Introduction
Different transcription factors serve various
functions in multiple physiological mechanisms, including cell
cycle progression, cell metabolism, growth and development
(1). Basic transcription factor 3
(BTF3) is involved in various biotic and abiotic stress processes,
as well as different physiological and developmental mechanisms
(2,3).
BTF3 is encoded by the human BTF3 gene and is
evolutionarily conserved in a range of organisms (4,5). BTF3 was
initially described as a member of the general transcription
machinery and forms a stable complex with the RNA polymerases
(6). BTF3 initiates transcription by
binding to promoter elements such as the TATA and CAAT box
sequences in the promoter region (7,8). Owing to
alternative splicing, BTF3 is present in two different isoforms:
BTF3a and BTF3b. BTF3a is the transcriptionally active form of
BTF3, whereas the BTF3b isoform, which lacks the 44 N-terminal
amino acids of BTF3a, is transcriptionally inactive, although it is
able to bind to RNA polymerase II (9).
A previous mouse embryonic development study
revealed that mice homozygous for a loss-of-function mutation in
the BTF3 gene succumbed early in development, indicating its
function in biological development (10). In addition to its functions as
transcription regulator, BTF3 also aids the regulation of the cell
cycle and apoptosis (11,12). Decreased BTF3 expression is associated
with increased apoptosis in lymphocytes (13), and downregulation of BTF3 inhibits
transcription and protein synthesis (14). In Caenorhabditis elegans,
overexpression of BTF3 prevents cell apoptosis, whereas the RNA
interference-mediated knockdown of BTF3 induces cell apoptosis
(11). In human cancer, BTF3 is
overexpressed in glioma (15),
hepatocarcinoma (16) and pancreatic
ductal adenocarcinoma (17).
Downregulation of BTF3 decreases the expression of several
cancer-associated genes, including ephrin receptor B2 (18), heparanase 2 (19) and the oncogene ABL proto-oncogene 2,
non-receptor tyrosine kinase (20).
In breast cancer, BTF3 interacts with either 17β-estradiol or
estrogen receptor α (ERα) through its AF1 domain and upregulates
the transcriptional response of ERα reporter genes (21,22). These
results suggest that BTF3 also serves an important function in
tumor occurrence and the development of tumor progression.
The molecular mechanism of BTF3 in colon cancer
remains unclear. In order to investigate the function of BTF3 in
colon cancer, the present study used a lentivirus-mediated short
hairpin RNA (shRNA) approach to target BTF3 to knockdown its
expression in human colon cancer cells. In addition, the effect of
BTF3-knockdown on cell proliferation, the cell cycle, cell
apoptosis and migration was investigated. The results of the
present study provide information on a novel molecular target for
the diagnosis and therapy of colon cancer.
Materials and methods
Cell culture
The colon cancer HCT116 and HT-29 cell lines, and
293 cells were purchased from the American Type Culture Collection
(Manassas, VA, USA). All cells were grown in complete Dulbecco's
medium Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), supplemented with 10% fetal bovine serum (HyClone;
GE Healthcare Life Sciences), and 100 U/ml penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Waltham, MA, USA),
and incubated at 37°C in a 5% CO2 atmosphere.
Plasmid and vector construction
The selected and optimized shRNA against human BTF3
(5′-GCAGCGAACACTTTCACCATT-3′) was transfected into the lentiviral
vector pLKO.1-pure (Addgene, Inc., Cambridge, MA, USA). The
constructed shBTF3 plasmid and control vector were further
co-transfected with Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.) into 293 cells with packaging plasmids (preserved in our lab)
to generate a shBTF3-expressing lentivirus or empty vector
lentivirus. Cells (1×106) were cultured in a 10-cm dish
and incubated at 37°C for 24 h. Control lentivirus and
BTF3-targeted shRNA lentivirus were then used to transfect the
HCT116 and HT-29 cells. Following selection in 2 µg/ml puromycin
(Thermo Fisher, Scientific, Inc.) for 1 week, cells were collected
to evaluate the knockdown efficiency.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BTF3-knockdown cell
lines (those transfected with BTF3-targeted shRNA), the empty
vector control cell line (Vector) and non-transfected cells using
an RNeasy mini kit (Qiagen China Co., Ltd., Shanghai, China),
according to the manufacturer's protocol. cDNA was generated by
reverse transcription of 1-µg aliquots of RNA using the Takara
PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. The cDNA was used
for qPCR using the SYBR Premix Ex-Taq kit (Takara Biotechnology
Co., Ltd.) on a CFX96 instrument qPCR system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The PCR was performed according to the
manufacturer's instructions: Initial denaturation was at 95°C for
10 min followed by 30 cycles at 95°C for 1 min, annealing at 53°C
for 1 min, extension at 72°C for 1 min, and final extension at 72°C
for 5 min. All expression data were normalized to β-actin levels
using 2−ΔΔCq method (23).
