Introduction
Leiomyosarcoma (LMS) is a relatively rare
soft-tissue tumor that can arise from any organ or structure
containing smooth muscle, most often occurs in the retroperitoneum,
the digestive tract, pelvis, skin and soft tissue (1,2). Primary
thyroid LMS is rare, and may be associated with smooth
muscle-walled vessels at the periphery of the thyroid gland
capsule; however, the pathogenesis of primary thyroid LMS remains
unclear (1–17). To the best of our knowledge, only 25
cases have been reported in English literature (1–17). LMS is
composed of cells with distinct smooth muscle histological
differentiation, and the diagnosis is confirmed by
immunohistochemical techniques combined with clinical history
(4). It is difficult to produce a
preoperative diagnosis of primary thyroid LMS and to differentiate
it from anaplastic thyroid carcinoma or other spindle cell sarcoma
types of either primary or metastatic origin (15). In 2016, Zou et al through
retrospective literature review, reported that the prognosis of
thyroid LMS is poor, with a 1-year survival rate of <10%
(1). In the present study, a rare
case of a 74-year-old female patient diagnosed with primary thyroid
LMS was reported and a brief review of the literature is
presented.
Case report
In October 2016, a 74-year-old Chinese female was
admitted to Shaoxing People's Hospital due to her exhibiting a
right anterior neck mass for 12 months, which rapidly enlarged for
the last 3 months. The present study was approved by the Ethics
Committee of the Shaoxing People's Hospital and the patient
provided written informed consent.
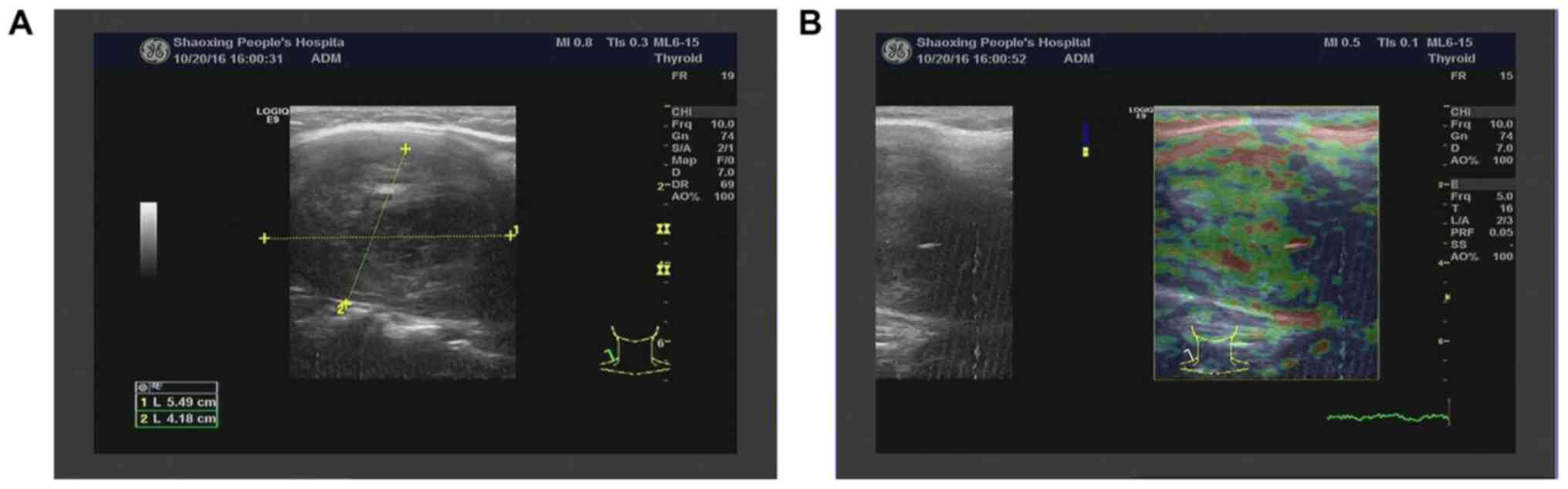
Ultrasound of the thyroid revealed a 55×42 mm
hypoechoic mass with clear margins in the right lobe (Fig. 1A and B). Color Doppler flow imaging
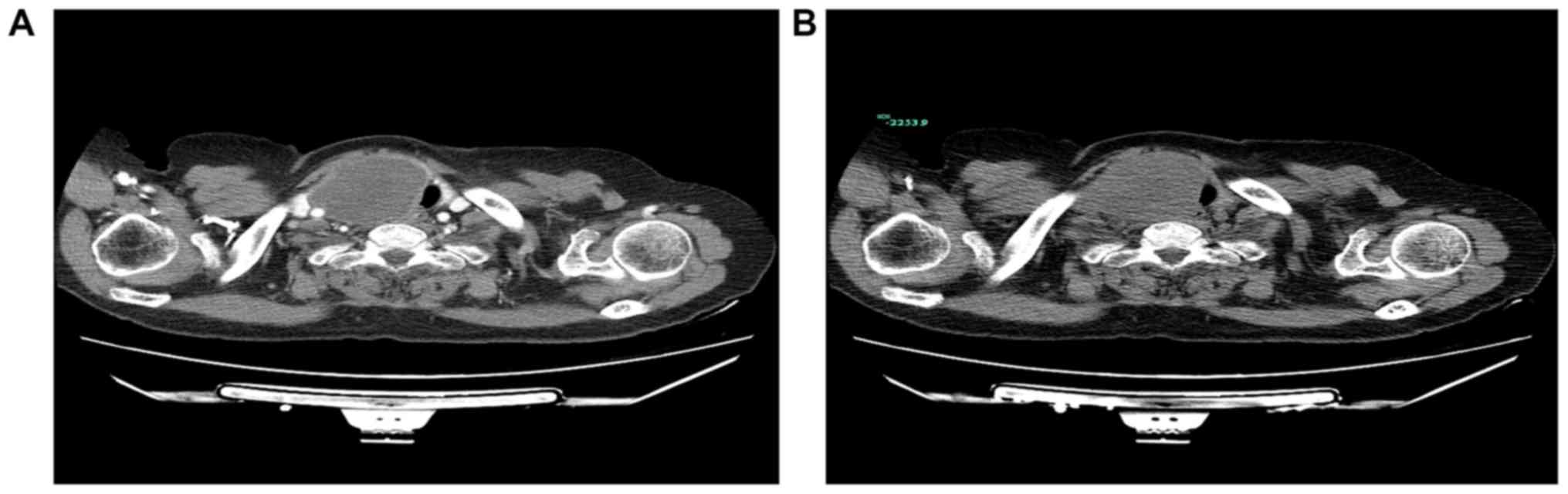
(CDFI) indicated no abnormal blood flow signal. Computed tomography
revealed a 66×46 mm soft tissue mass at the entrance of the
thoracic trachea, adjacent to the right side of the soft tissue
(Fig. 2A), and there was no notable
reinforcement following enhanced scanning (Fig. 2B). There were no abnormal findings in
the chest or pelvis as demonstrated by imaging examination.
Physical examination demonstrated a hard mass of ~6.0 cm in the
right lobe of the thyroid gland. There were no palpable cervical or
supraclavicular lymph nodes. There was no history of radiation
