to our knowledge, of an urothelial-bladder cancer
metastasizing to the retro-bulbar region and infiltrating the
lacrimal gland. Furthermore, we provide a systematic qualitative
review of the current literature on eye metastases from urothelial
bladder cancer using the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) (24). Finally, we aim to clarify the
features, medical interventions, outcomes and we try to describe
the natural course of the disease in this uncommon group of
patients.
Case report
A 70 years old man came to the hospital in March
2017 because of visual disorders in the right eye, diplopia and
diffuse pain in retro-bulbar region. His past medical history was
characterized by chronic obstructive pulmonary disease (COPD) on
treatment with Broncho-dilatators and arterial hypertension on
treatment with ACE-inhibitor. In June 2014, patient had received
radical cystectomy with lymphadenectomy for grade 3, urothelial
bladder cancer, stage pT4N0M0. Despite preoperative staging
detected a muscle invasive cancer, the patient strongly preferred a
surgical approach instead of neoadjuvant chemotherapy. After
radical surgery, adjuvant chemotherapy with cisplatin plus
gemcitabine combination was administered for 4 cycles. At the time
of hospitalization, the patient was undergoing to a follow up
program that was negative for both local recurrence and distant
metastases up to six months before. Eye clinical examination
detected any cystic neo-formation but evidenced reduced motility.
At the abdomen palpation liver was at 2.5 cm from the right costal
