Introduction
Bladder cancer (BCa) is the most common malignancy
of the urinary system and is associated with a high mortality rate
(1). Approximately 90% of BCa cases
manifest as transitional cell carcinoma (TCC) of the urinary tract.
TCC can be clinically divided into two groups: Non-muscle-invasive
TCC, which can be treated by transurethral resection and bladder
irrigation chemotherapy with satisfactory clinical outcome in terms
of prognosis; and muscle-invasive TCC, which even with radical
cystectomy, adjuvant chemotherapy and other treatments, remains to
have a high mortality rate due to its high incidence of metastasis
(2). Therefore, there is an urgent
need to investigate the biological characteristics of TCC to
improve the therapeutic outcome of TCC.
The short (−22 nt) non-coding RNAs known as
microRNAs (miRNA/miRs) regulate target gene expression
post-transcriptionally (3–5). Recent studies show that miRNAs control
genes involved in cell apoptosis, differentiation, stress
responses, migration and the cell cycle, and that their
dysregulation is related to the initiation and progression of many
cancers. MiR-506, a miRNA cluster located in chromosome X, has been
implicated in several types of cancer. Previous studies confirmed
that miR-506 exerted anti-tumor effects in cervical, ovarian and
breast cancers (6–9). By contrast, miR-506 played the role of
an oncogene in lung cancer and melanoma (10,11).
However, how miR-506 is involved in BCa remains largely unknown,
and its specific role in BCa requires further study.
In the present study, we aimed to identify the
biological roles of miR-506 in BCa. We reported that miR-506
expression was decreased in human BCa tissues and cell lines.
Furthermore, overexpressed miR-506 had antitumor effects that
manifested as inhibited BCa cell proliferation, migration, invasion
and EMT, which were indicated to occur through targeting of RWD
domain containing (4) (RWDD4).
Materials and methods
Cell lines
TCC cell lines (T24, J82, and UM-UC-3) and the
bladder epithelial immortalized cell line SV-HUC-1 were provided by
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The T24 and SV-HUC-1 cells were maintained in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), and the others were cultured in MEM medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS,
1.5 g/l NaHCO3 and 0.1 g/l sodium pyruvate. All the cells were
cultured in a humidified incubator containing 5% CO2 at
37°C.
Tumor tissues
This study was approved by the ethical committee of
the Fourth People's Hospital of Shenyang (ethics review approval
no: 20140124-8). Informed consent was provided by all participants.
A total of 40 patients who were pathologically diagnosed with
muscle-invasive TCC were enrolled in this study. A total of 40
pairs of primary TCC tissues and paracarcinoma specimens were
obtained from surgical resection specimens and stored in liquid
nitrogen. Hematoxylin-eosin (HE) staining was performed and
evaluated by at least two experienced pathologists.
Total RNA extraction and quantitative
PCR
Total RNA from the TCC cell lines and tissues was
extracted with 1 ml TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The relative expression of miR506 or RWDD4 was
detected by a real-time PCR system using SYBR Green Master Mix
(Takara Bio, Inc., Otsu, Japan) and quantified by the
2−ΔΔCq method (12), with
target expression normalized to the level of U6 or GAPDH. The
primers designed and used for the assay were as follows:
MiR-506-forward: GACATGCATAAGGCACCCTTC MiR-506-reverse:
GTGCAGGGTCCGAGGT U6-forward: CGCTTCGGCAGCACATATACU6-reverse:
CAGGGGCCATCCTAATCTTRWDD4-forward:
TGGTGATCCCAAAGCCTTCTRWDD4-reverse:
CATCAACCCAGTTCCAGCCTGAPDH-forward:
ACAACTTTGGTATCGTGGAAGGGAPDH-reverse: GCCATCACGCCACAGTTTC
Transfection
MiR-506 mimic and a negative control of the mimic
(miR-Ctrl) were obtained from GenePharma (Shanghai, China).
According to the manufacturer's instructions, T24 cells were
transiently transfected with miR-506 mimic or miR-Ctrl by using 30
nM Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.).