exposure or another primary tumor. Routine laboratory
investigations were normal, including complete blood count and
electrolyte levels. A serum thyroid function test was also normal.
Tumor markers, including carcinoembryonic antigen, α-fetoprotein,
carbohydrate antigen 125, carbohydrate antigen 19-9 and lactate
dehydrogenase were all within normal limits. The patient underwent
thyroid neoplasm resection and the frozen examination demonstrated
a malignant spindle-cell tumor. Subsequently, the patient underwent
bilateral thyroid radical resection and neck lymphadenectomy.
Following surgery, the patient refused all treatment and succumbed
to the disease after 2 months.
The tissue was fixed in 10% buffered formalin for 6
h at room temperature, and embedded in paraffin, following which
4-µm thin sections were cut and stained with hematoxylin (for 5 min
at room temperature) and eosin (for 1 min at room temperature).
Sections, which were deparaffinized and subsequently rehydrated in
a descending alcohol series (100% alcohol for 5 min, 95% alcohol
for 4 min, 85% alcohol for 2 min). Antigens were heat-retrieved at
98°C in EDTA solution. Following cooling to room temperature, the
tissue sections were quenched with 3% hydrogen peroxidase and
non-specific binding sites were blocked with 5% goat serum
(Zhongshan Bio, Beijing, China) at 37°C for 30 min. Subsequently,
the sections were incubated with the following primary antibodies
(listed in Table I).
Immunohistochemical staining was performed by EnVision (Zhongshan
Bio, Beijing, China) according to the manufacturer's protocol. All
antibodies were incubated at room temperature for 20–30 min, then
observed under an OLYMPUS microscope at ×40, ×100 and ×400
magnification. Immunohistochemical staining was performed using
commercially available antibodies to the following antigens:
Epithelial membrane antigen (EMA) (dilution, 1:100; cat. no. GP1.4;
Guangzhou Ascend Biotechnology Co., Ltd., Guangzhou, China),
pan-cytokeratin (CKpan) (dilution, 1:100; cat. no. V9; Ascend
Biotechnology Co., Ltd.), vimentin (dilution, 1:100; cat. AE1/AE3;
Ascend Biotechnology Co., Ltd.), smooth muscle actin (SMA)
(dilution, 1:800; cat. no. 1A4; Fuzhou Maixin Biotech, Fuzhou,
China), desmin (dilution, 1:600; cat. no. D33; Zhongshan Bio,
Beijing, China), paired box (Pax)-8 (dilution, 1:100; rabbit. no.