margin with an irregular surface. Complete blood count was within
normal limits and biochemical evaluation showed liver impairment:
Aspartate aminotransferase 470 U/l, alanine aminotransferase 527
U/l, gamma-glutamyl transferase 435 U/l. Contrast-enhanced computed
tomography (CT) of the orbit showed an involvement of the right
periorbital fat, retro bulbar spaces and lacrimal gland. Excisional
biopsy was performed and samples from retro-bulbar fibro-adipose
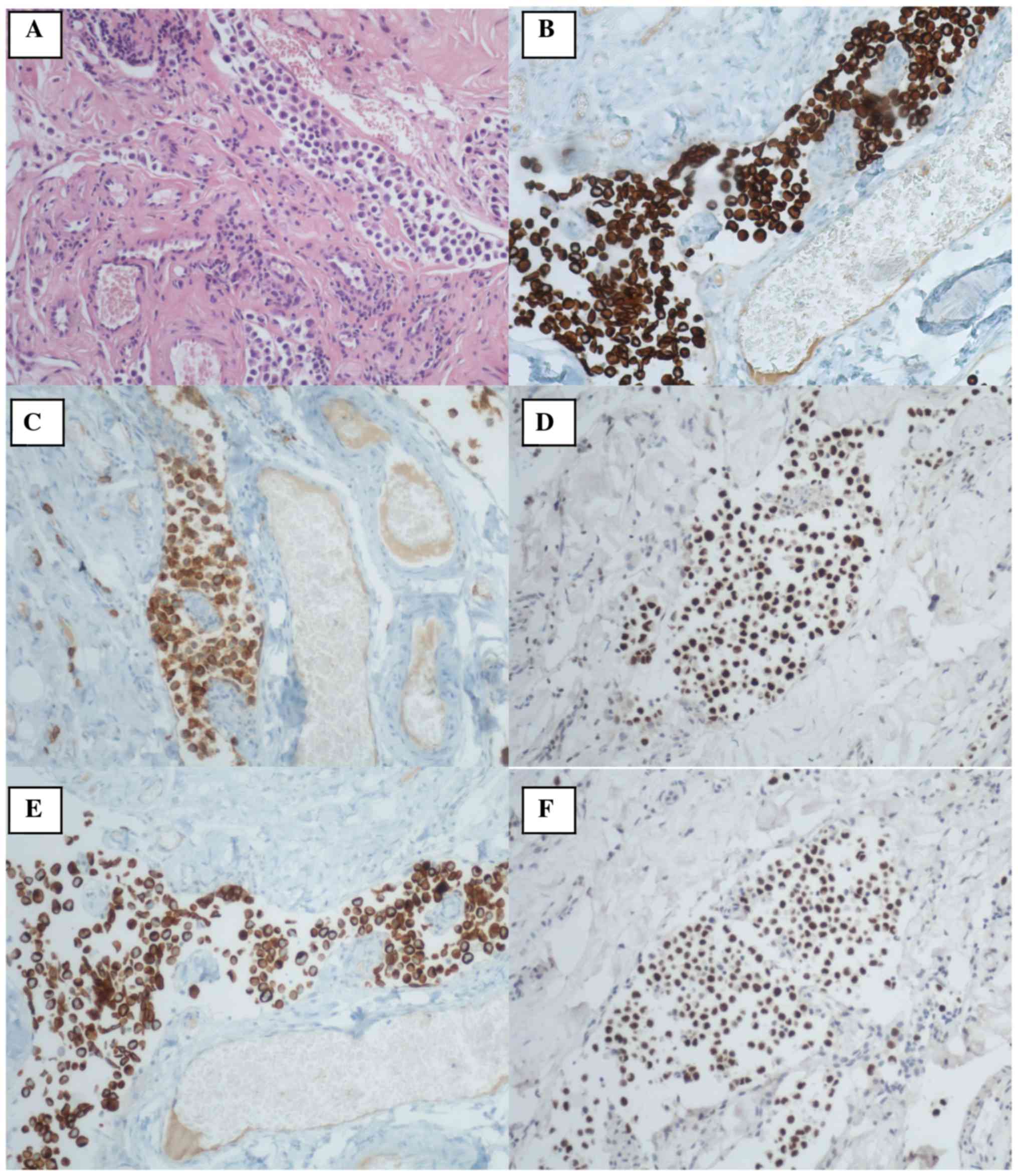
tissue and lacrimal gland were collected. Histological examination
showed neoplastic infiltration of fibro adipose tissue
characterized by diffuse population of cellular elements with a
high eosinophilic cytoplasm and eccentric nuclei. Diffuse
angiolymphatic invasion was also present. Immunohistochemistry
stains were positive for GATA3, CKAE1/AE3, CK5, CK7, CK20, CD138,
DNP63 and negative for LCA and CD79α (Fig. 2). Finally, the histological
examination was diagnostic for retro bulbar metastases from
urothelial carcinoma. Subsequently, full body CT scan showed
multiple liver metastases. Due to the patient's clinical condition
(Eastern Cooperative Oncology Group-ECOG performance status 2) and
the liver impairment evidenced by the biochemistry, no chemotherapy
was administered and best supportive care was started.
Unfortunately, because of widespread metastatic disease the patient
died three months after the diagnosis of the ocular
involvement.
Patient's medical history including comorbidities,
concomitant medications, bladder cancer diagnosis, previous
treatments and ocular metastasis detection were taken from clinical