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to measure cell
viability following transfection. T24 cells transfected with
miR-506 mimic or si-RWDD4 were seeded in 96-well dishes
(1×104 cells per well) and cultured for 24, 48 and 72 h.
Cells transfected with miR-Ctrl or si-Ctrl were used as negative
controls. After incubation, 10 µl CCK-8 was added and the cells
were incubated at 37°C for 3 h. Absorbance was then assessed at a
wavelength of 450 nm.
Transwell migration assay
T24 cells transfected with miR-506 mimic or miR-Ctrl
(1×105 cells/well) were resuspended in 200 µl medium
without FBS and seeded in the upper chambers of a Transwell insert
containing an 8-µm pore filter (BD Biosciences, Franklin Lakes, NJ,
USA). The lower chamber was filled with medium supplemented with
10% FBS. After 72 h of incubation, cells on the lower side of the
membrane were removed and fixed. The cells were then stained with
0.1% crystal violet and counted.
Invasion assay
T24 cells transfected with miR-506 mimic or miR-Ctrl
were plated in the upper Transwell chambers containing
Matrigel-treated 8-µm pore filters (BD Biosciences). The invasion
assay was performed using the same procedures described for the
Transwell migration assay.
Luciferase assay
T24 cells were co-transfected miR-506 and
recombinant vectors. The transfection groups were established as
follows: A: miR-506 mimics + pmirGLO-RWDD4 WT; B: miR-Ctrl +
pmirGLO-RWDD4 WT; C: miR-506 mimics + pmirGLO-RWDD4 mutant; D:
miR-Ctrl + pmirGLO-RWDD4 mutant. A total of 20 µl PLB lysate was
added to each group and the groups were incubated at room
temperature for 15 min. A Dual-Luciferase Reporter Assay System
(Promega Corporation, Madison, WI, USA) was used to compare the
luciferase activities of the transfected cells. Relative
fluorescence was determined as the ratio of firefly luciferase
fluorescence to Renilla luciferase fluorescence.
Western blot analysis
T24 cells transfected with miR-506 mimic or miR-Ctrl
were lysed and then processed for western blot analysis as
previously described (13). The
primary antibodies were used at 1:1,000 dilution with incubation at
4°C overnight, after which the blots were incubated with secondary
antibodies (goat anti-mouse IgG-HRP, 1:10,000 and goat anti-rabbit
IgG-HRP 1:15,000) for 2 h at room temperature. Immunodetection was
performed using Super Signal West Pico PLUS Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc.) and detected with a
Bio-Rad GelDoc XR+ system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Primary antibodies against E-cadherin, N-cadherin, Snail,
and GAPDH were purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA), and the primary RWDD4 antibody, goat anti-mouse
IgG-HRP and goat anti-rabbit IgG-HRP were obtained from Abcam
(Cambridge, MA, USA).