Primer sequences were as follows: β-actin, 5′-CGAGCGCGGCTACAGCT-3′
(forward) and 5′-TCCTTAATGTCACGCACGATTT-3′ (reverse); and BTF3
5′-AGCTTGGTGCGGATAGTCTGA-3′ (forward) and
5′-GTGCTTTTCCATCCACAGATTG-3′ (reverse).
Western blotting
Cells were lysed in radioimmunoprecipitation buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA), containing
Complete protease inhibitors (Roche Applied Science, Penzberg,
Germany), phosphatase inhibitors (Roche Applied Science), 5 mM
dithiothreitol (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck KGaA) for
15 min, and then centrifuged at 15,000 × g for 10 min at 4°C. The
supernatant was collected and protein concentration was quantified
using the Bio-Rad Protein assay kit II #5000002 (Bio-Rad
Laboratories, Inc.). The proteins were separated by SDS-PAGE (10%
gel), blotted onto a polyvinylidene fluoride membrane (Merck KGaA),
and then blocked for 1 h at room temperature in TBST with 2%
non-fat milk, followed by overnight incubation at 4°C with
anti-BTF3 (Abnova, Taipei, Taiwan; Catalog no. H00000689-R01) or
anti-β-actin (Cell Signaling Technology, Inc.; Catalog no. 3700)
antibodies (1:1,000). Membranes were rinsed with Tris-buffered
saline containing Tween-20 (TBST) with 0.05% Tween-20 and incubated
for 2 h with a 1:5,000 diluted HRP-conjugated goat anti-human IgG
secondary antibody (Thermo Fisher Scientific, Inc.; Catalog no.
A18847). Following another three washes with TBST, target proteins
were detected using an Enhanced Chemiluminescence detection kit
(Pierce; Thermo Fisher Scientific, Inc.). Quantification of the
western blot analysis was performed using ImageJ software v1.8.0
(National Institutes of Health, Bethesda, MD, USA).
MTT assay
The shBTF3 shBTF3-expressing lentivirus or empty
vector lentivirus was transfected into HCT116 and HT-29 cells the
knockdown efficiency were examined. HCT116 and HT-29 cells were
seeded at 3×103 cells per well at 37°C in 96-well plates
and cell viability was assessed every day for 4 days. In brief, at
the start of each assay, 20 µl MTT (with 5 mg/ml thiazolyl blue
tetrazolium bromide; Sigma-Aldrich; Merck KGaA) was added to each
well and then the plate was incubated for an additional 4 h.
Culture medium was removed from the wells, 100 µl dimethylsulfoxide
(Sigma-Aldrich; Merck KGaA) was added to each well and plates were
incubated for 20 min at 37°C. Absorbance was determined using a
microplate reader (Bio-Rad Laboratories, Inc.) at a wavelength of
495 nm. Each experiment was performed three times.
Transwell assay
The cell migration assay was carried out using a
modified Boyden chamber plate with 8-µm pore size polycarbonate
membrane filters (Corning Incorporated, Corning, NY, USA) (24). ShBTF3- and empty vector-transfected
HTC116 and HT-29 cells, along with non-transfected HTC116 and HT-29
cells, were incubated at 37°C in DMEM for 6 h. Subsequently,
1×104 cells from each group and cell type were added to
the upper part of the Boyden chamber, and the bottom chamber was
filled with DMEM containing 20% serum. The cells were allowed to
migrate to the underside of the membrane during incubation for 48 h
at 37°C. Next, the cells on the membrane filter were fixed with 4%
paraformaldehyde and stained with 0.05% Giemsa (Sigma-Aldrich;
Merck KGaA). The migration index was defined as the number of cells
that had migrated to the membrane filter by cell counting in at
least three random fields using a light microscope (magnification,
×200) per filter.
Flow cytometric assays
Cells were harvested using trypsin/EDTA (Gibco;
Thermo Fisher Scientific, Inc.) from 6-cm-diameter dishes.
Following centrifugation 1,200 × g for 15 min at 4°C, pellets were
then washed with PBS and centrifuged again. Following resuspension
in binding buffer (10 mmol/l HEPES, 140 mmol/l NaCl, 5 mmol/l
CaCl2), cells were incubated with annexin V and
propidium iodide (PI) according to the manufacturer's protocol
(BioVision, Inc., Milpitas, CA, USA). Cells were analyzed using a
FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and FlowJo software v10.0 (Tree Star, Inc., Ashland, OR, USA).
For cell cycle analysis, cells were washed twice with ice-cold PBS
and fixed using 70% ethanol overnight at 4°C. The cells were then
digested with 50 µg/ml RNase A in 100 µl PBS and stained with 20
µg/ml PI for 30 min at 37°C. Cells were then analyzed using a
FACSCanto II flow cytometer (BD Biosciences) and FlowJo
software.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical comparisons were made using one-way analysis of
variance with a least significant difference post hoc test or an
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was performed
at least three times.