records. Written informed consent for the case publication was
obtained from the patient at the time of retro-bulbar metastasis
histological confirmation. The Ethics Committee approved all
procedures.
Review criteria
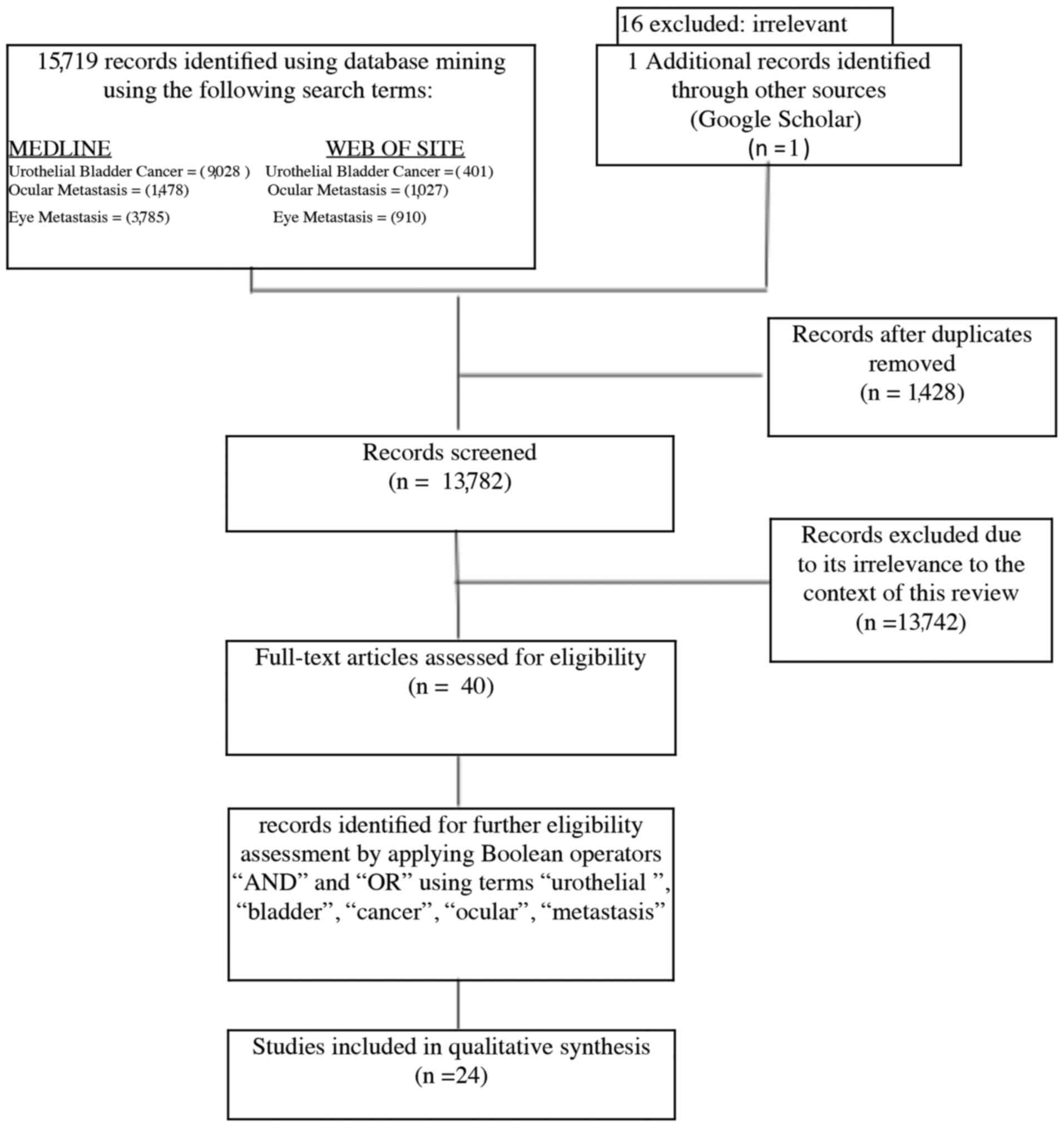
A systematic review of the literature was performed
in compliance with the PRISMA guidelines. Screening was performed
by reviewing article titles or full text up to February 2018 using
electronic database: MEDLINE and WEB OF SCIENCE. The primary search
terms included ‘ocular metastasis’, ‘eye metastasis’ and
‘urothelial bladder cancer’ in the article titles. The extracted
citations were then screened for duplicates. Later operators ‘and’
and ‘or’ were applied on the extracted records by use of the terms
‘urothelial,’ ‘bladder’, ‘cancer’, ‘ocular’, ‘eye’, ‘metastasis’ to
narrow the scope of the review (Fig.
1). Twenty-four articles met eligibility criteria for our
qualitative systematic review and 23 previously reported cases were
identified. This population was described for median age, gender,
ocular site, both systemic and ocular specific treatment. We also
evaluated the time to ocular metastases, defined as the time
elapsed between the urothelial bladder cancer diagnosis and the
ocular metastases occurrence. Overall survival from ocular
metastases was defined as the time elapsed from the eye involvement
to death. Kaplan Meier analysis for survival was performed using
GraphPad Prism 5.04 for Windows (GraphPad Software, Inc., La Jolla,
CA, USA). This evaluation included only patients whose survival
data were available.
