Introduction
Primary liver cancer is the fifth most common
malignancy and the second leading cause of cancer-associated
mortality worldwide (1).
Hepatocellular carcinoma (HCC) accounts for >80% of primary
liver cancer cases (2). Patients with
chronic hepatitis B virus (CHB) have been reported to frequently
progress to cirrhosis and liver failure (3). Furthermore, it has been demonstrated
that CHB is associated with the development of HCC. It has been
estimated that CHB-associated HCC accounts for >80% of HCC cases
in areas with high hepatitis B virus (HBV) incidence (4). HCC is prone to invade the intrahepatic
vessels, particularly the portal vein system (4). It has been reported that portal vein
tumor thrombus (PVTT) occurs frequently in patients with HCC
(5). Patients with HCC and PVTT often
present with portal vein hypertension, ascites, tumor dissemination
and deterioration of liver function. In addition, poor prognosis
and a survive rate of 2–3 months has been reported for these
patients when no treatment is received (6). Since radical resection cannot be
performed in patients with HCC and PVTT, minimally invasive
treatment is widely used for treating patients with HCC and PVTT
(7,8).
The development of minimally invasive treatments in the past 20
years has greatly improved (9). It
has been reported that the most widely used minimally invasive
treatments include transarterial chemoembolization (TACE) and
radiofrequency ablation (RFA) (10).
It has been reported that TACE is the optimal treatment recommended
by the European Association for the study of the liver and by the
American Association for the study of liver diseases in the HCC
management guidelines for patients with intermediate HCC (11). According to the guidelines for the
management of HCC, RFA is one of the first-line treatments for
patients with Barcelona clinic liver cancer (BCLC)-0 which is in
the earliest stages of cancer, and BCLC-A grade (12).
Liver function is one of the important factors that
affect tumor prognosis. The model for end-stage liver disease score
(MELD), which includes serum creatinine, bilirubin and the
international normalized prothrombin time ratio, serves as an
alternative non-invasive biomarker of liver function (13). In the present study, the associations
between liver function and minimally invasive treatment and the
survival rate of patients with liver cancer were determined.
However, the association between MELD score and minimally invasive
treatment requires further investigation. The purpose of the
present study was to analyze the demographic characteristics,
laboratory indicators and imaging data in patients with
HBV-associated HCC and PVTT, and to further examine the effect of
MELD score and minimally invasive treatment on the 1-year overall
survival rate of the aforementioned patients.
Materials and methods
Patients
A total 173 patients (18 to 80 years old, the median
age was 56 years) with HBV-associated primary liver cancer and PVTT
were included in the present retrospective study. Samples (n=173)
were collected from Beijing Ditan Hospital (Beijing, China) between
January 2012 and January 2015. The present study was approved by
the Ethics Committee of the Beijing Ditan Hospital, Capital Medical
University, in accordance with the Declaration of Helsinki
(approval no. 7142081). Written informed consent was obtained from
all individual participants included in the present study.
Inclusion criteria
Patients with hepatitis B surface antigen-positive
primary liver cancer were included in the present study. The
pathological diagnostic criteria for primary liver cancer were as