Results
Lentivirus-mediated knockdown of BTF3
in human colon cancer cells
In order to investigate the function of BTF3 in
colon cancer, a lentivirus vector was used to generate
BTF3-knockdown stable HCT116 and HT-29 cell lines. Following
selection in puromycin for 1 week and once stable cell
proliferation was achieved, qPCR and western blot analysis were
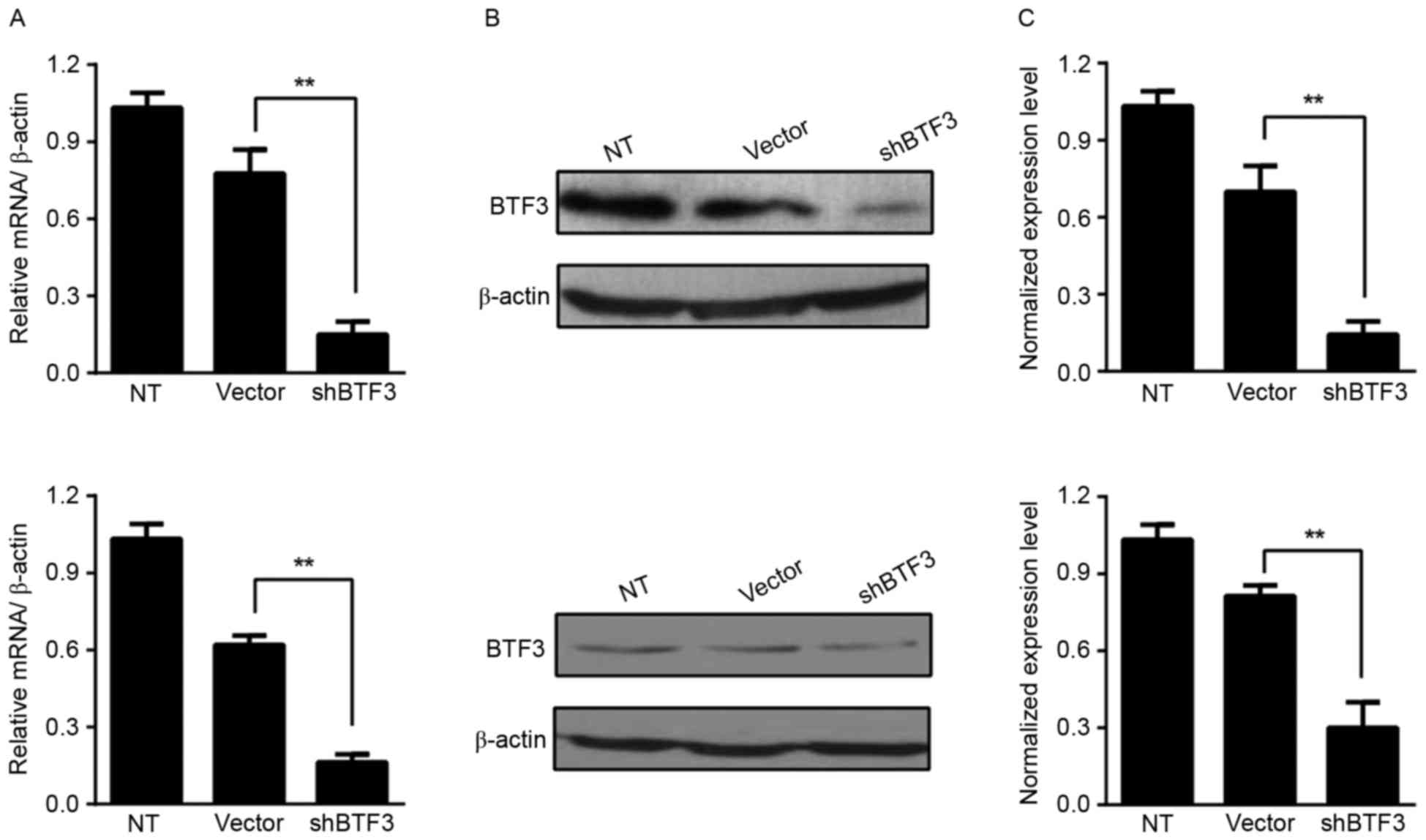
performed to assess the efficiency of BTF3-knockdown. As presented
in Fig. 1A, expression of BTF3 mRNA
in HCT116 cells transfected with shBTF3 lentivirus was 80% lower
compared with that in non-transfected cells and 60% lower compared
with that in cells transfected with the empty vector control
(P<0.01). Similar results were obtained in the HT-29 cell line
(Fig. 1A). Comparing BTF3-knockdown
and empty vector-treated cells, the BTF3 protein level appeared to
be markedly decreased in HCT116 and HT-29 cells (Fig. 1B). The semi-quantitative results of
western blot densitometry analysis revealed that there was a
significant difference in the BTF3 protein expression level between
shBTF3 and empty vector-treated cells (P<0.01; Fig. 1C).