Statistical analysis
Data were statistically analyzed with IBM SPSS
statistics software v21 (IBM Corp., Armonk, NY, USA) and expressed
as the mean ± SD. To analyze miR-506 expression in human TCC cell
lines, one-way analysis of variance (ANOVA) was used and multiple
comparisons between the groups were performed using Dunnett's least
significant difference (LSD) test. Differences the control group
and the experimental group were compared by using the Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MiR-506 expression is decreased in
human TCC cell lines and tissues
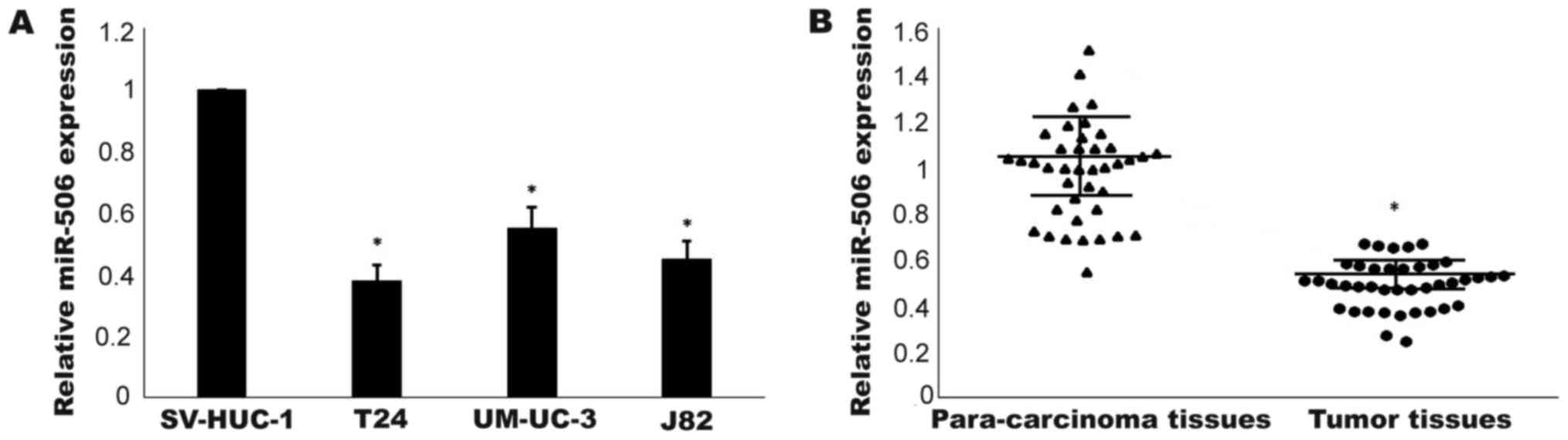
The expression levels of miR-506 in three TCC cell
lines (T24, J82 and UM-UC-3) and a normal human bladder epithelial
cell line (SV-HUC-1) were detected by RT-qPCR. As shown in Fig. 1A, compared with in the human normal
bladder cells, miR-506 expression was downregulated in the TCC cell
lines (P<0.05). T24 cells exhibiting the lowest expression level
were used in the following experiments. Furthermore, as depicted in
Fig. 1B, the expression level of
miR-506 in TCC tissues was significantly lower than that in
non-tumor tissues (P<0.05). Taken together, these results
indicated that miR-506 was downregulated in TCC.
MiR-506 suppresses TCC cell
proliferation, invasion, migration and EMT
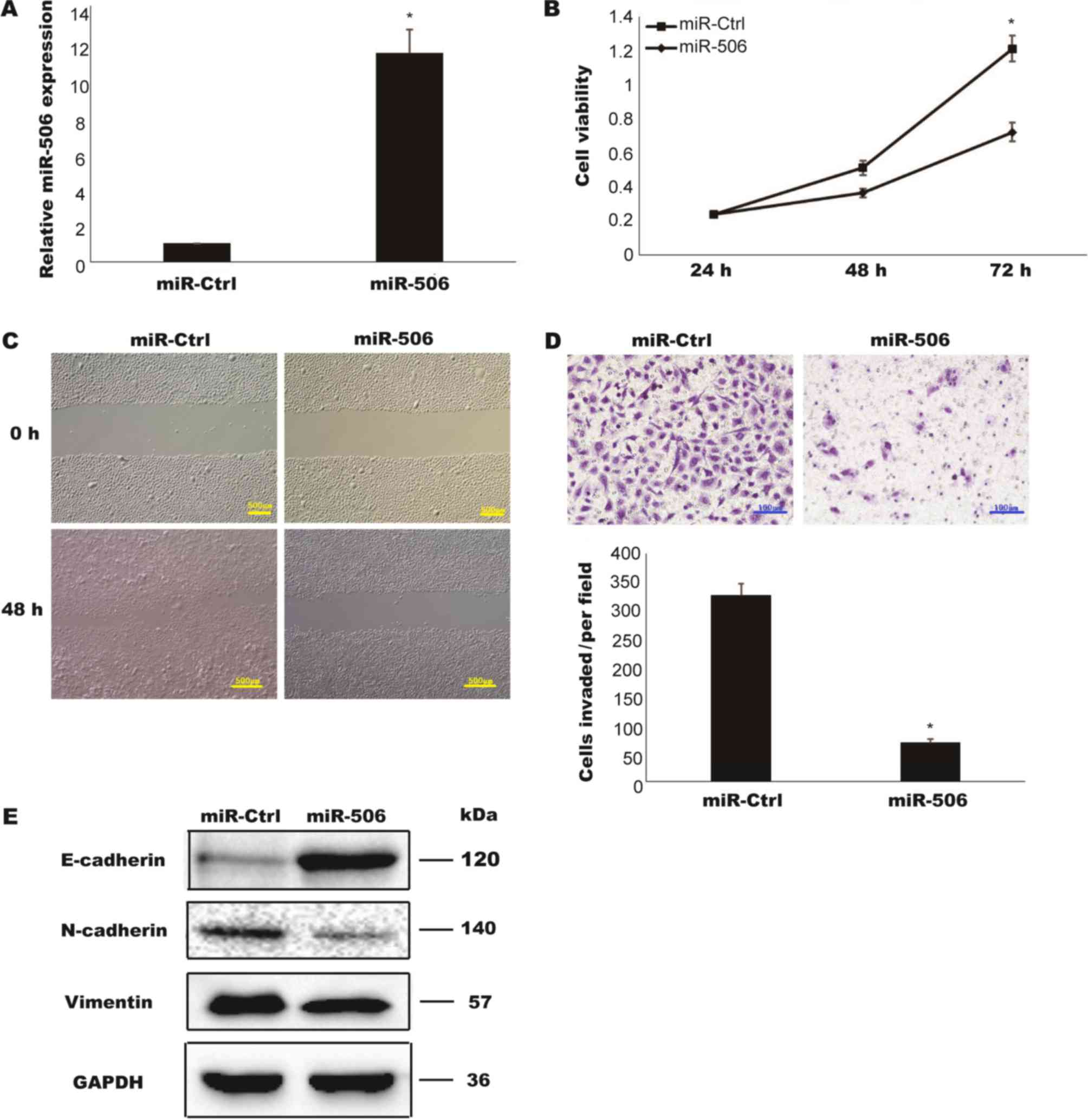
To assess the biological function of miR-506 in TCC,