IR1; Zhongshan Bio, Beijing, China), thyroid transcription factor-1
(TTF-1) (dilution, 1:400; cat. no. SPT24; Ascend Biotechnology Co.,
Ltd.), 34βE12(dilution, 1:200; cat.; Ascend Biotechnology Co.,
Ltd.), cytokeratin (CK)5/6 (dilution, 1:1,600; cat. no. 007;
Zhongshan Bio, Beijing, China), cluster of differentiation (CD)117
(dilution, 1:1,000; rabbit. no. EP10; Fuzhou Maixin Biotech,
Fuzhou, China), CD34 (dilution, 1:1,000; rabbit. no. QBEnd110;
Zhongshan Bio, Beijing, China), CD68 (dilution, 1:400; cat.no. EP2;
Ascend Bio, Guangzhou, China), myoglobin (dilution, 1:100; cat. no.
MY018; Zhongshan Bio, Beijing, China), S100 (dilution, 1:800; cat.
no. poly; Fuzhou Maixin Biotech, Fuzhou, China), p53 (dilution,
1:800; rabbit.no. EP9; Ascend Biotechnology Co., Ltd.), Ki-67
(dilution, 1:200; cat.no. MIB1; Ascend Bio, Guangzhou, China),
progesterone receptor (PR) (dilution, 1:800; rabbit. no. EP2;
Ascend Biotechnology Co., Ltd.) and estrogen receptor (ER)
(dilution, 1:800; rabbit. no. EP1; Ascend Biotechnology Co., Ltd.).
Simultaneously, in situ hybridization for the presence of
small Epstein-Barr virus (EBV)-encoded RNA (EBER) was performed to
identify the association between this tumor and EBV. All protocols
were employed according to the manufacturer's protocols (Table I).
 | Table I.Summary of primary antibodies and
results of immunohistochemistry. |
Table I.
Summary of primary antibodies and
results of immunohistochemistry.
| Antibody | Source | Dilution | Result |
|---|
| CK | Ascend Bio,
Guangzhou, China | 1:100 | – |
| EMA | Ascend Bio | 1:100 | – |
| Vimentin | Ascend Bio | 1:100 | + |
| TTF-1 | Ascend Bio | 1:400 | – |
| S100 | Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA | 1:3,200 | – |
| Pax-8 | Ascend Bio | 1:100 | – |
| Desmin | Zhongshan Bio,
Beijing, China | 1:600 | + |
| SMA | Dako; Agilent
Technologies, Inc. | 1:3,200 | + |
| p53 | Ascend Bio | 1:800 | + |
| 34βE12 | Ascend Bio | 1:200 | – |
| CK5/6 | Zhongshan Bio | 1:1,600 | – |
| CD117 | Dako; Agilent
Technologies, Inc. | 1:1,000 | – |
| CD34 | Zhongshan Bio | 1:1,000 | – |
| CD68 | Ascend Bio | 1:400 | – |
| Myoglobin | Zhongshan Bio | 1:100 | – |
| PR | Genetic Tech,
Shanghai, China | 1:1,600 | – |
| ER | Epitomics; Abcam,
Cambridge, MA, USA | 1:800 | – |
| Ki-67 | Ascend Bio | 1:200 | 40% in the most
concentrated spot |
| EBER | Triplex
International Biosciences, Fuzhou, China | RTU | – |
Results
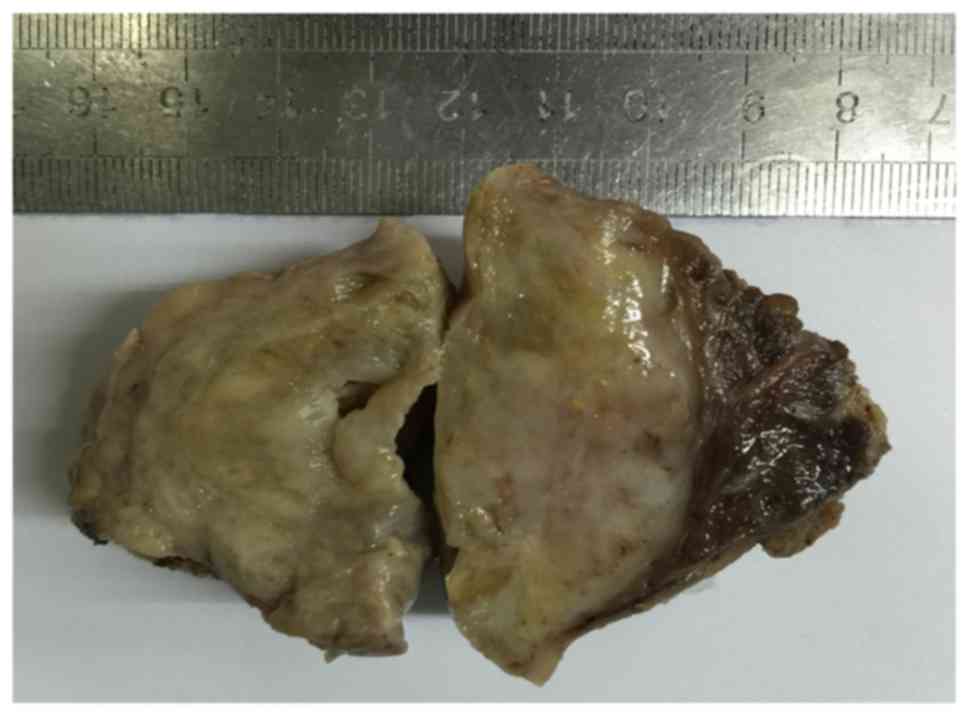
The size of the surgical specimen was 7.0×5.5×5.0
cm, and the largest diameter of the tumor was 6.5 cm. In cross
sections, the tumor was yellowish-white with focal areas of
hemorrhage, cystic change and myxoid degeneration (Fig. 3). There was identifiable thyroid
tissue around the tumor. Microscopically, there was no capsule
between the tumor and the surrounding normal thyroid tissue, and
the tumor infiltrated into the adjacent thyroid (Fig. 4A), fat and perineural (Fig. 4B). There was a visible thick-walled
blood vessel located around the tumor, and the neoplastic cells
scroll off the muscle wall, where tumor cells grew around the
periphery of blood vessels (Fig. 4C),
which indicated that the tumor may originate from smooth muscle of
the walled vessels. The tumor consisted of spindle cells arranged
in interlacing fascicles, and the cells had cigar shaped,
blunt-ended nuclei (Fig. 4D).