Discussion
Eye metastases are uncommon, accounting for between
2.5 and 8.1% of all orbital space-occupying lesions (25). The majorities are adenocarcinoma and
the most common primary site is breast (26). Prostate, colon, and lung cancer can
also metastasize to the eye. Bladder carcinoma is an extremely rare
source of eye metastases. Since the first case reported in 1965,
only 23 additional cases of eye metastases from bladder cancer have
been reported in literature prior to the present case. This case is
extremely rare since the patient developed retro bulbar metastasis
with lacrimal gland infiltration and it is the first described in
literature. Seventy per cent of bladder tumors do not extend beyond
the lamina propria at the time of diagnoses. Among the patients
treated with radical cystectomy 57% are characterized by muscular
infiltration while the remaining 43% show non-infiltrative disease
that progress to an infiltrative carcinoma. Around a quarter of
patients receiving radical surgery has lymph-nodes involvement and
one third of the cases with infiltrative carcinoma could have micro
metastatic disease at the time of primary tumor treatment (25). In the present case patient obtained
cystectomy and lymphadenectomy three years before the occurrence of
retrobulbar and liver metastases. The current state of the art for
muscle invasive bladder cancer treatment is neoadjuvant,
platinum-based chemotherapy. Nevertheless, in this case the patient
chose an immediate surgical approach. At the time of diagnosis, he
showed pT4N0M0, G3 urothelial bladder carcinoma. Despite of
infiltrative disease no lymph nodes were involved at the time of
surgery. Notably, the patient received adjuvant chemotherapy but he
developed multiple liver and ocular metastases. Therefore, the
concept of an aggressive, micro-metastatic disease from the
beginning should be taken into account. Usually, bladder cancer
metastasizes to lymph nodes, liver, lung and bones. However this
case is particular because widespread disease was diagnosed without
lymph node involvement, suggesting an hematogenous diffusion. The
median age of patients with eye metastases from bladder cancer
reported so far is 58 years (range, 43–75) (Table I). In the majority of cases, eye
involvement is metachronous and is associated to other metastatic
sites (Table I). Five of 24 cases are
reported as synchronous metastases but 4 out of 19 described as
metachronous show a time to eye metastases less than 1 month. At
the light of this observation, it should be recommended a careful
and correct staging of the disease at the time of diagnosis. In
fact, in patients with ocular symptoms the probability of eye
involvement should be considered, even if it is uncommon. Clinical
presentation may differ depending on ocular or orbital localization
and it could be peculiar for each metastatic site (5). In fact exophthalmos, proptosis and pain
are likely related to orbital involvement. Moreover, as reported in
literature, other signs or symptoms like visual disorders,
diplopia, paralysis of the VI cranial nerve, hyperesthesia of the
trigeminal nerve, amaurosis, inflamed conjunctiva, retinal
detachment and even palpable mass should not be underestimated.
Because of the rarity of eye involvement, there is no diagnostic
algorithm: Specific clinical visit and CT scan are recommended and
differential diagnosis includes: Retro bulbar region or lacrimal