follows: Tissue specimens were diagnosed as primary liver cancer by
histopathology and cytology, and tissue specimens were acquired
from liver lesions or extrahepatic metastases by biopsy or surgical
resection. Clinical diagnostic criteria for primary liver cancer
were in accordance with the ‘primary liver cancer diagnosis and
treatment norms’ (14). PVTT was
diagnosed on the basis of a filling defect in the portal vein or
its branch on contrast-enhanced computed tomography or magnetic
resonance imaging.
Exclusion criteria
Patients with the following characteristics were
excluded from the present study: i) Hepatitis C, human
immunodeficiency virus infection, autoimmune liver disease, genetic
metabolic liver disease, drug-induced liver disease and other
chronic liver diseases; ii) diseases affecting the heart, lungs,
kidneys, brain, blood and other vital organs; iii) severe mental
illness; iv) pregnancy and lactation; v) metastatic liver cancer;
vi) treatment with radiotherapy or chemotherapy; and vii)
incomplete clinical data.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Mean ± standard deviation
was used to fit the normal distribution. Student's t-test was used
to compare continuous variables and the significant differences
between two groups (15). In
non-normal distribution data, the two groups were compared using
the Wilcoxon rank sum test. Frequency represented the count data,
which were compared with the χ2 test. In univariate
analyses, the χ2 test or Fisher exact test were utilized
to compare categorical data and the two sample groups (16). Logistic regression analysis was used
for multivariate analysis and interaction between MELD and
minimally invasive treatment. In multivariate regression analysis,
single factor (P<0.05) was used to calculate the independent
risk factors. P<0.05 was considered to indicate a statistically
significant difference. Overall survival rates were calculated by
the Kaplan-Meier method with the log-rank test applied for
comparison. Bonferroni's test was used to correct for the multiple
comparisons. Kaplan-Meier survival curves were drawn using GraphPad
5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patients and disease
characteristics
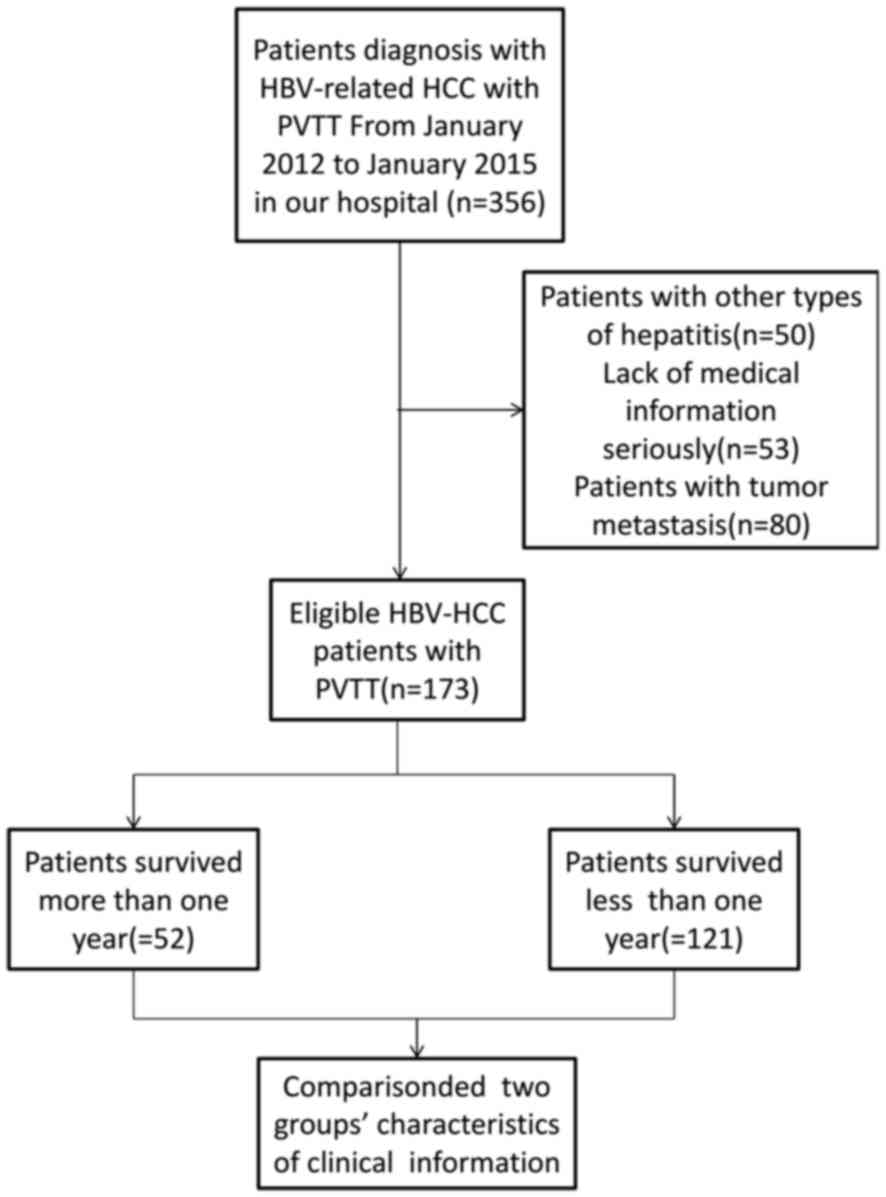
Patients and disease characteristics are summarized
in Table I. The present study
included 173 patients with HBV-associated HCC who received PVTT
treatment in the Beijing Ditan Hospital between January 2012 and
January 2015 (Fig. 1). Complete
follow-up of the patients (n=173) was available until the time of
patient mortality or for the following 2 years. Out of the 173
patients participating in the present study, a total of 121
succumbed within a year and 52 survived. The survival rate was
42.98% and the median survival time was 5 months. Baseline
characteristics were compared between the survival group and the
mortality group. The alanine transaminase, aspartate transaminase,
direct bilirubin, glutamyl transferase, alkaline phosphatase and
MELD scores were significantly higher in the mortality group
(P<0.05), while the cholinesterase and percentage of cluster of
differentiation (CD)4+/CD8+ T cells were
significantly lower in the mortality group relative to the survival
group (P<0.05). In addition, there was an increased number of
patients with α-fetoprotein (AFP) >1,000 ng/ml, HBV-DNA >500
copies and abdominal effusion in the mortality group compared with
that in the survival group (P<0.001, Table I).
 | Table I.Baseline characteristics of the study
cohort. |
Table I.
Baseline characteristics of the study
cohort.