Additionally, in a number of areas, the appearance of neoplastic
cells ranged from spindled to plump or pleomorphic cells (Fig. 4E), and they exhibited notable nuclear
pleomorphism, atypical, giant cell formation (Fig. 4F), with >3 abnormal mitotic figures
per each of 10 high-power fields (Fig.
4G), large areas of hemorrhage and coagulative necrosis
(Fig. 4H).
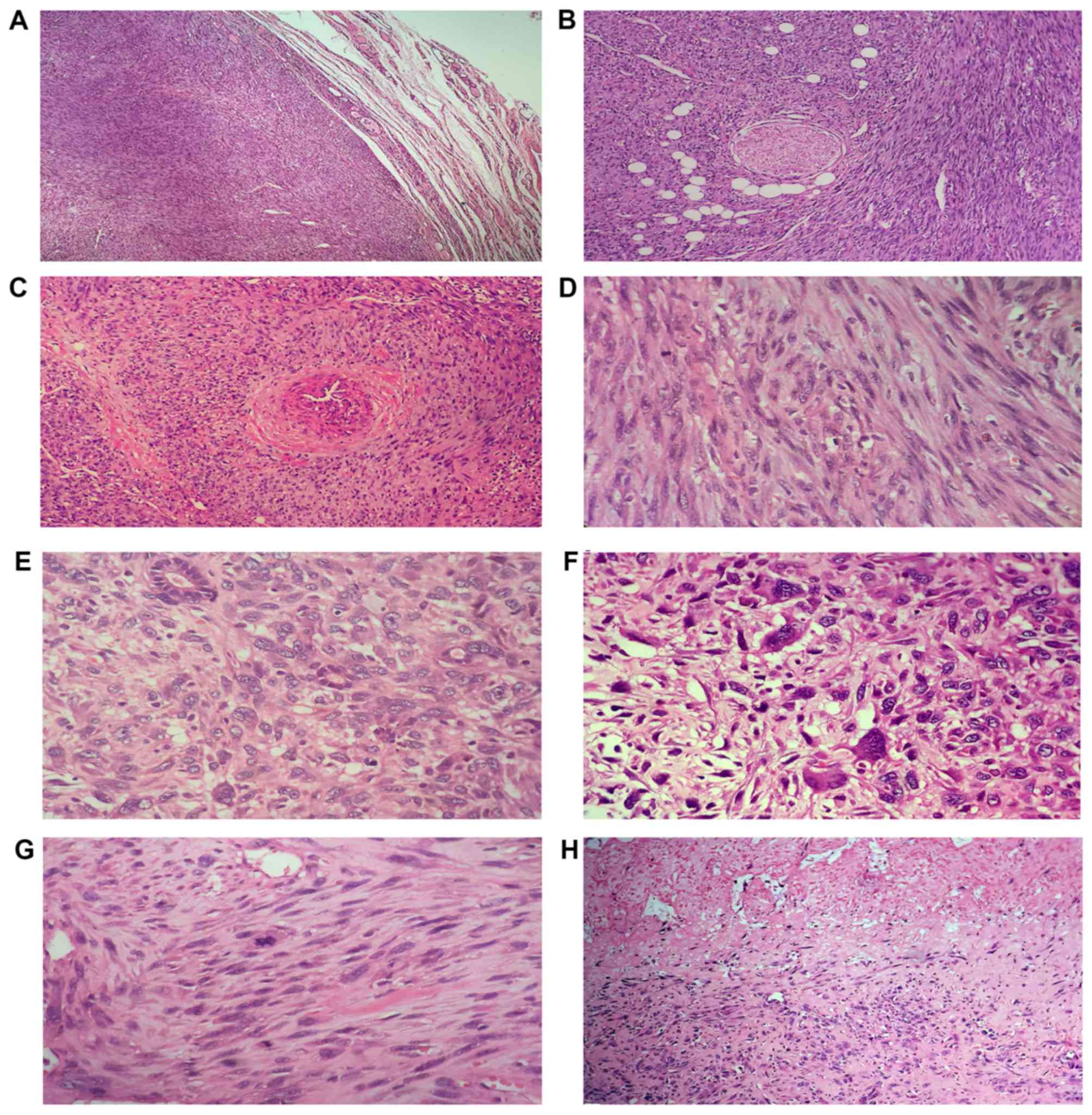 | Figure 4.Morphological characteristics of LMS.