gland tumors, retinal abscess, metastasis from carcinoma, and
granuloma (25). In this patient,
retro-bulbar region biopsy was performed because of visual
disorders that occurred before liver involvement diagnosis. Only
few patients among the cases reported in literature had received a
cytological or histological examination. Immunohistochemical
staining (IHC) of the nuclei of the tumor cells was intensely
positive for transcription factor GATA3, which is specific for
urothelial and breast carcinoma. The cells were also strongly
immunoreactive for epithelial markers CKAE1/AE3, CK5, CK7 and CK20.
Co-expression of CK7 and CK20 is a differential feature of
urothelial carcinoma and excludes breast carcinoma that is positive
only for CK7. Positive CD138, DNP63 and negative LCA, CD79α
completed the diagnosis. Metastatic eye involvement indicates a
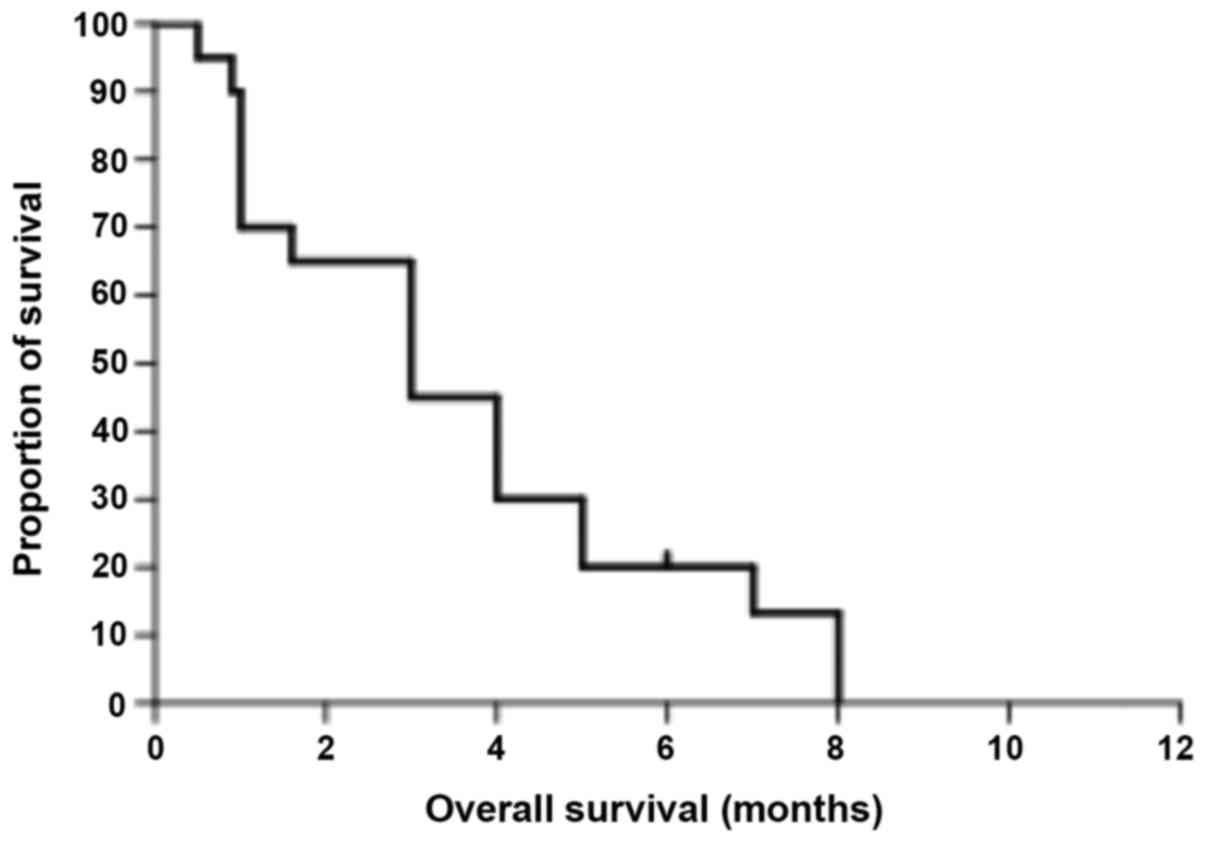
later stage of the disease with an extremely poor prognosis. In the
literature, median time to eye metastases is 11 months (Table I) and the median overall survival is 3
months (Fig. 3). In the present case,
no chemotherapy was administered due to the poor ECOG PS and liver
impairment showed by laboratory examinations. There is no specific
treatment for eye metastases and the aim of the cure is palliative.
Orbital and ocular metastatic sites could receive theoretically
different approaches but treatment should be evaluated case by case
because of the lack of evidences. Only 5 out of 24 patients
reported so far received local radiotherapy and 1 out of 24
received surgical decompression in order to improve the ocular
symptoms. Conversely, the majority of patients received
chemotherapy for metastatic disease without clinical or survival
benefit.
 | Table I.Summary of reported cases of
urothelial-bladder cancer metastasizing to the eye. |
Table I.
Summary of reported cases of
urothelial-bladder cancer metastasizing to the eye.
| Author, year | Age (years) | Gender | Eye localization | Time to ocular
metastasis (months) | Treatment | Treatment for ocular
metastasis | OS (months) | (Refs.) |
|---|
| Smiley, 1965 | 75 | M | Retrobulbar, L | Synchronous | BSC | No | 8 | (7) |
| Resnick et al,
1975 | 46 | M | Orbit, L | 1 | ADRIA+5FU | No | 4 | (8) |
| Resnick et al,
1975 | 51 | M | Choroid, R | 15 | No | RT | 4 | (8) |
| Cieplinski et al,
1982 | 57 | M | Choroid, B | 8 | CDDP+ADRIA+CTX | RT | 0.5 | (9) |
| Krauss et al,
1982 | 64 | F | Orbit, R | 15 | No | RT | 1 | (10) |
| Prats et al,
1989 | 58 | M | Orbit, L | Synchronous | No | Surgical
decompression | 4 | (11) |
| Felip et al,
1991 | 58 | M | Orbit | 0,5 | NA | No | 3 | (12) |
| Felip et al,
1991 | 62 | M | Orbit | 36 | NA | No | LF | (12) |
| Angulo et al,
1991 | 61 | M | Retro-orbital | 11 | BSC | No | 0.9 | (13) |
| Hugkulstone et al,
1994 | 45 | M | Orbit, B | Synchronous | MVAC | RT | 5 | (14) |
| Scott and Williams,
1994 | NA | NA | Orbit | Synchronous | NA | NA | 8 | (15) |
| Fynn-Thompson et
al, 2003 | 68 | M | Orbital + optic
nerve, L | 48 | BSC | No | 1 | (16) |
| Nabi et al,
2002 | 43 | F | Optic canal L | 6 | MVAC | No | 3 | (17) |
| Nabi et al,
2002 | 79 | M | Choroid R | 8 | MVAC | No | 1.6 | (17) |
| Amemiya et al,
2002 | 55 | M | Orbital B | NA | NA | NA | NA | (18) |
| Amemiya et al,
2002 | 73 | M | Orbital R | NA | NA | NA | NA | (18) |
| Souza Filho et al,
2005 | 53 | M | Orbit L | 3 weeks | BSC | no | 1 | (19) |
| Levecq et al,