Knockdown of BTF3 inhibits
proliferation of colon cancer cells
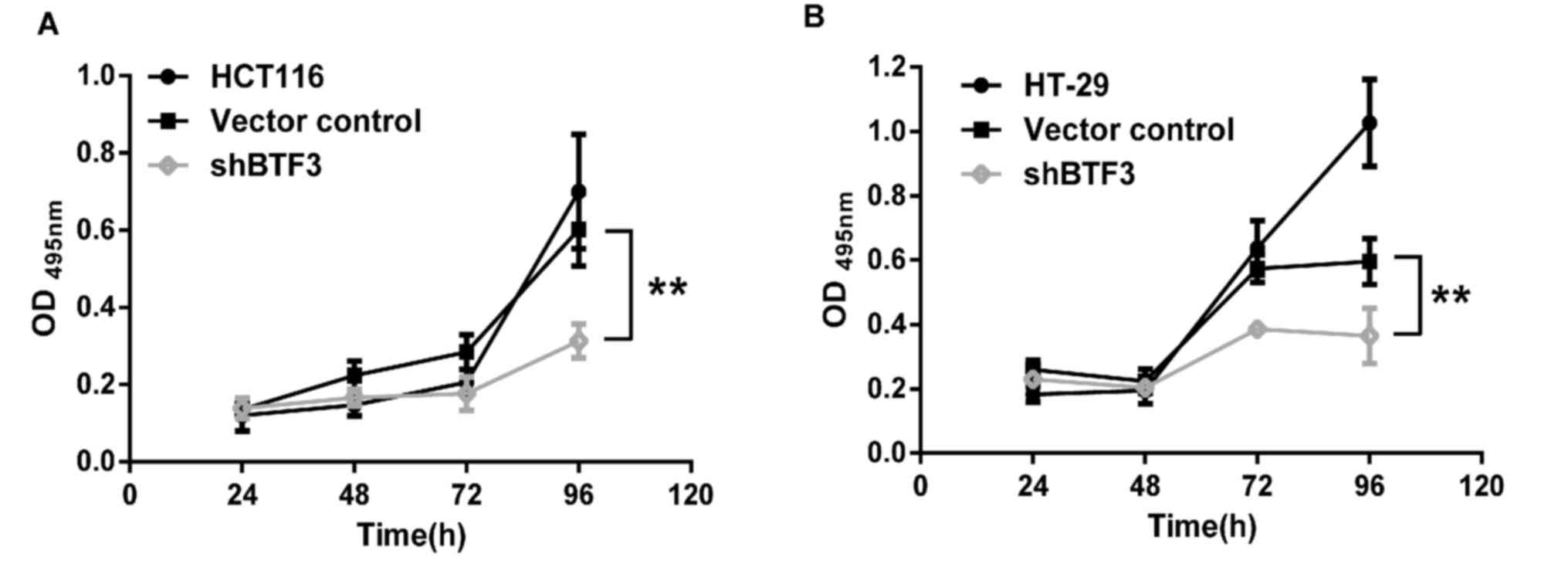
To assess the effect of BTF3 on colon cancer cell
proliferation, BTF3-knockdown, empty vector control- and
non-transfected HCT116 and HT-29 cells were seeded in
96-well-plates and a series of MTT assays were performed. The
results of these assays revealed that cell proliferation in
BTF3-knockdown HCT116 and HT-29 cells was significantly decreased
over the course of the 4 days, compared with controls (P<0.01;
Fig. 2A and B). These results suggest
that BTF3 knockdown significantly decreased the proliferation of
colon cancer cells.
BTF3 knockdown induces early apoptosis
and arrests cells in G0-G1 phase
To explore further the mechanism by which BTF3
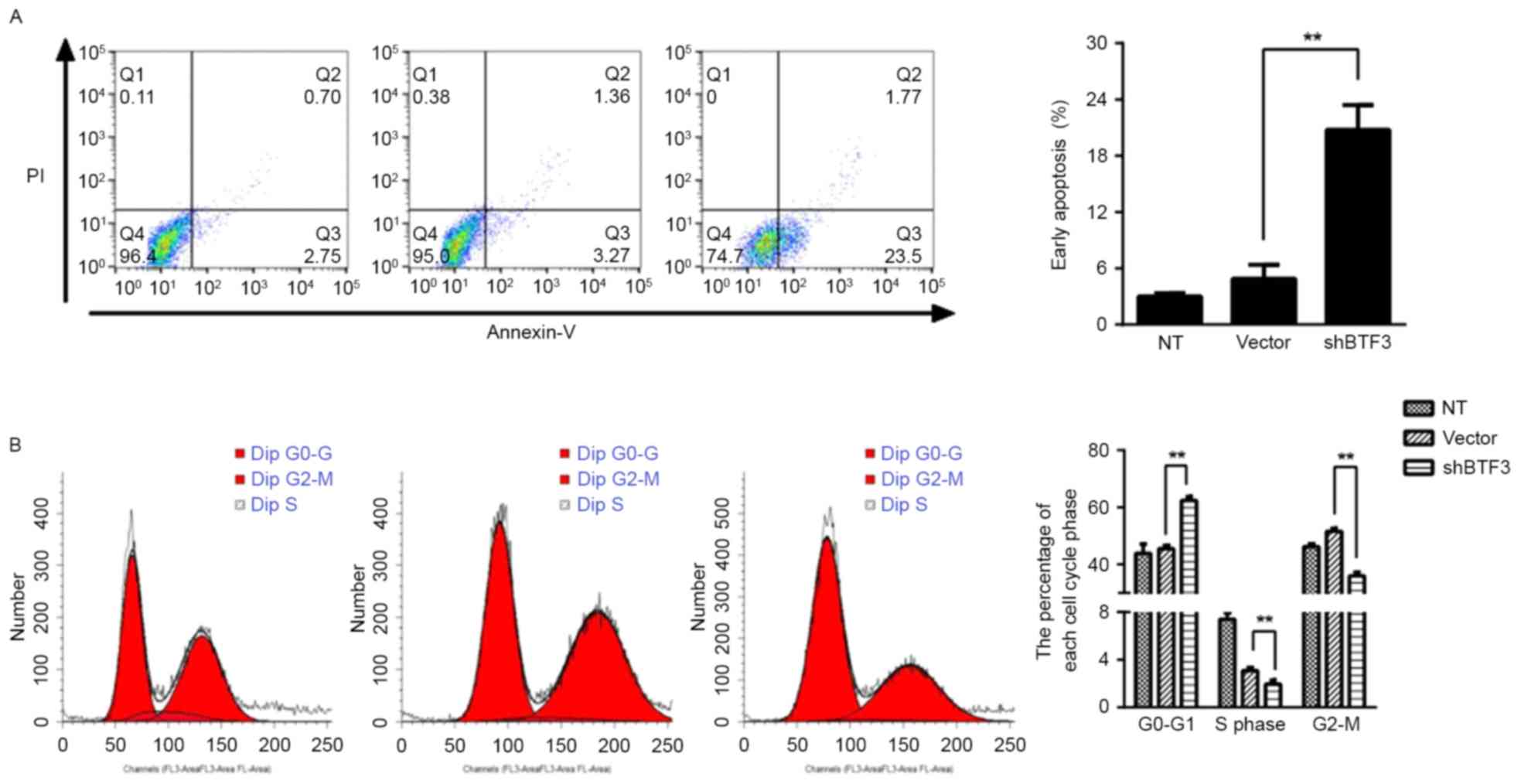
knockdown inhibits cell proliferation, cell apoptosis was examined
through annexin V/PI double staining for apoptotic cells. There was
a significant increase in early apoptosis (annexin V-positive,
PI-negative) in BTF3-knockdown compared with NT (non-transfected)
control cells, from 4.42±1.33 to 25.13±1.65% (P<0.01; Fig. 3). This result indicated that BTF3 may
be involved in colon cancer cell survival. In order to determine
the function of BTF3 in cell-cycle progression, flow cytometry was
used to assess the changes in cell cycle distribution prior to and
following BTF3-knockdown in HTC116 cells. In vector control cells,
the cell distribution was: G0-G1 phase,
45.52±1.09%; S phase, 3.08±0.27%; G2-M phase,
51.58±1.06%. Following BTF3 knockdown, cell distribution altered
to: G0-G1 phase, 62.41±1.33%; S phase,
1.95±0.32%; G2-M phase, 36.01±1.21% (Fig. 3B). Comparing the control and
BTF3-knockdown cells revealed that cell distribution in the
G0-G1 phase was significantly increased by
BTF3-knockdown (P<0.01), and the proportion of cells in S and
G2-M phase was significantly decreased (P<0.01).
These results indicated that BTF3 knockdown may be involved in
cell-cycle regulation as the BTF3-knockdown cells were arrested at
G0-G1 phase, decreasing the proportion of
cells undergoing mitosis and therefore inhibiting cell
proliferation.