Microscopically, there was no capsule between the tumor and the
surrounding normal thyroid tissue, and the tumor infiltrated the
(A) adjacent thyroid, (B) fat and perineural (H&E staining,
×40). (C) There was a visible thick-walled blood vessel located
around the tumor, and the neoplastic cells scroll off the muscle
wall (H&E staining, ×100). (D) The tumor consisted of spindle
cells arranged in interlacing fascicles, and the cells had cigar
shaped, blunt-ended nuclei (H&E staining, ×400). (E) In a
number of areas, the appearance of neoplastic cells ranged from
spindled to plump or pleomorphic cells, and there were residual
thyroid follicles (H&E staining, ×400). (F) Additionally, it
exhibited notable nuclear pleomorphism and atypical, giant cell
formation (H&E staining, ×400). Furthermore, the tissue
exhibited (G) abnormal mitotic figures (H&E staining, ×400) and
(H) coagulative necrosis (H&E staining, ×40). H&E,
hematoxylin and eosin. |
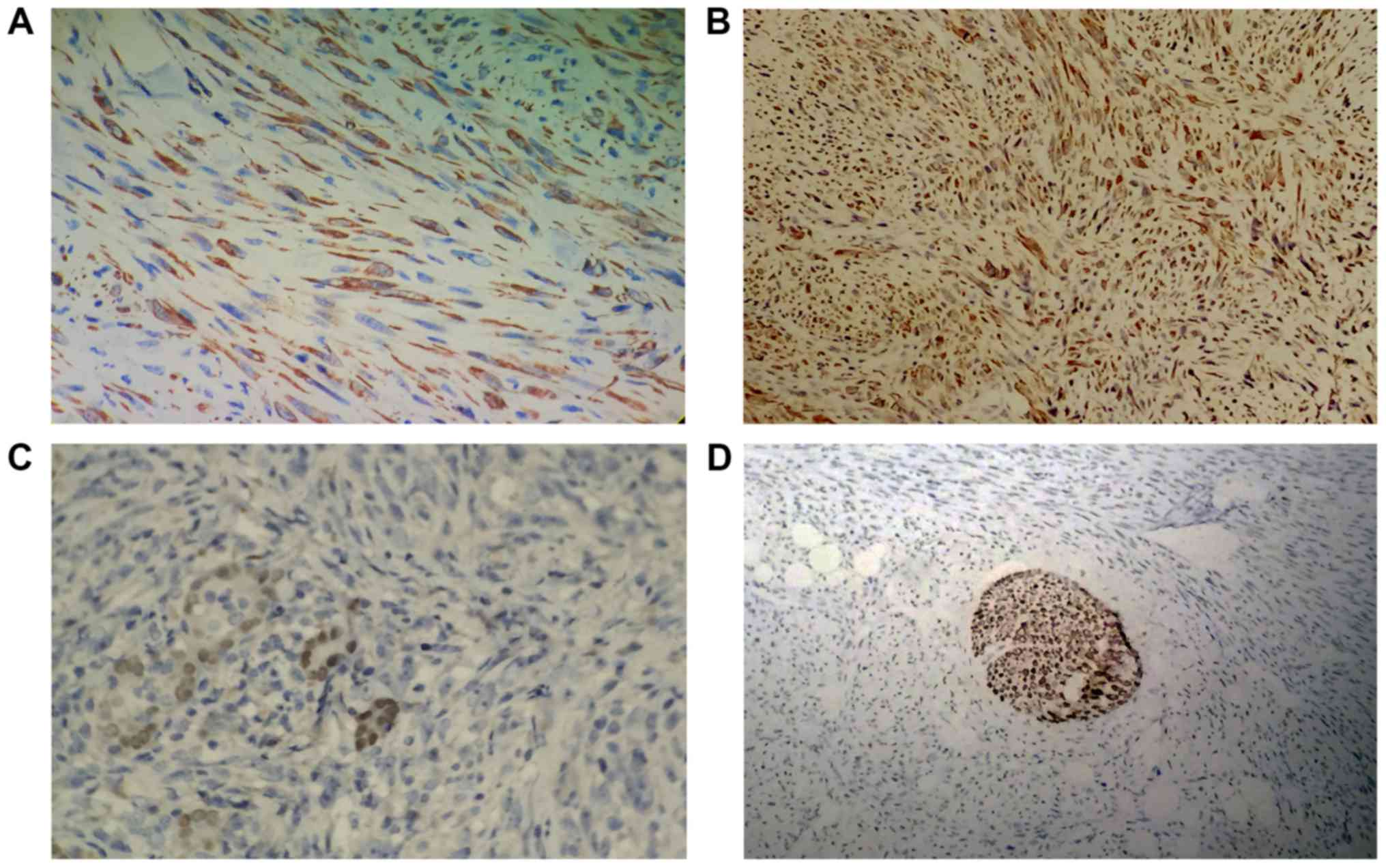
Immunohistochemically, the tumor cells were strongly
positive for desmin (Fig. 5A), SMA
(Fig. 5B), p53 and vimentin
expression, but negative for CKpan, EMA, TTF-1 (Fig. 5C), Pax-8, 34βE12, CK5/6, CD117, CD34,
CD68, myoglobin, S100 (Fig. 5D), p16,
PR and ER. The Ki-67 labeling index reached 40% in the most
concentrated location. Furthermore, EBV (in situ
hybridization) was negative.