T24 cells were transiently transfected with miR-506 mimic, and the
relative expression of miR-506 was determined to be successfully
upregulated compared with in a negative control group (miR-Ctrl)
(P<0.05; Fig. 2A). A CCK-8 assay
was performed to determine the cell viabilities of the transfected
cells. As presented in Fig. 2B, the
proliferation rate of T24 cells overexpressing miR-506 was markedly
decreased compared with that of the cells transfected with miR-Ctrl
(P<0.05). In vitro Transwell assays were further utilized
to examine whether miR-506 is involved in TCC cell invasion and
migration. The overexpression of miR506 lead to decreases in the
abilities of cells to invade and migrate (Fig. 2C and D). EMT is considered an early
and key step in the metastatic cascade. The loss of epithelial
protein E-cadherin and the increase of mesenchymal proteins
N-cadherin and Vimentin are hallmarks of EMT (14). To analyze whether miR-506 suppressed
cell migration through inhibition of EMT, the expressions of these
markers were detected by western blotting. Compared with the cells
transfected with miR-Ctrl, the overexpression of miR-506 lead to
upregulation of E-cadherin and downregulation of N-cadherin and
Vimentin (Fig. 2E). Taken together,
these data indicated that miR-506 inhibited TCC cell proliferation,
invasion, migration and EMT.