| Variables | Survival group
(n=52) | Mortality group
(n=121) | P-value |
|---|
| Age, years | 53.560±1.960 | 54.780±1.479 | 0.859 |
| Sex, n (%) |
|
|
|
| Male | 42 (29.000) | 103 (79.000) | 0.503 |
|
Female | 10 | 18 |
|
| Alcohol consumption,
n | 19 | 52 | 0.410 |
| WBC,
×109/l | 4.500 (2.000,
7.000) | 5.000 (3.500,
7.000) | 0.100 |
| HGB, g/l | 109.00±7.499 | 119.332±3.745 | 0.900 |
| PLT,
×109/l | 118.75±17.009 | 122.457±9.356 | 0.440 |
| NLR | 3.150 (2.278,
5.237) | 3.404 (2.293,
4.986) | 0.210 |
| ALT, U/l | 35.500(15.500,
51.750) | 45.000 (32.000,
62.000) | 0.004a |
| AST, U/l | 40.000 (23.750,
51.750) | 85.000 (50.000,
168.000) |
<0.001a |
| DBIL, µmol/l | 8.000 (5.000,
13.500) | 11.00 (7.000,
25.000) | 0.010a |
| GGT, U/l | 107.000 (58.000,
235.250) | 148.000 (95.500,
271.500) |
<0.001a |
| ALP, U/l | 134.188±12.234 | 167.530±11.367 |
<0.001a |
| CHE, U/l |
4426.938±606.710 |
3855.385±224.471 | 0.039a |
| ALB, g/l | 33.000 (29.250,
37.750) | 34.000 (30.000,
40.500) | 0.687 |
| Cr, µmol/l | 69.500 (52.250,
76.500) | 63.000 (55.000,
73.000) | 0.284 |
| PTA, % | 70.000 (57.750,
76.750) | 75.000 (66.000,
81.500) | 0.650 |
|
CD4+/CD8+, % | 2.500 (2.000,
3.000) | 2.000 (1.000,
2.000) | 0.007a |
| AFP (>1,000
ng/ml), n (%) | 9 (12.676) | 62 (87.324) |
<0.001a |
| HBV DNA (>500
copies/l), n (%) | 9 (14.754) | 52 (85.246) |
<0.001a |
| Treatment method, n
(%) |
|
| 0.010a |
|
Minimally invasive | 33 (63.462) | 61 (50.413) |
|
|
Conservative | 19 (36.538) | 60 (49.587) |
|
| MELD score, n
(%) |
|
|
|
|
≤17.85 | 30 (37.975) | 49 (62.025) | 0.037a |
|
>17.85 | 22 (23.404) | 72 (76.595) |
|
| Complication, n
(%) |
|
|
|
|
Abdominal effusion | 27 (23.894) | 86 (76.106) | 0.030a |
|
Abdominal infection | 12 (22.222) | 42 (77.778) | 0.150 |
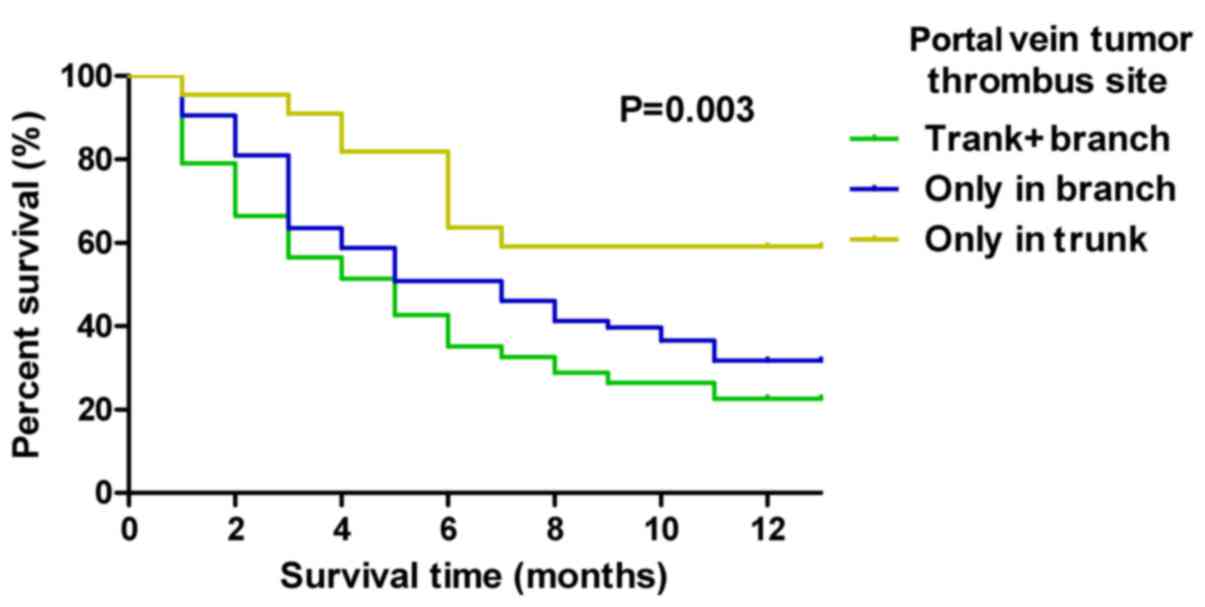
Site of tumor thrombus invasion is
associated with mortality rate
The association was analyzed between the site of
invasion of the tumor thrombus and patient mortality. As indicated
in Table II, the highest mortality
rate (54.5%) was exhibited in patients where the tumor thrombus had
invaded the trunk and the branches of the portal vein, whereas the
lowest mortality rate (7.4%) was indicated in patients where the
tumor thrombus had only invaded the trunk. Kaplan-Meier survival
curves illustrated the survival time of patients with tumor
thrombus invasion on a 1-year mortality scale. The tumor thrombus
invasion site significantly affected the survival time of patients
with HBV-associated HCC (P=0.003; Fig.
2). The patients with a tumor thrombus invasion site only in
the trunk of the portal vein experienced a longer survival time
(P=0.003; Fig. 2).
 | Table II.Baseline distribution of tumor
thrombus. |
Table II.
Baseline distribution of tumor
thrombus.