2007 | 58 | M | Choroidal | 96 | no | Enucleation,
RT | 7 | (20) |
| Lin et al,
2007 | 60 | M | Orbit R at 6 | 8 | MVAC | No | Alive | (4) |
| Wettach and Steele
2008 | 66 | M | Orbit R | Synchronous | CBDCA+GEM | No | 1 | (21) |
| Mitsui et al,
2014 | 48 | M | Choroid R | 17 | MVAC | No | 5 | (2) |
| SooHoo et al,
2012 | 53 | F | Orbit R | 1 | BSC | No | 3 | (22) |
| Magrath et al,
2015 | 57 | F | Orbit L | Synchronous | BSC | No | LF | (23) |
| Present case | 70 | M | Retrobulbar R,
lacrimal gland | 33 | BSC | No | 3 | – |
To the best of our knowledge, this is the first
qualitative systematic review about eye metastases caused by
urothelial bladder cancer. Furthermore, the case described in this
article represents the first report of retro-bulbar involvement
that infiltrates the lacrimal gland from urothelial bladder cancer.
Another novelty of this case is represented by an accurate IHC
evaluation in order to clarify the differential diagnoses among the
different primary tumor sites. Muscular infiltrative bladder cancer
should be considered as a potential micro metastatic disease since
the beginning. Therefore, Oncologist should be aware about the
possibility of eye involvement in patients with a past medical
history of urothelial bladder cancer associated with eye disorders.
Multidisciplinary approach is required for an early detection of
this condition that should lead to a prompt therapeutic
intervention. The rarity of metastatic eye localization does not
provide high level of evidence for a specific treatment. Actually,
majority of patients receive chemotherapy with poor outcomes.
Molecular and biological aspects of both urothelial bladder cancer
and metastatic sites should be further investigated in order to
improve therapeutic options and patients' prognosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by SAMAR Laboratorio di
Istopatologia di Roma.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GG, NO and PP conceived the research, wrote the
paper, and assessed the figures and tables. LC and EDS collected
the case report data. LC, EM, AR and MB reviewed and confirmed the
histological diagnosis. MP, GC, TDR, MRDA and AS performed the
literature research and critically reviewed the manuscript for
important intellectual content. PP and GG supervised the
project.
Ethics approval and consent to
participate
The Ethics Committee ‘LAZIO 1’ n. 2001/2017 (Rome,
Italy; prot. no. 2198) approved all procedures.
Patient consent for publication
The patient provided written informed consent for
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NA
|
not available
|
|
M
|
male
|
|
F
|
female
|
|
L
|
left
|
|
R
|
right
|
|
B
|
bilateral
|
|
BSC
|
best supportive care
|
|
ADRIA
|
adriamycin
|
|
5FU
|
5-fluoroiracil
|
|
CDDP
|
cisplatin
|
|
CTX
|
cyclophosphamide
|
|
MVAC
|
methotrexate + vinblastine +
adriamycin + cisplatin
|
|
CBDCA
|
carboplatin
|
|
GEM
|
gemcitabine
|
|
RT
|
radiotherapy
|
|
OS
|
overall survival
|
|
LF
|
lost to follow up
|
|
CT
|
computed tomography
|
References
|
1
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitsui Y, Arichi N, Inoue K, Hiraki M,
Nakamura S, Hiraoka T, Ishikawa N, Maruyama R, Yasumoto H and
Shiina H: Choroidal and cutaneous metastasis from urothelial
carcinoma of the bladder after radical cystectomy: A case report
and literature review. Case Rep Urol. 2014:4915412014.PubMed/NCBI
|
|
3
|
Cohen VM: Ocular metastases. Eye (Lond).