RWDD4 is a target of miR506 in human
TCC cell lines
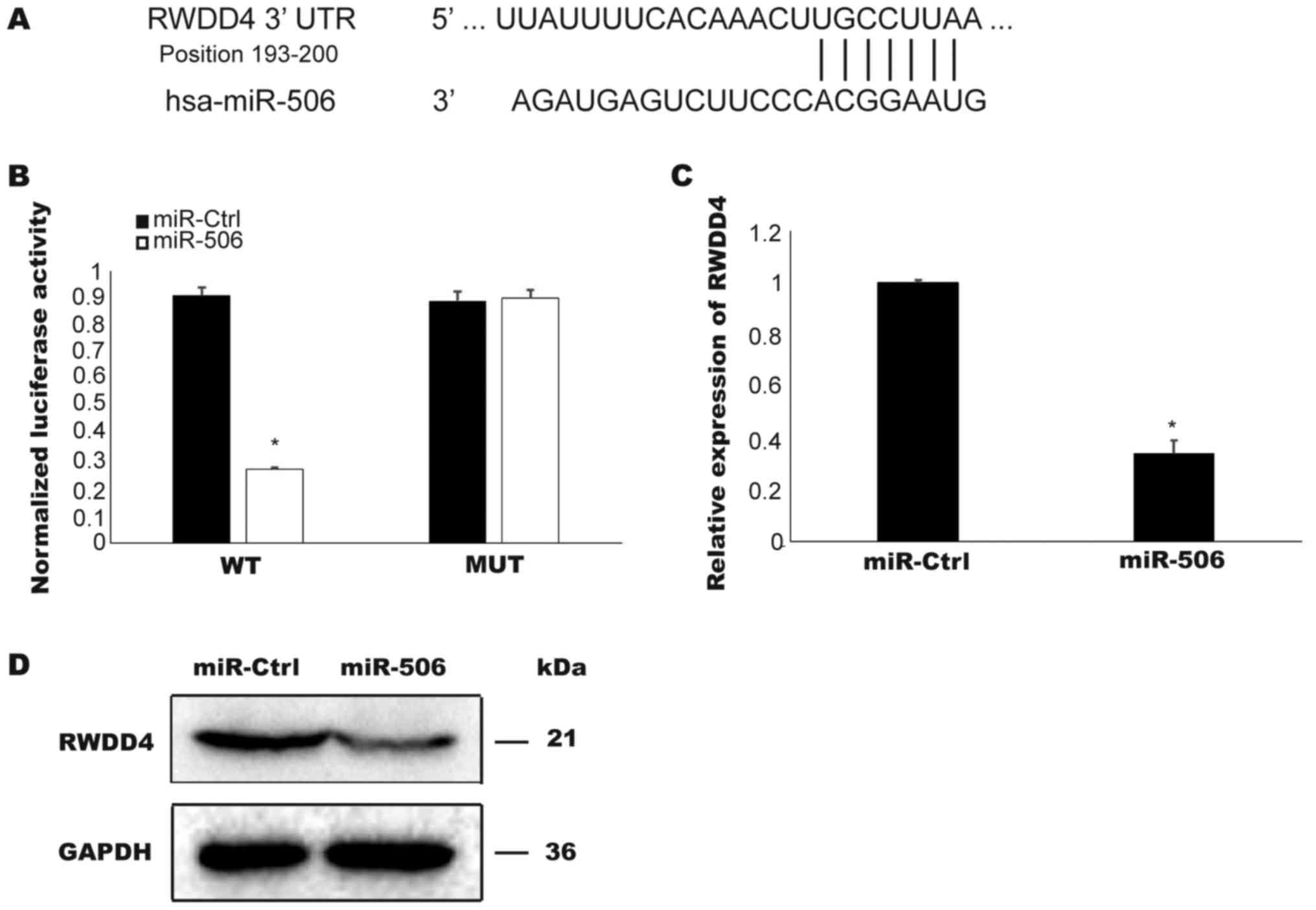
To determine the potential targets of miR506,
TargetScan software was used to search for conserved sites. As
depicted in Fig. 3A, the 3′UTR region
of RWDD4 was identified to contain a highly conserved miR506
binding site. To confirm that RWDD4 is a target of miR506 in TCC
cells, a luciferase activity assay was performed. Overexpressed
miR506 suppressed the luciferase activities of pmirGLO-WT-
RWDD4-3′-UTR plasmid in the T24 cells but did not affect the
activities controlled by the mutant RWDD4-3′-UTR plasmid
(P<0.05; Fig. 3B). Furthermore,
overexpressed miR506 in the T24 cells inhibited the expression of
RWDD4 mRNA and protein (P<0.05; Fig.