Discussion
Primary thyroid LMS is a rare malignant tumor type,
and to the best of our knowledge, only 25 cases of primary thyroid
LMS have been previously reported in English literature (1–17). The
etiology of primary thyroid LMS remains unclear, particularly the
role of radiation exposure (1,3,7,9,11). It may be associated with smooth
muscle-walled vessels at the periphery of the thyroid gland capsule
(1–5).
A single case of an EBV-associated thyroid smooth muscle tumor has
been reported in a child with a congenital immunodeficiency disease
(12). A previous study demonstrated
that LMS of other sites have also been associated with acquired
immunodeficiency syndrome or EBV infection (12). It appears that LMS has a high
probability of occurring in immunosuppressed patients (1,12).
However, the present patient had no history of radiation exposure.
Microscopically, there was a visible thick-walled blood vessel
located around the tumor, and the neoplastic cells scroll off the
muscle wall, where tumor cells grew around the periphery of blood
vessels, which indicated that the tumor may originate from the
smooth muscle of the walled vessels. Additionally, the present
patient was negative for human immunodeficiency virus and EBV;
therefore, we hypothesized that there was no association between
them.
In retrospective review of the small number of
reported cases (1–17), limited information was available for 1
patient (16). The ages of all the
patients ranged between 39 and 90 years, excluding a 6-year-old
patient who had immune system deficiency, (mean age, 59.7; median
age, 65), indicating that the tumor has been principally determined
in adults. Additionally, the majority of patients were elderly and
the female: male ratio was 1.5:1, demonstrating a slight
predilection for female patients. The majority of thyroid LMS were
unilateral, with only 1 patient exhibiting bilateral thyroid LMS
(2). Clinical manifestations included
a painless, rapidly growing neck mass, or dysphagia, hoarseness,
odynophagia, dyspnea and weight loss (1–17). The
majority of reported patients were euthyroid (1,3,5–17). The
present patient was an older female, and exhibited a right anterior
neck mass for 12 months, which rapidly enlarged over the last 3
months. Furthermore, a serum thyroid function test was also
normal.
There are no imaging characteristics or tumor
markers that allow a preoperative diagnosis, and all patients have
been diagnosed following surgical resection. The diagnosis is based
entirely on histopathological and immunohistochemical evaluations.
Additionally, the tumor boundary was not clear, and the
cross-sections were grayish white, or with hemorrhage, necrosis and
cystic change (1,2,6). The tumor
diameter range was 1.9–12.0 cm (average diameter, 6.3 cm), with a
diameter of >5 cm in of cases (1–17). The
tumor of the present patient exhibited a typical interlacing
fascicular growth pattern with spindle cells, and the cells had
cigar shaped, blunt-ended nuclei. Additionally, there were a number
of areas with diffuse pleomorphic neoplastic cells containing large
nuclei or osteoclast-like giant cells, and the nuclei was notably
atypical and pleomorphic. Furthermore, the tumor infiltrated the
adjacent thyroid, fat and perineural. The tumor cells were positive
for desmin, SMA, p53 and vimentin; therefore, the final diagnosis
was primary thyroid LMS.
The major differential diagnosis included anaplastic
(undifferentiated) thyroid carcinoma, metastatic LMS (18,19),
spindle epithelial tumor with thymus-like differentiation (SETTLE)
(20), spindle cell variant of
medullary thyroid carcinoma (21) or
other primary and metastatic malignant mesenchymal tumor types,
including rhabdomyosarcoma (22),
synovial sarcoma (23), malignant
peripheral nerve sheath tumor (MPNST) (24) or undifferentiated pleomorphic sarcoma
(25). Thyroid LMS should be
diagnosed only when there is a complete lack of all epithelial
differentiation and there is definite evidence (histologic,
imninophenotypic, or ultrastructural) of specific sarcomatous
differentiation (1,6,17).
Anaplastic thyroid carcinoma also frequently occurs in older
patients with longstanding history of a pre-existing thyroid lesion
that has rapidly enlarged (26).