| Location | Survival group
(n=52) | Mortality group
(n=121) | P-value |
|---|
| Trunk + branch, n
(%) | 19 (36.538) | 66 (54.545) | 0.001a |
| Branch, n (%) | 20 (38.462) | 46 (38.017) |
|
| Trunk, n (%) | 13 (25.000) | 9 (7.438) |
|
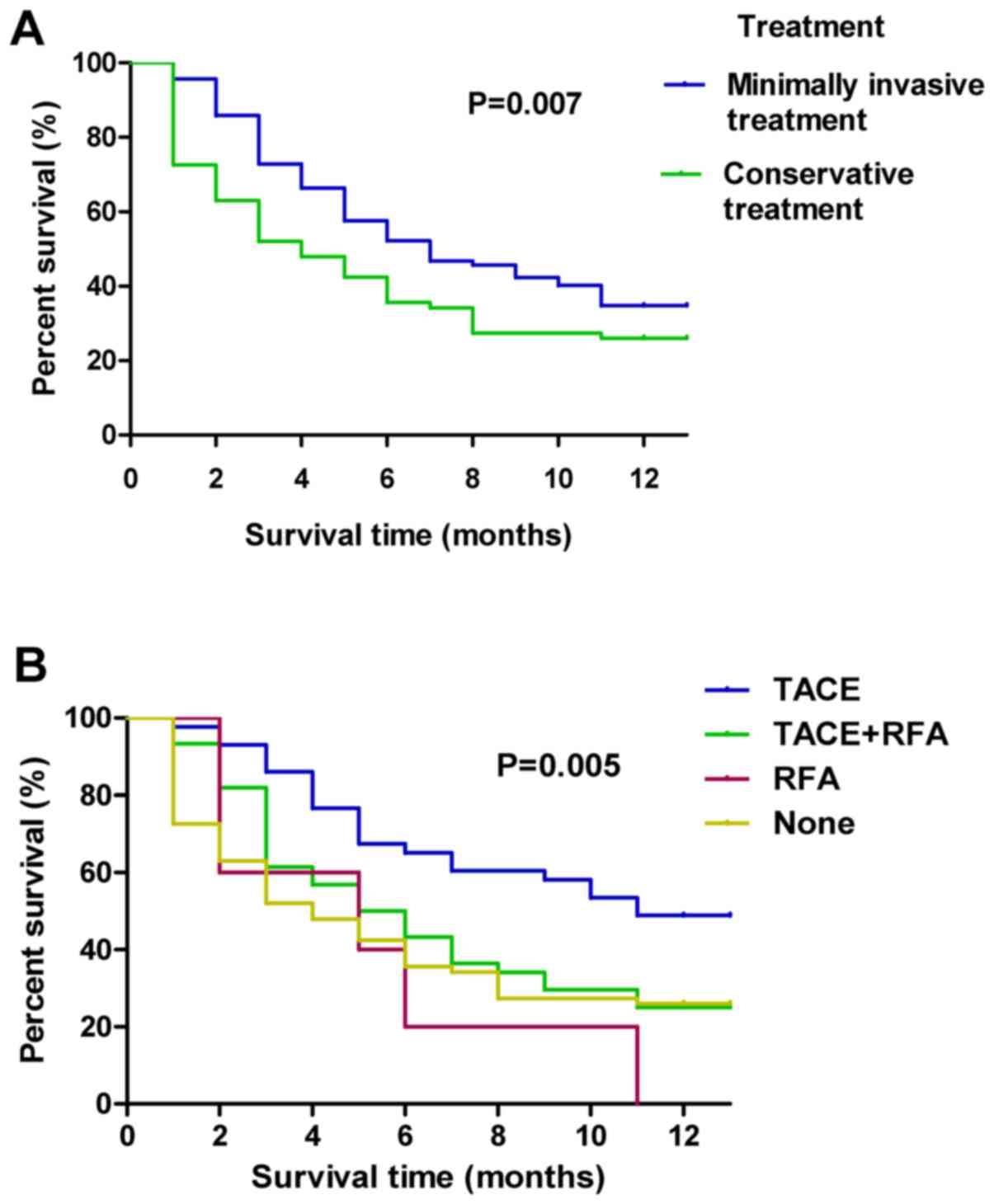
Association between minimally invasive
treatment and mortality
The effect of minimally invasive treatment for a
cancer embolus on patient mortality rate was analyzed. The
mortality rates of conservative treatment and minimally invasive
treatment were 75.95 and 64.89%, respectively, indicating a
significant difference (P<0.01) between the two types of
treatment. By comparing different minimally invasive treatment
methods, the lowest mortality rate of 52.3% was indicated in
patients with the combination treatment of TACE and RFA (Table III). The mortality rate was 75.9% in
patients without minimally invasive treatment.
 | Table III.Minimally invasive treatment of
baseline distribution. |
Table III.
Minimally invasive treatment of
baseline distribution.
| Treatment | Survival group
(n=52) | Mortality group
(n=121) | P-value |
|---|
| TACE + RFA, n
(%) | 21 (40.385) | 23 (19.008) | 0.016a |
| TACE, n (%) | 12 (23.077 | 33 (27.273) |
|
| RFA, n (%) | 0 (0.000) | 5 (4.132) |
|
| None, n (%) | 19 (36.538) | 60 (51.240) |
|
A Kaplan-Meier curve for 1-year mortality was used
to compare the survival times among patients with conservative
treatment and patients with different types of minimally invasive
treatment. Results indicated that the patients with minimally
invasive treatment exhibited a significantly longer survival time
compared with patients with conservative treatment (P=0.007;
Fig. 3A). Furthermore, the minimally
invasive treatments were further divided into the TACE, RFA,
combined TACE and RFA, and conservative treatment groups. It was
indicated that the minimally invasive treatment of TACE plus RFA
exerted the best survival time of patients with HBV-associated HCC
in all group (P=0.005; Fig. 3B).