Knockdown of BTF3 inhibits colon
cancer cell migration
Cell proliferation and migration are two
characteristics of tumor cells. The aforementioned results
indicated that BTF3 knockdown is able to inhibit the proliferation
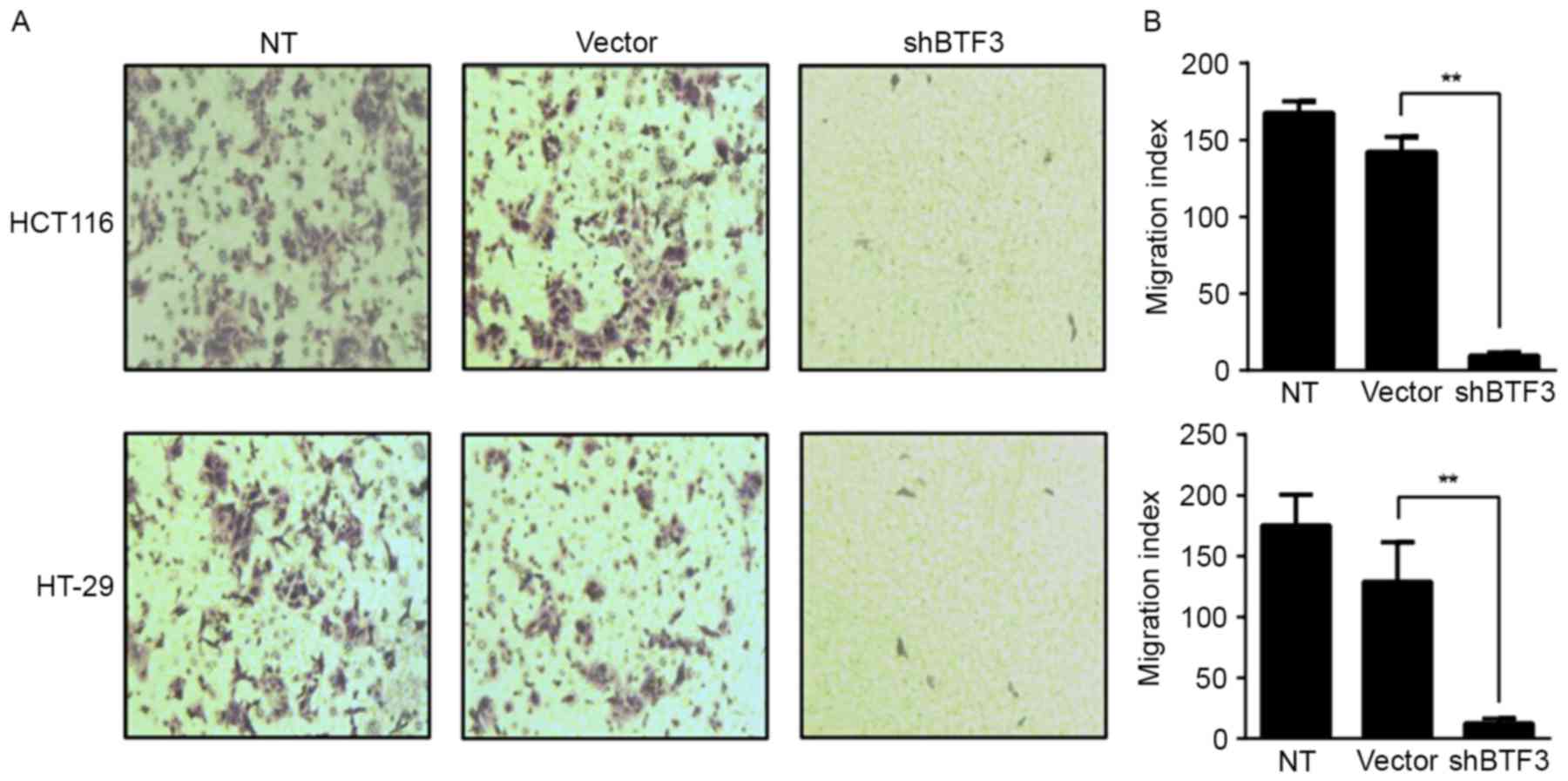
of colon cancer cells. Whether BTF3 knockdown alters cell migration
was assessed using a Transwell assay. A change in cell migratory
ability was observed following BTF3 knockdown in HTC116 and HT-29
cells (Fig. 4A). Compared with empty
vector-transfected control cells, the migration index of
BTF3-knockdown cells decreased significantly by 79.7 and 66.3%, in
HTC116 and HT-29, respectively (P<0.01; Fig. 4B). These results indicated that BTF3
knockdown may significantly inhibit the migration of colon cancer
cells.
Discussion
BTF3, which functions as an additional transcription
factor II-associated protein, does not bind to proximal promoter
regions directly, but forms a stable complex with RNA polymerase II
and is a part of the gene transcription initiation complex
(4,7).
Several studies have indicated that the expression pattern of BTF3
is frequently disordered in tumors (13,17). On
the other hand, BTF3 is also highly expressed in malignant tissue
and affects the invasion and proliferation of tumor cells, which
indicates that BTF3 may serve as an independent diagnostic factor
(25). By analyzing clinical data
from colorectal cancer patients, Wang et al (26) identified that BTF3 overexpression may
be an early event in colon cancer and may be a useful biomarker for
early-stage colon cancer. In addition, BTF3 expression is
associated with the expression of nuclear factor κB, RAD50
double-strand break repair protein, MRE11 homolog, double-strand
break repair nuclease, nibrin and metadherin (26). This change in BTF3 expression may
affect these signaling pathways, although the molecular mechanism
of BTF3 in colon cancer remains unknown.