Histologically, the presence of residual well-differentiated
thyroid carcinoma favors anaplastic thyroid carcinoma (6,12,26). Anaplastic thyroid carcinomas
frequently express epithelial markers to different degrees, and a
few rare cases may lose the epithelial phenotype, and express SMA
and desmin (27). Prior to the
diagnosis of primary thyroid LMS, the possibility of LMS
metastasising to other sites, including the uterus, lung, or
gastrointestine or soft tissue, must be ruled out (2,18,19). The clinical history and radiographic
evaluations are beneficial to the differential diagnosis of
LMS.
Immunohistochemistry has important value in the
diagnosis and differential diagnosis of LMS. The lack of CD5, CD117
and p63 expression ruled out SETTLE. The lack of calcitonin and
neuroendocrine markers expression ruled out medullary thyroid
carcinoma. The lack of S100 protein expression ruled out MPNST. The
lack of MyoD1 and myogenin protein expression ruled out
rhabdomyosarcoma. Synovial sarcoma is frequently expresses a
different degree of CK and EMA. Additionally, undifferentiated
pleomorphic sarcoma neither expresses epithelial markers nor
mesenchymal markers. When tumors were positive for SMA and desmin,
support the diagnosis of LMS. CD117 is rarely expressed in LMS,
although a case of CD117 overexpression in primary thyroid LMS has
been previously reported (9). A
number of reports demonstrated that uterus LMS can express ER and
p16; therefore, ER and p16 may have important value in identifying
primary or metastatic uterine LMS (18,19,26). In
the present case, the final diagnosis of LMS was supported by
histopathological findings plus the positive immunostaining for
desmin and SMA, and radiological and clinical observations.
There is no consensus on standardized treatment
strategy for thyroid LMS, but radical surgical resection is
considered to be the most effective treatment (1,2,6). Adjuvant chemotherapy, radiation therapy
and immunotherapy have not proven beneficial. The prognosis of the
patients with thyroid LMS is poor (3–5,7,16).
According to the literature review, it was determined that 2
patients had lymph node metastasis (2,16), and
10/25 patients had distant metastases (1,11–14). In 2016, Zou et al (1) through retrospective literature review,
reported that thyroid LMS is primarily fatal and survival rates are
<10% in the first year worldwide (1). A total of 16/25 patients succumbed
within 8 months due to the disease, with the longest disease-free
duration of a patient being 5 years (17).
In conclusion, a thyroid mass with spindle cells and
abnormal pleomorphic cells should raise the suspicion of LMS,
particularly in older patients exhibiting a rapidly growing mass at
the anterior portion of the neck. Immunohistochemistry is required
to differentiate it from anaplastic thyroid carcinoma or other
primary and metastatic malignant mesenchymal tumor types. The
prognosis of thyroid LMS is notably poor, and the necessity of an
aggressive oncological and effective treatment approach remains
controversial.
Acknowledgements
Not applicable.
Funding
The present study was supported by Project of
natural science foundation of Zhejiang province (grant no.
LY16H160058), Medical and health science and technology project of
Zhejiang province (grant no. 201512180), Shaoxing Public Welfare
Technology Application Research Project (grant no. 2017B70020) and
the Youth Research Foundation of Shaoxing People's Hospital of
Zhejiang Province, China (grant no. 2017A09) supported the present
study.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors read and approved the final manuscript.
JGW made substantial contributions to conception and design, or
acquisition of data, or analysis and interpretation of data, and
drafted the article and revising it critically for important
intellectual content; JFY, WQL and CWX contributed to the
acquisition of data or analysis and interpretation of data; YYW
revised the article critically for important intellectual content,
and contributed to the conception and design, approved the final
version to be published.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Shaoxing People's Hospital and written informed
consent was obtained from the patient.
Patient consent for publication
The patient signed written informed consent for the
publication of any data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zou ZY, Ning N, Li SY, Li J, DU XH and Li
R: Primary thyroid leiomyosarcoma: A case report and literature
review. Oncol Lett. 11:3982–3986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Şahin Mİ, Vural A, Yüce İ, Çağlı S, Deniz
K and Güney E: Thyroid leiomyosarcoma: Presentation of two cases
and review of the literature. Braz J Otorhinolaryngol. 82:715–721.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conzo G, Candela G, Tartaglia E,
Gambardella C, Mauriello C, Pettinato G, Bellastella G, Esposito K
and Santini L: Leiomyosarcoma of the thyroid gland: A case report
and literature review. Oncol Lett. 7:1011–1014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ege B and Leventoğlu S: Primary
leiomyosarcoma of the thyroid. J Korean Surg Soc. 85:43–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanboon J and Keskool P: Leiomyosarcoma: A
rare tumor of the thyroid. Endocr Pathol. 24:136–143. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amal B, El Fatemi H, Souaf I, Moumna K and
Affaf A: A rare primary tumor of the thyroid gland: Report a new
case of leiomyosarcoma and literature review. Diagn Pathol.