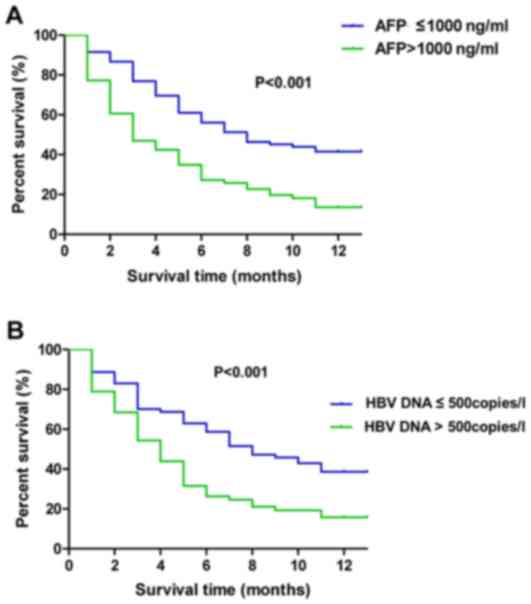
Multivariate analysis
The Kaplan-Meier curve for 1-year mortality was used
to compare the survival time between patients with AFP >1,000
ng/ml and AFP ≤1,000 ng/ml, and between patients with HBV-DNA
>500 copies/l and HBV-DNA ≤500 copies/l. As indicated in
Fig. 4A, in patients with AFP ≤1,000
ng/ml refer to a significantly higher survival rate compared with
patients with AFP >1,000 ng/ml (P<0.001). In addition,
patients with HBV-DNA ≤500 copies/l refer to a significantly higher
survival rate compared with patients with HBV-DNA >500 copies/l
(P=0.001) (Fig. 4B).
Using multivariate logistic regression analyses
(Table IV), the independent risk
factors of mortality of HBV-associated HCC with PVTT were HBV-DNA
>500 copies/l, (P=0.013; odds ratio, 4.582) and AFP >1,000
ng/ml (P=0.012; odds ratio, 2.167). Furthermore, the interaction
between MELD score and minimally invasive treatment was indicated
(P=0.021; odds ratio, 2.167; 95% confidence interval, 1.124–4.177)
in Table IV.
 | Table IV.Logistic regression analysis of
significant variables. |
Table IV.
Logistic regression analysis of
significant variables.
| Variables | B | OR | 95% CI | P-value |
|---|
| HBV DNA,
copies/l |
|
|
|
|
|
≤500 |
| Reference |
|
|
|
>500 | 1.522 | 4.582 | 1.377–15.242 | 0.013 |
| AFP, ng/ml |
|
|
|
|
|
≤1,000 |
| Reference |
|
|
|
>1,000 | 1.598 | 4.945 | 1.420–17.223 | 0.012 |
| MELD score*
minimally invasive treatment |
| Reference |
|
|
|
| 0.773 | 2.167 | 1.124–4.177 | 0.021 |
In order to verify the interaction between the
treatment and the MELD score, the mean survival times of patients
with minimally invasive treatments or conservative therapy were
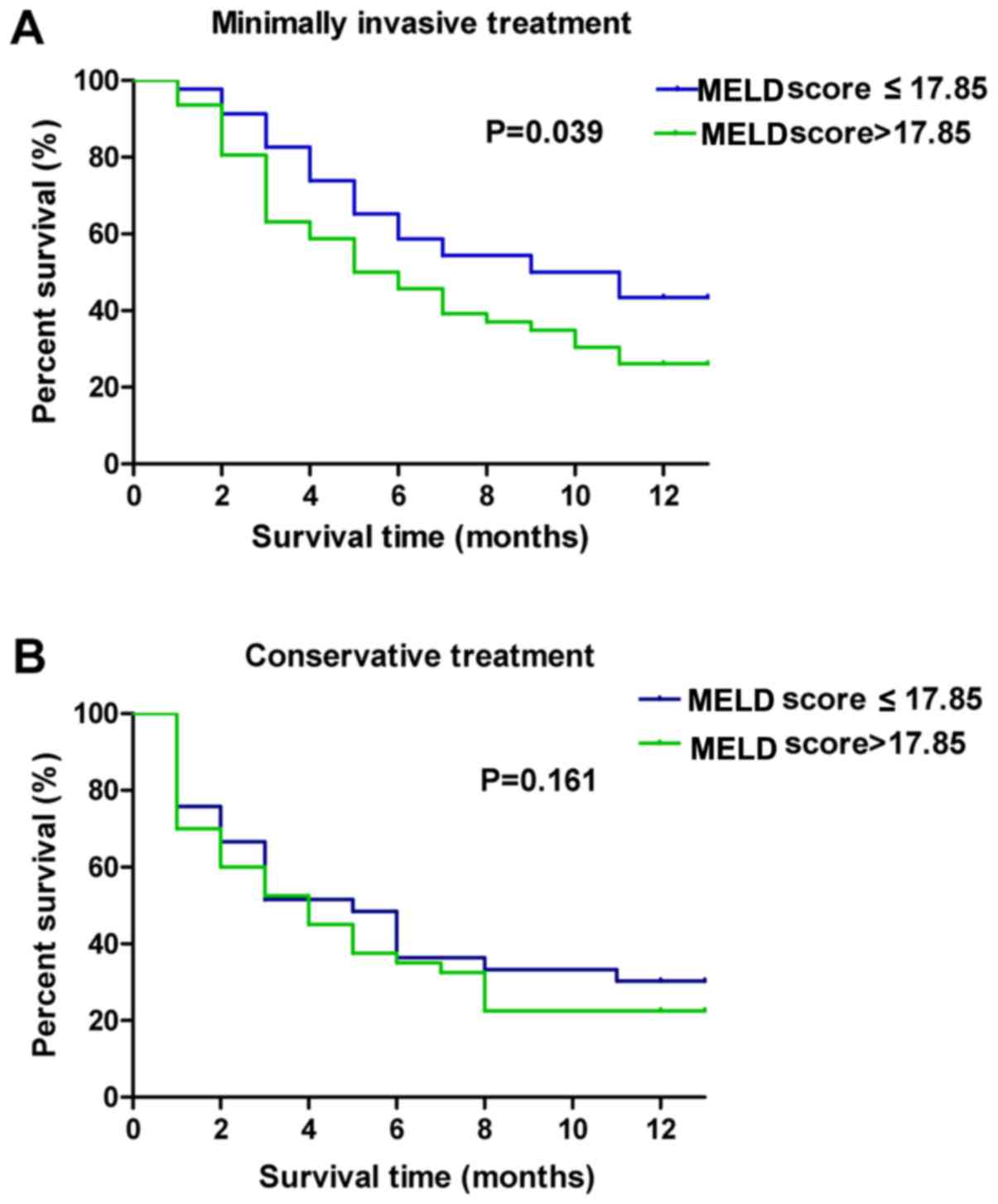
analyzed when the MELD score was different (Table V). The cut-off value of the MELD score
(17.85) was obtained by using Jorden index. The present study also
indicated the survival curves of different types of treatment for
different MELD scores (Fig. 5A and
B). The results indicated that patients with minimally invasive
treatment had a longer survival time compared with patients with
conservative treatment (Table V). The
patients in the minimally invasive treatment group with a MELD
score ≤17.85, experienced a significantly longer survival time
compared with that of patients with a MELD score >17.85
(Table V; Fig. 5A and B). In addition, the survival
time of patients receiving different types of minimally invasive
treatments, in addition to having different MELD scores, was
analyzed (Table VI). Results
indicated that the patients with a MELD score ≤17.85 experienced a
significantly longer survival time for all types of minimally
invasive treatments.
 | Table V.Survival time (months) of patients
with minimally invasive and conservative treatment and different
MELD scores. |
Table V.
Survival time (months) of patients
with minimally invasive and conservative treatment and different
MELD scores.
| MELD score | Minimally
invasive | Conservative | P-value |
|---|
| ≤17.85 | 8.652±0.645 | 6.273±0.863 | 0.012 |
| >17.85 | 6.714±0.565 | 4.739±0.690 |
|
 | Table VI.Survival time (months) of patients
with different minimally invasive treatments and different MELD
scores. |
Table VI.
Survival time (months) of patients
with different minimally invasive treatments and different MELD
scores.
| Treatment | MELD score
≤17.85 | MELD score
>17.85 | P-value |
|---|
| TACE + RFA | 9.680±0.815 | 8.000±1.094 | 0.008 |
| TACE | 7.667±1.051 | 5.977±0.817 |