27:137–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin HC, Chang CH, Li WM, Hsiao HL, Chang
TH, Wu WJ and Huang CH: Orbital metastasis from urothelial
carcinoma of the urinary bladder. Kaohsiung J Med Sci. 23:84–88.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bita E: Ophthalmic Oncology. MD Anderson
Solid Tumor Oncology Series. Spinger; New York, NY: 2011
|
|
6
|
Goldberg RA, Rootman J and Cline R: Tumors
metastatic to the orbit: A changing picture. Surv Ophthalmol.
35:1–24. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smiley SS: An orbital metastasis from the
urinary bladder. Arch Opthalmol. 74:809–810. 1965. View Article : Google Scholar
|
|
8
|
Resnick MI, O'Conor VJ Jr and Grayhack JT:
Metastases to the eye from transitional cell carcinoma of the
bladder. J Urol. 114:722–724. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cieplinski W, Ciesielski TE, Haine C and
Nieh P: Choroid metastases from transitional cell carcinoma of the
bladder. A case report and a review of the literature. Cancer.
50:1596–1600. 1982.
|
|
10
|
Krauss HR, Slamovits TL, Sibony PA, Dekker
A and Kennerdell JS: Orbital metastasis of bladder carcinoma. Am J
Ophthalmol. 94:265–267. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prats J, Bellmunt J, Calvo MA, Sarrias F
and Toran N: Orbital metastasis, by transitional cell carcinoma of
the bladder. Int Urol Nephrol. 21:389–392. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Felip E, Rovirosa MA, Salud A, Capdevila
F, Bellmunt J and Giralt J: Orbital metastases from
transitional-cell cancer of the urinary bladder. Urol Int.
46:82–84. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Angulo JC, Lopez JI, Larrinaga JR and
Flores N: Metastasising carcinoma of the urinary bladder presenting
as a retro-orbital mass. Case report. Scand J Urol Nephrol.
25:83–84. 1991. View Article : Google Scholar
|
|
14
|
Hugkulstone CE, Winder S and Sokal M:
Bilateral orbital metastasis from transitional cell carcinoma of
the bladder. Eye (Lond). 8:580–582. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scott JA and Williams R: Orbital
metastasis from bladder carcinoma. Eye. 6:664–666. 1994.
|
|
16
|
Fynn-Thompson N, McKiernan JM and Fay A:
Transitional cell carcinoma of the urinary bladder metastatic to
the orbit. Ophthalmic Plast Reconstr Surg. 19:165–167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nabi G, Dadeya S, Dogra PN and Lal H: Eye
metastasis form urothelial tumours. Int Urol Nephrol. 34:51–54.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amemiya T, Hayashida H and Dake Y:
Metastatic orbital tumors in Japan: A review of the literature.
Ophthalmic Epidemiol. 9:35–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Souza Filho JP, Odashiro AN, Pereira PR,
Al-Buloushi A, Codere F and Burnier MN: Orbital metastasis of
urinary bladder carcinoma: A clinicopathologic report and review of
the literature. Orbit. 24:269–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levecq L, De Potter P, Godfraind C,
Guagnini AP and Kozyreff A: Choroidal metastasis from carcinoma of
the bladder. Retin Cases Brief Rep. 1:251–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wettach GR and Steele EA: Urothelial cell
carcinoma of the bladder presenting as orbital metastasis. Arch
Pathol Lab Med. 132:12242008.PubMed/NCBI
|
|
22
|
SooHoo JR, Gonzalez MO, Siomos VJ and
Durairaj VD: Urothelial carcinoma with orbital metastasis. Urology.
80:e45–e46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magrath GN, Proctor CM, Reardon WA, Patel
KG, Lentsch EJ and Eiseman AS: Esophageal adenocarcinoma and
urothelial carcinoma orbital metastases masquerading as infection.
Orbit. 34:51–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; The PRISMA group, . Preferred reporting items for systematic
reviews and meta-analyses: PRISMA statement. Ann Intern Med.
151:264–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shields JA, Bakewell B, Augsburger JJ and
Flanagan JC: Classification and incidence of space-occupying
lesions of the orbit. A survey of 645 biopsies. Arch Ophthalmol.
102:1606–1611. 1984. View Article : Google Scholar
|
|
26
|
Konstantinidis L and Damato B: Intraocular
metastases-a review. Asia Pac J Ophthalmol (Phila). 6:208–214.
2017. View Article : Google Scholar : PubMed/NCBI
|