A shRNA targeting BTF3 was lentivirally transfected
into HCT116 and HT-29 human colon cancer cells. The results
demonstrated that knockdown of BTF3 significantly inhibited the
proliferation of colon cancer cells, and the amount of early
apoptotic cells was significantly increased. The cell-cycle
distribution of BTF3-knockdown cells was also altered. The
percentage of cells in G0-G1 phase was
significantly increased in BTF3-knockdown colon cancer cells,
whereas those in S and G2-M phases was significantly
decreased, which indicated that BTF3-knockdown cells underwent
cell-cycle arrest in interphase G0-G1. As a
result of this arrest, cells could not enter mitosis and early
apoptosis was induced, thus leading to a decrease in the
proliferation of colon cancer cells. Previous studies revealed that
regulation of apoptosis is associated with cell-cycle regulation
(27,28). Although apoptosis may be induced at
any point in the cell cycle, the propensity for apoptosis to be
induced differs markedly depending location of the cell within the
cell cycle (29). Progression through
the cell cycle is also subject to a number of regulatory proteins.
For example, the progression through G1 phase depends on
the balance of cyclin D1 and cyclin-dependent kinase inhibitor 2A
expression, owing to their identity as positive and negative
regulators of progression through G1 phase, respectively
(30). Therefore, whether BTF3 caused
cells to arrest at G0-G1 phase owing to its
regulation of cyclins warrants further research.
BTF3 also has an important function in other types
of cancer. Liu et al (31)
identified that BTF3 is potentially associated with the development
and progression of gastric cancer. BTF3 is expressed at different
levels in different stages of gastric cancer; low expression or
gene silencing of BTF3 inhibited tumor growth and may be beneficial
for gastric cancer treatment (31).
In pancreatic ductal carcinoma, overexpression of BTF3 may be
involved in cell-cycle progression, cell proliferation and
extracellular matrix degradation (17). Using an immunohistochemical tissue
array for the diagnosis and stratification of prostate cancer,
Symes et al (32) identified
that BTF3 expression was significantly upregulated in malignant
prostate cancer tissue compared with non-malignant tissue.
Therefore, BTF3 has the potential to be used as a specific
molecular marker for the diagnosis and stratification of prostate
cancer (32). The results of the
present study indicated that, in colon cancer cells, BTF3 knockdown
inhibited cell proliferation and promoted early apoptosis,
suggesting an association between BTF3 expression and colon
cancer.
In conclusion, the results of the present study shed
light on the biological function of BTF3 in colon cancer. The
results of the present study demonstrated that BTF3 knockdown is
able to inhibit the proliferation of colon cancer cells, suggesting
that BTF3 may promote the occurrence of colon cancer. BTF3 may
therefore serve as a biomarker for the diagnosis of colon cancer
and provide a molecular target for tumor gene therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81702297).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL carried out the design of the study, drafted the
manuscript. JS conducted the cellular function experiments. JX
performed molecular genetics examination. FC carried out the
statistical analysis. HW helped the design the study and draft the
manuscript. CF and HTW conceived the study, and participated in its
design and coordination. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Hussain SS, Kayani MA and Amjad M:
Transcription factors as tools to engineer enhanced drought stress
tolerance in plants. Biotechnol Prog. 27:297–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang W, Xu M, Wang Y and Jamil M: Basal
transcription factor 3 plays an important role in seed germination
and seedling growth of rice. Biomed Res Int.
2014:4657392014.PubMed/NCBI
|
|
3
|
Wang Y, Zhang X, Lu S, Wang M, Wang L,
Wang W, Cao F, Chen H, Wang J, Zhang J and Tu J: Inhibition of a
basal transcription factor 3-like gene Osj10gBTF3 in rice results
in significant plant miniaturization and typical pollen abortion.
Plant Cell Physiol. 53:2073–2089. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng XM, Moncollin V, Egly JM and Chambon
P: A general transcription factor forms a stable complex with RNA
polymerase B (II). Cell. 50:361–368. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cavallini B, Faus I, Matthes H, Chipoulet
JM, Winsor B, Egly JM and Chambon P: Cloning of the gene encoding
the yeast protein BTF1Y, which can substitute for the human TATA
box-binding factor. Proc Natl Acad Sci USA. 86:9803–9807. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng XM, Black D, Chambon P and Egly JM:
Sequencing and expression of complementary DNA for the general
transcription factor BTF3. Nature. 344:556–559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cavallini B, Huet J, Plassat JL, Sentenac
A, Egly JM and Chambon P: A yeast activity can substitute for the
HeLa cell TATA box factor. Nature. 334:77–80. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno M, Chalut C and Egly JM: Genomic
structure of the putative BTF3 transcription factor. Gene.