|
| RFA | 6.000±2.646 | 4.000±2.000 |
|
| None | 6.273±0.863 | 4.739±0.690 |
|
Discussion
Liver cancer is prone to metastasis and confers a
poor prognosis (17). Portal vein
invasion is common in HCC with intrahepatic metastasis, and is one
of the leading causes for tumor recurrence and tumor-associated
mortality subsequent to surgery (16). A retrospective study of 601 patients
indicated that PVTT significantly affected the survival rate of
patients independently (18).
However, there have been few studies on prognostic models of HCC
with PVVT. The purpose of the present study was to identify
independent risk factors for HBV-associated HCC and to establish a
predictive model. It was indicated that minimally invasive
treatment is the optimal treatment choice for patients with HCC and
PVTT plus a low MELD score in comparison to patients with a high
MELD score (threshold value, 17.85).
Previous studies have demonstrated the effect of
PVTT on patient prognosis. It was reported that patients with
HBV-associated HCC and PVTT experienced a low median survival time
of 2–4 months (19,20). A recent study conducted by Kokudo
et al (21) indicated that the
median survival time of patients in the liver resection group was
1.77 years longer than that of patients in the non-liver resection
group and 0.88 years longer than that of patients in the non-liver
resection group in a propensity score-matched cohort. In addition,
a survival benefit of chemoembolization plus iodine-125 seed
implantation has been reported in unresectable HBV-associated HCC
with PVTT (22,23). In the present study, it was indicated
that the median survival time was 5 months in patients with
HBV-associated HCC and PVTT. The median survival time was
significantly longer compared with results reported in previous
studies (19,20), suggesting that improvements in
treatment methods may have increased patient survival time. In the
present study, AFP levels were significantly associated with a poor
prognosis in patients with HCC. Since AFP levels have been reported
to reflect tumor progression, this marker is frequently measured
during patient treatment (24). In
previous studies, AFP response had been reported as a predictive
factor for radiological response, recurrence and survival in early
and advanced HCC cases (25–28). Consistent with previous studies, the
present study indicated that AFP >1,000 ng/ml was one of the
independent risk factors for survival time of patients with
HBV-associated HCC and PVTT. The copies of HBV-DNA represent viral
load, which are risk factors for the development of cirrhosis and
HCC. The copies of HBV-DNA have also been indicated to be
associated with a poor prognosis in patients with HCC (29,30). The
present study demonstrated that patients with HBV-DNA >500
copies/l experienced a shorter survival time compared with patients
with HBV-DNA ≤500 copies/l. The aforementioned result was
consistent with previous studies (20,30).
Hirooka et al (31) indicated that the cumulative survival
rates at 6, 12 and 24 months were 100, 89.7 and 78.8%,
respectively, with the combined treatment of TACE and RFA and that
the median survival time was 953 days. For patients treated only
with TACE, the cumulative survival rates at 6, 12 and 24 months
were 84.9, 56.1 and 16.9%, respectively, and the median survival
time was 352 days. In the present study, it was demonstrated that
the combination of TACE and RFA significantly increased the
survival time in patients with HCC.