3C and D). Collectively these results confirmed RWDD4 as a
target of miR506.
Knockdown of RWDD4 inhibits TCC cell
proliferation, migration and invasion
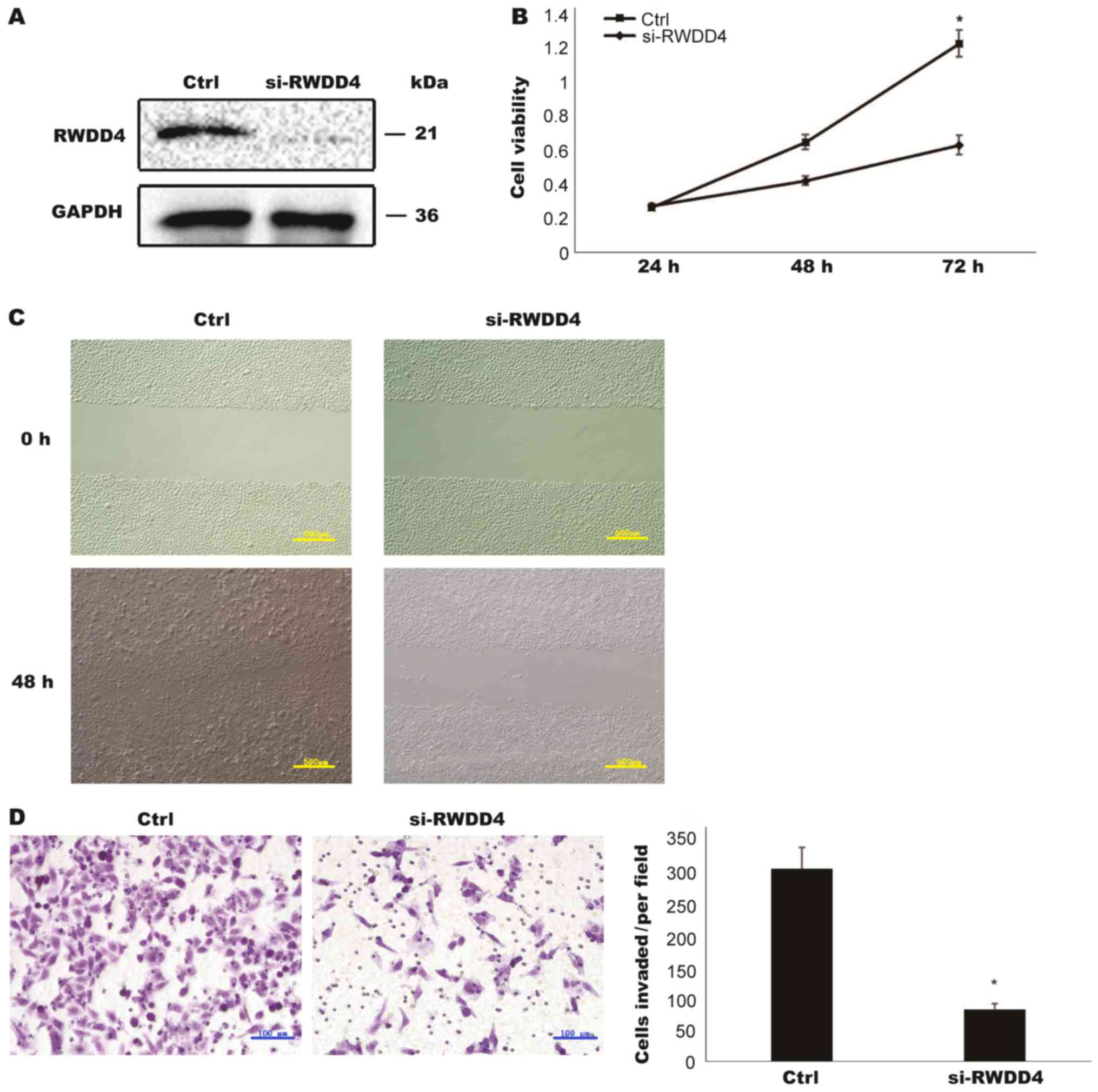
Many functions of the RWDD4 gene have not yet been
fully elucidated. RWDD4 contains a RWD domain that is involved in
protein-protein interactions (15).