117:219–228. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parvin JD, Shykind BM, Meyers RE, Kim J
and Sharp PA: Multiple sets of basal factors initiate transcription
by RNA polymerase II. J Biol Chem. 269:18414–18421. 1994.PubMed/NCBI
|
|
10
|
Deng JM and Behringer RR: An insertional
mutation in the BTF3 transcription factor gene leads to an early
postimplantation lethality in mice. Transgenic Res. 4:264–269.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bloss TA, Witze ES and Rothman JH:
Suppression of CED-3-independent apoptosis by mitochondrial betaNAC
in Caenorhabditis elegans. Nature. 424:1066–1071. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiede B, Dimmler C, Siejak F and Rudel T:
Predominant identification of RNA-binding proteins in Fas-induced
apoptosis by proteome analysis. J Biol Chem. 276:26044–26050. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brockstedt E, Otto A, Rickers A, Bommert K
and Wittmann-Liebold B: Preparative high-resolution two-dimensional
electrophoresis enables the identification of RNA polymerase B
transcription factor 3 as an apoptosis-associated protein in the
human BL60-2 Burkitt lymphoma cell line. J Protein Chem.
18:225–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li R, Liu XL, Du QF, Zhang S, Luo RC and
Zhou SY: Proteome analysis of apoptotic K562 cells induced by
harringtonine. Zhonghua Xue Ye Xue Za Zhi. 25:323–327. 2004.(In
Chinese). PubMed/NCBI
|
|
15
|
Odreman F, Vindigni M, Gonzales ML,
Niccolini B, Candiano G, Zanotti B, Skrap M, Pizzolitto S, Stanta G
and Vindigni A: Proteomic studies on low- and high-grade human
brain astrocytomas. J Proteome Res. 4:698–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roy L, Laboissiere S, Abdou E, Thibault G,
Hamel N, Taheri M, Boismenu D, Lanoix J, Kearney RE and Paiement J:
Proteomic analysis of the transitional endoplasmic reticulum in
hepatocellular carcinoma: An organelle perspective on cancer.
Biochim Biophys Acta. 1804:1869–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kusumawidjaja G, Kayed H, Giese N, Bauer
A, Erkan M, Giese T, Hoheise JD, Friess H and Kleeff J: Basic
transcription factor 3 (BTF3) regulates transcription of
tumor-associated genes in pancreatic cancer cells. Cancer Biolo
Ther. 6:367–376. 2007. View Article : Google Scholar
|
|
18
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Surawska H, Ma PC and Salgia R: The role
of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev.
15:419–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH,
Wu LF, Zhu AL, Wang XQ and Wu M: Profiling of differentially
expressed genes in human gastric carcinoma by cDNA expression
array. World J Gastroenterol. 8:580–585. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Green CD, Thompson PD, Johnston PG and
El-Tanani MK: Interaction between transcription factor, basal
transcription factor 3, and the NH2-terminal domain of human
estrogen receptor alpha. Mol Cancer Res. 5:1191–1200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
el-Tanani MK and Green CD: Transcription
factor, BTF3, and the AF-1 function of the estrogen receptor.
Biochem Soc Trans. 26:S2521998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu HH, Kuo WW, Ju DT, Yeh YL, Tu CC, Tsai
YL, Shen CY, Chang SH, Chung LC and Huang CY: Estradiol agonists
inhibit human LoVo colorectal-cancer cell proliferation and
migration through p53. World J Gastroenterol. 20:16665–16673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Zhu ZG, Ji J, Yuan F, Yu YY, Liu
BY and Lin YZ: Transcription factor Sp1 expression in gastric
cancer and its relationship to long-term prognosis. World J
Gastroenterol. 11:2213–2217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang CJ, Franbergh-Karlson H, Wang DW,
Arbman G, Zhang H and Sun XF: Clinicopathological significance of
BTF3 expression in colorectal cancer. Tumour Biol. 34:2141–2146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meikrantz W and Schlegel R: Suppression of
apoptosis by dominant negative mutants of cyclin-dependent protein
kinases. J Biol Chem. 271:10205–10209. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gil-Gomez G, Berns A and Brady HJ: A link
between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2
activation during thymocyte apoptosis. EMBO J. 17:7209–7218. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leach SD, Scatena CD, Keefer CJ, Goodman
HA, Song SY, Yang L and Pietenpol JA: Negative regulation of Wee1
expression and Cdc2 phosphorylation during p53-mediated growth
arrest and apoptosis. Cancer Res. 58:3231–3236. 1998.PubMed/NCBI
|
|
30
|
Hara K, Ueda S, Ohno Y, Tanaka T, Yagi H,
Okazaki S, Kawahara R, Masayuki T, Enomoto T, Hashimoto Y, et al:
NIH3T3 cells overexpressing CD98 heavy chain resist early G1 arrest
and apoptosis induced by serum starvation. Cancer Sci.
103:1460–1466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Q, Zhou JP, Li B, Huang ZC, Dong HY,
Li GY, Zhou K and Nie SL: Basic transcription factor 3 is involved
in gastric cancer development and progression. World J
Gastroenterol. 19:4495–4503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Symes AJ, Eilertsen M, Millar M, Nariculam
J, Freeman A, Notara M, Feneley MR, Patel HR, Masters JR and Ahmed
A: Quantitative analysis of BTF3, HINT1, NDRG1 and ODC1 protein
over-expression in human prostate cancer tissue. PLoS One.
8:e842952013. View Article : Google Scholar : PubMed/NCBI
|