The MELD scoring system has been widely used to
assess the prognosis of liver function and liver-associated
diseases (13). The cut-off value of
the MELD score (17.85) was obtained by using Jorden index
(https://www.scalelive.com/youden-index.html). The
results of the present study indicated that a MELD score ≤17.85 in
patients with HCC and PVTT displayed a better prognosis compared
with a MELD score >17.85. Furthermore, patient prognosis became
worse as MELD score increased. The aforementioned result was
consistent with previous studies (32,33). In
addition, the present study also indicated an interaction between
MELD score and minimally invasive treatment, suggesting that
minimally invasive treatment may improve the prognosis in patients
with a MELD score ≤17.85. However, minimally invasive treatment did
not improve the prognosis in patients with a meld score
>17.85.
In conclusion, the present study provided a
theoretical basis for the treatment of patients with HCC and PVTT.
However, the number of samples in the present study was limited and
therefore, further investigation is required to expand the sample
size, verifying the present study results.
Acknowledgements
We would like to thank Dr. Lingling He from Beijing
Ditan Hospital, Capital Medical University (Beijing, China) and Dr.
Shuan Zhang from the Digestive department, Chinese Medicine
Hospital of Zhengzhou (Zhengzhou, China) for assistance with the
follow-up information.
Funding
The present study was funded by the Capital Health
Research and Development of Special (grant no. 2016-2-2171;
Beijing, China), the Science and Technology Project of Beijing
Municipal Education Commission (grant no. SQKM201610025026;
Beijing, China) and the Beijing Municipal Science and Technology
Commission (grant no. Z171100001017082; Beijing, China).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZYY and XLL designed the study; YLZ, XLL and MGL
collected and analyzed the data; MGL and XLL wrote the manuscript;
XLL acquired, analysed and interpreted the data; XHW and ZBD
provided patients' data; YYJ, XLL and ZYY were responsible for the
interpretation of data and revision. ZYY and XLL approved the final
version.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Beijing Ditan Hospital, Capital Medical University. Written
informed consent was obtained from each patient.
Patient consent for publication
Written informed consent was obtained from each
patient. Information that could identify individual participants
during or after data collection was not accessible.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taketomi A: Clinical trials of
antiangiogenic therapy for hepatocellular carcinoma. Int J Clin
Oncol. 21:213–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Facciorusso A, Di Maso M and Muscatiello
N: Drug-eluting beads versus conventional chemoembolization for the
treatment of unresectable hepatocellular carcinoma: A
meta-analysis. Dig Liver Dis. 48:571–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z,
Wan BJ, Liu LM, Tian ZH, Deng H, Sun QH and Chen XP: The strategies
for treating primary hepatocellular carcinoma with portal vein
tumor thrombus. Int J Surg. 20:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bucci L, Garuti F, Lenzi B, Pecorelli A,
Farinati F, Giannini EG, Granito A, Ciccarese F, Rapaccini GL, Di
Marco M, et al: The evolutionary scenario of hepatocellular
carcinoma in Italy: An update. Liver Int. 37:259–270. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramirez AG, Munoz E, Holden AE, Adeigbe RT
and Suarez L: Incidence of hepatocellular carcinoma in Texas
Latinos, 1995-2010: An update. PLoS One. 9:e993652014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakazawa T, Adachi S, Kitano M, Isobe Y,
Kokubu S, Hidaka H, Ono K, Okuwaki Y, Watanabe M, Shibuya A, et al:
Potential prognostic benefits of radiotherapy as an initial
treatment for patients with unresectable advanced hepatocellular
carcinoma with invasion to intrahepatic large vessels. Oncology.
73:90–97. 2017. View Article : Google Scholar
|
|
8
|
Tanaka Y, Nakazawa T, Komori S, Hidaka H,
Okuwaki Y, Takada J, Watanabe M, Shibuya A, Minamino T, Yamamoto H,
et al: Radiotherapy for patients with unresectable advanced
hepatocellular carcinoma with invasion to intrahepatic large
vessels: Efficacy and outcomes. J Gastroenterol Hepatol.
29:352–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stippel DL, Wahba R, Bruns CJ, Bunck A,
Baues C and Persigehl T: Image-guided, minimally invasive surgery
and other local therapeutic procedures for primary liver tumors.
Chirug. 2018.(In German).
|
|
10
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, . Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen A, Tang C, Wang Y, Chen Y, Yan X,
Zhang C, Liu R, Wei X, Zhu Y, Zhang H and Wu Z: A systematic review
of sorafenib in Child-Pugh A patients with unresectable
hepatocellular carcinoma. J Clin Gastroenterol. 47:871–880. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheah YL and Chow PKH: Liver
transplantation for hepatocellular carcinoma: An appraisal of
current controversies. Liver Cancer. 1:183–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malinchoc M, Kamath PS, Gordon FD, Peine
CJ, Rank J and ter Borg PC: A model to predict poor survival in
patients undergoing transjugular intrahepatic portosystemic shunts.