Compared with si-Ctrl, si-RWDD4 inhibited RWDD4 protein expression
(Fig. 4A). The knockdown of RWDD4
inhibited TCC cell proliferation, migration and invasion, which was
consistent with the results of miR-506 overexpression (Fig. 4B-D). Therefore, we concluded that
miR-506 may inhibit TCC development through its relationship with
RWDD4.
Discussion
Short (−22 nt) non-coding miRNAs play essential
roles in the regulation of gene expression post-transcriptionally.
Recent studies showed that miRNAs play vital roles in the
regulation of tumor growth and progression in various tumor types
including TCC (16,17). The essential functions of miRNAs have
prompted increasing investigations into the use of miRNA-targeted
strategies for human cancer treatment (13,18). As
invasive BCa is a disease that can affect the whole bladder
(19), HE staining was performed
before RT-qPCR detection to verify that the controls were non-tumor
tissues pathologically. However, due to the characteristics of
invasive BCa, there is still the possibility that genetic
alterations were present in the non-tumor tissues. MiR-506 plays
distinct roles in different cancers through the regulation of
specific genes. Consistent with previous findings in breast,
cervical and ovarian cancer, we revealed that miR-506 was notably
decreased in human TCC cell lines and TCC tissues compared with in
a normal human bladder cell line and paired non-tumor samples,
respectively. In vitro studies also demonstrated that
overexpressed miR-506 suppressed TCC cell proliferation, migration
and invasion. Moreover, we identified RWDD4 to be a biological
target of miR-506. Decreased RWDD4 expression via siRNA
transfection showed comparable results to those obtained with
miR-506 overexpression. Therefore, our findings suggested that
miR-506 functioned as a tumor suppressor though targeting RWDD4 in
human TCC.
The effects of therapies for advanced TCC are still
not up to expectations due to the high incidence of metastasis in
TCC (20). EMT, which was originally
established as a process in normal cell differentiation, is divided
into three types. Type 3 EMT is involved in formation of cancer
stem cells and cancer progression (21). The loss of epithelial features and the
acquisition of mesenchymal characteristics enable cancer cells to
diffuse more rapidly and thus more invasive (22). Therefore, we also investigated the
effects of miR-506 on the expression of proteins involved in the
regulation of EMT. We found that the overexpression of miR-506
upregulated E-cadherin and downregulated N-cadherin and Vimentin.
Several transcription factors including snail family zinc finger
(Snail), twist basic helix-loop-helix transcription factor 1
(TWIST1), and zinc finger E-box-binding homeobox (ZEB1) have been
reported to induce EMT by suppressing E-cadherin expression
(23). To the best of our knowledge,
only one previous study has revealed an association of RWDD4 with
prostate cancer aggression (15).
Consistent with this previous study, we found downregulated RWDD4
inhibited TCC cell proliferation, migration and invasion in
vitro. Based on large-scale data analyses, cancer metastasis
has been established as a complicated procedure driven by more than
100 genes (24,25). Whether and how RWDD4 is involved in
the miR-506-mediated regulation of EMT remains to be clarified in
further studies. However, the characterization of RWDD4 gene
function in the present study has in part enriched our knowledge on
cancer metastasis in TCC.
In conclusion, this study has identified that
miR-506 plays critical roles in TCC cell proliferation, migration,
invasion and EMT through targeting RWDD4. Our data warrant further
investigation into the potential of miR-506 as a novel target in
human TCC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and performed the all experiments.
Ethics approval and consent to
participate
The present study was approved by the ethical
committee of the Fourth People's Hospital of Shenyang (ethics
review approval no: 20140124-8). Informed consent was provided to
the all participants.