8:362013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang TS, Ocal IT, Oxley K and Sosa JA:
Primary leiomyosarcoma of the thyroid gland. Thyroid. 18:425–428.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Just PA, Guillevin R, Capron F, Le
Charpentier M, Le Naour G, Menegaux F, Leenhardt L, Simon JM and
Hoang C: An unusual clinical presentation of a rare tumor of the
thyroid gland: Report on one case of leiomyosarcoma and review of
literature. Ann Diagn Pathol. 12:50–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Day AS, Lou PJ, Lin WC and Chou CC:
Over-expression of c-kit in a primary leiomyosarcoma of the thyroid
gland. Eur Arch Otorhinolaryngol. 264:705–708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takayama F, Takashima S, Matsuba H,
Kobayashi S, Ito N and Sone S: MR imaging of primary leiomyosarcoma
of the thyroid gland. Eur J Radiol. 37:36–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsugawa K, Koyanagi N, Nakanishi K, Wada
H, Tanoue K, Hashizume M and Sugimachi K: Leiomyosarcoma of the
thyroid gland with rapid growth and tracheal obstruction: A partial
thyroidectomy and tracheostomy using an ultrasonically activated
scalpel can be safely performed with less bleeding. Eur J Med Res.
4:483–487. 1999.PubMed/NCBI
|
|
12
|
Tulbah A, Al-Dayel F, Fawaz I and Rosai J:
Epstein-Barr virus-associated leiomyosarcoma of the thyroid in a
child with congenital immunodeficiency: A case report. Am J Surg
Pathol. 23:473–476. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozaki O, Sugino K, Mimura T, Ito K, Tamai
S and Hosoda Y: Primary leiomyosarcoma of the thyroid gland. Surg
Today. 27:177–180. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iida Y, Katoh R, Yoshioka M, Oyama T and
Kawaoi A: Primary leiomyosarcoma of the thyroid gland. Acta Pathol
Jpn. 43:71–75. 1993.PubMed/NCBI
|
|
15
|
Kawahara E, Nakanishi I, Terahata S and
Ikegaki S: Leiomyosarcoma of the thyroid gland. A case report with
a comparative study of five cases of anaplastic carcinoma. Cancer.
62:2558–2563. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaur A and Jayaram G: Thyroid tumors:
Cytomorphology of medullary, clinically anaplastic, and
miscellaneous thyroid neoplasms. Diagn Cytopathol. 6:383–389. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mouaqit O, Belkacem Z, Ifrine L, Mohsine R
and Belkouchi A: A rare tumor of the thyroid gland: Report on one
case of leiomyosarcoma and review of literature. Updates Surg.
66:165–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gauthé M, Testart Dardel N, Nascimento C,
Trassard M, Banal A and Alberini JL: Uterine leiomyosarcoma
metastatic to thyroid shown by 18F-FDG PET/CT imaging.
Rev Esp Med Nucl Imagen Mol. 36:113–115. 2017.(In English,
Spanish). PubMed/NCBI
|
|
19
|
Woo Young K, Young Ran K, Sang Uk W and
Jae Bok L: Pulmonary leiomyosarcoma metastatic to the thyroid
gland: Case report and review of the literature. Ann Endocrinol
(Paris). 72:314–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ippolito S, Bellevicine C, Arpaia D,
Peirce C, Ciancia G, Vigliar E, Troncone G and Biondi B: Spindle
epithelial tumor with thymus-like differentiation (SETTLE):
Clinical-pathological features, differential pathological diagnosis
and therapy. Endocrine. 51:402–412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papi G, Corrado S and LiVolsi VA: Primary
spindle cell lesions of the thyroid gland; an overview. Am J Clin
Pathol. 125 Suppl:S95–S123. 2006.PubMed/NCBI
|
|
22
|
Febrero B, Oviedo I, Ríos A and Rodríguez
JM: Primary rhabdomyosarcoma of the thyroid in an adult with
auricular thrombosis. Eur Ann Otorhinolaryngol Head Neck Dis.
134:49–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi RL, Qu N, Gao LL, Lu ZW, Sun GH and Ji
QH: Primary synovial sarcoma of the thyroid with locally repeated
relapses in short periods: A case report. Biomed Rep. 5:79–82.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pallares J, Perez-Ruiz L, Ros S, Panades
MJ, Pardo-Mindan J, Lloreta J and Matias-Guiu X: Malignant
peripheral nerve sheath tumor of the thyroid: A clinicopathological
and ultrastructural study of one case. Endocr Pathol. 15:167–174.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Postovsky S, Vlodavsky E, Kuten A,
Shendler Y, Doweck I and Ben Arush MW: Undifferentiated sarcoma of
the thyroid in a child. Pediatr Blood Cancer. 54:1038–1040.
2010.PubMed/NCBI
|
|
26
|
Baloch ZW and LiVolsi VA: Special types of
thyroid carcinoma. Histopathology. 72:40–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hakverdi S, Güngören A, Yaldiz M, Hakverdi
AU and Toprak S: Immunohistochemical analysis of p16 expression in
uterine smooth muscle tumors. Eur J Gynaecol Oncol. 32:513–515.
2011.PubMed/NCBI
|