Hepatology. 31:864–871. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eguchi S, Kanematsu T, Arii S, Omata M,
Kudo M, Sakamoto M, Takayasu K, Makuuchi M, Matsuyama Y and Monden
M; Liver Cancer Study Group of Japan, . Recurrence-free survival
more than 10 years after liver resection for hepatocellular
carcinoma. Br J Surg. 98:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou Z, Yuan Z, Zhang Q, Long Z, Chen J,
Tang Z, Zhu Y, Chen S, Xu J, Yan M, et al: Aurora kinase A
inhibition-induced autophagy triggers drug resistance in breast
cancer cells. Autophagy. 8:1798–1810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Zou Z, Gao J, Zhang H, Lin Z, Zhang
Y, Luo X, Liu C, Xie J and Cai C: Increased expression of HMGB3: A
novel independent prognostic marker of worse outcome in patients
with esophageal squamous cell carcinoma. Int J Clin Exp Pathol.
8:345–352. 2015.PubMed/NCBI
|
|
17
|
Tandon P and Garcia-Tsao G: Prognostic
indicators in hepatocellular carcinoma: A systematic review of 72
studies. Liver Int. 29:502–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang TT, Zhao XQ, Liu Z, Mao ZY and Bai
L: Factors affecting the recurrence and survival of hepatocellular
carcinoma after hepatectomy: A retrospective study of 601 Chinese
patients. Clin Transl Oncol. 18:831–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schöniger-Hekele M, Müller C, Kutilek M,
Oesterreicher C, Ferenci P and Gangl A: Hepatocellular carcinoma in
Central Europe: Prognostic features and survival. Gut. 48:103–109.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Llovet JM, Bustamante J, Castells A,
Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J and Bruix J: Natural
history of untreated nonsurgical hepatocellular carcinoma:
Rationale for the design and evaluation of therapeutic trials.
Hepatology. 29:62–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kokudo T, Hasegawa K, Matsuyama Y,
Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima
O, et al: Survival benefit of liver resection for hepatocellular
carcinoma associated with portal vein invasion. J Hepatol.
65:938–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang M, Lin Q, Wang H, Chen J, Bai M,
Wang L, Zhu K, Jiang Z, Guan S, Li Z, et al: Survival benefit of
chemoembolization plus Iodine125 seed implantation in unresectable
hepatitis B-related hepatocellular carcinoma with PVTT: A
retrospective matched cohort study. Eur Radiol. 26:3428–3436. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu XJ, Dong J, Ji LJ, Luo JH, Cao HM, Xiao
LX, Zhou J and Ling CQ: Safety and efficacy of TACE and gamma knife
on hepatocellular carcinoma with portal vein invasion. Gut.
65:715–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riaz A, Ryu RK, Kulik LM, Mulcahy MF,
Lewandowski RJ, Minocha J, Ibrahim SM, Sato KT, Baker T, Miller FH,
et al: Alpha-fetoprotein response after locoregional therapy for
hepatocellular carcinoma: Oncologic marker of radiologic response,
progression, and survival. J Clin Oncol. 27:5734–5742. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB,
Ho WM, Lam KC, Chan AT, Mok TS and Yeo W: New utility of an old
marker: Serial alpha-fetoprotein measurement in predicting
radiologic response and survival of patients with hepatocellular
carcinoma undergoing systemic chemotherapy. J Clin Oncol.
27:446–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MH, Kim SU, Kim DY, Ahn SH, Choi EH,
Lee KH, Lee DY, Seong J, Han KH, Chon CY and Park JY: Early
on-treatment predictions of clinical outcomes using
alpha-fetoprotein and des-gamma-carboxy prothrombin responses in
patients with advanced hepatocellular carcinoma. J Gastroenterol
Hepatol. 27:313–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nobuoka D, Kato Y, Gotohda N, Takahashi S,
Nakagohri T, Konishi M, Kinoshita T and Nakatsura T: Postoperative
serum alpha-fetoprotein level is a useful predictor of recurrence
after hepatectomy for hepatocellular carcinoma. Oncol Rep.
24:521–528. 2010.PubMed/NCBI
|
|
28
|
Bujold A, Massey CA, Kim JJ, Brierley J,
Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et
al: Sequential phase I and II trials of stereotactic body
radiotherapy for locally advanced hepatocellular carcinoma. J Clin
Oncol. 31:1631–1639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kubo S, Hirohashi K, Tanaka H, Tsukamoto
T, Shuto T, Yamamoto T, Ikebe T, Wakasa K, Nishiguchi S and
Kinoshita H: Effect of viral status on recurrence after liver
resection for patients with hepatitis B virus-related
hepatocellular carcinoma. Cancer-Am Cancer Soc. 88:1016–1024.
2000.
|
|
30
|
Ohkubo K, Kato Y, Ichikawa T, Kajiya Y,
Takeda Y, Higashi S, Hamasaki K, Nakao K, Nakata K and Eguchi K:
Viral load is a significant prognostic factor for hepatitis B
virus-associated hepatocellular carcinoma. Cancer. 94:2663–2668.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirooka M, Koizumi Y, Kisaka Y, Abe M,
Murakami H, Matsuura B, Hiasa Y and Onji M: Mass reduction by
radiofrequency ablation before hepatic arterial infusion
chemotherapy improved prognosis for patients with huge
hepatocellular carcinoma and portal vein thrombus. AJR Am J
Roentgenol. 194:W221–W226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai JC, Covinsky KE, Dodge JL, Boscardin
WJ, Segev DL, Roberts JP and Feng S: Development of a novel frailty
index to predict mortality in patients with end-stage liver
disease. Hepatology. 66:564–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benko T, Gallinat A, Minor T, Saner FH,
Sotiropoulos GC, Paul A and Hoyer DP: The postoperative model for
end stage liver disease score as a predictor of short-term outcome
after transplantation of extended criteria donor livers. Eur J
Gastroenterol Hepatol. 29:716–722. 2017. View Article : Google Scholar : PubMed/NCBI
|