Patient consent for publication
All the patient, or parent, guardian or next of kin
provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leow JJ, Martin-Doyle W, Rajagopal PS,
Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL,
Choueiri TK and Bellmunt J: Adjuvant chemotherapy for invasive
bladder cancer: A 2013 apdated systematic review and meta-analysis
of randomized trials. Eur Urol. 66:42–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-A brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wen SY, Lin Y, Yu YQ, Cao SJ, Zhang R,
Yang XM, Li J, Zhang YL, Wang YH, Ma MZ, et al: miR-506 acts as a
tumor suppressor by directly targeting the hedgehog pathway
transcription factor Gli3 in human cervical cancer. Oncogene.
34:717–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du J, Zheng X, Cai S, Zhu Z, Tan J, Hu B,
Huang Z and Jiao H: MicroRNA506 participates in pancreatic cancer
pathogenesis by targeting PIM3. Mol Med Rep. 12:5121–5126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang
D, Parker Kerrigan BC, Shmulevich I, Chen K, Sood AK, et al:
MiR-506 suppresses proliferation and induces senescence by directly
targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol.
233:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arora H, Qureshi R and Park WY: miR-506
regulates epithelial mesenchymal transition in breast cancer cell
lines. PLoS One. 8:e642732013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin M, Ren X, Zhang X, Luo Y, Wang G,
Huang K, Feng S, Bao X, Huang K, He X, et al: Selective killing of
lung cancer cells by miRNA-506 molecule through inhibiting NF-kB
p65 to evoke reactive oxygen species generation and p53 activation.
Oncogene. 34:691–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Streicher KL, Zhu W, Lehmann KP,
Georgantas RW, Morehouse CA, Brohawn P, Carrasco RA, Xiao Z, Tice
DA, Higgs BW, et al: A novel oncogenic role for the miRNA-506-514
cluster in initiating melanocyte transformation and promoting
melanoma growth. Oncogene. 31:1558–1570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Sun D, Tai J and Wang L: Ganoderic
acid A inhibits proliferation and invasion, and promotes apoptosis
in human hepatocellular carcinoma cells. Mol Med Rep. 16:3894–3900.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Winter JM, Gildea DE, Andreas JP, Gatti
DM, Williams KA, Lee M, Hu Y, Zhang S; NISC Comparative Sequencing
Program5, ; Mullikin JC, et al: Mapping complex traits in a
diversity outbred f1 mouse population identifies germline modifiers
of metastasis in human prostate cancer. Cell Syst. 4:31–45 e6.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enokida H, Yoshino H, Matsushita R and
Nakagawa M: The role of microRNAs in bladder cancer. Investig Clin
Urol. 57 Suppl 1:S60–S76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fendler A, Stephan C, Yousef GM,
Kristiansen G and Jung K: The translational potential of microRNAs
as biofluid markers of urological tumours. Nat Rev Urol.
13:734–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhardwaj A, Singh S and Singh AP:
MicroRNA-based cancer therapeutics: Big hope from small RNAs. Mol
Cell Pharmacol. 2:213–219. 2010.PubMed/NCBI
|
|
19
|
Sanli O, Dobruch J, Knowles MA, Burger M,
Alemozaffar M, Nielsen ME and Lotan Y: Bladder cancer. Nat Rev Dis
Primers. 3:170222017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Froehner M, Koch R, Heberling U, Novotny
V, Oehlschlaeger S, Hubler M, Baretton GB, Hakenberg OW and Wirth
MP: Decreased overall and bladder cancer-specific mortality with
adjuvant chemotherapy after radical cystectomy: Multivariable
competing risk analysis. Eur Urol. 69:984–987. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: Concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian CN, Mei Y and Zhang J: Cancer
metastasis: Issues and challenges. Chin J Cancer. 36:382017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mei Y, Yang JP and Qian CN: For robust big
data analyses: A collection of 150 important pro-metastatic genes.
Chin J Cancer. 36:162017. View Article : Google Scholar : PubMed/NCBI
|