Introduction
Primary liver cancer is one of the most common
malignant tumors in the world, with particularly high incidence
rates in Asia and Africa (1,2). It is the third leading cause of
cancer-associated mortality worldwide (3). The major histopathologic types of liver
cancer include hepatocellular carcinoma (HCC) and intrahepatic
cholangiocellular carcinoma (ICC) (2,4). Although
various methods have been implemented to improve the survival of
patients with liver cancer, including surgical resection, liver
transplantation, transarterial chemoembolization, local ablation,
radiotherapy, chemotherapy and molecular targeted drug treatment,
the prognosis remains poor, particularly for ICC (5–8).
ICC is the second most common malignancy worldwide
and accounts for 15–20% of primary liver cancer (9). Due to its rising incidence and poor
prognosis, research into ICC is receiving increasing attention
(10,11). Surgical resection remains one of the
most effective ways to treat ICC (12,13).
However, the clinical outcomes are limited due to the high
recurrence and metastasis rates following operation (14). Thus, effective indicators that may
predict the prognosis of ICC are essential for the treatment of
these patients.
Previous studies have demonstrated that inflammation
serves an important role in the development and progression of
liver cancer (15,16). It has previously been demonstrated
that a number of inflammation-based indicators in the blood are
associated with the prognosis of HCC, such as the Glasgow
Prognostic Score (GPS), the systemic inflammation score, the
neutrophil to lymphocyte ratio (NLR), the lymphocyte to monocyte
ratio (LMR), the platelet-to-lymphocyte ratio (PLR), prognostic
nutritional index (PNI), prognostic index and red cell distribution
width (RDW) (3,17–19). In
addition, there are various serum enzyme-associated parameters that
have been found to be associated with the clinical outcomes of ICC,
including the gamma-glutamyltransferase to platelet ratio (GPR),
albumin (ALB) to alkaline phosphatase ratio (APPR),
γ-glutamyltransferase to alanine aminotransferase ratio (GAR) and
the ALB to γ-glutamyltransferase ratio (AGR) (20,21).
However, few studies have considered the prognostic significance of
serum inflammatory-based indicators for ICC.
The aims of the present retrospective analysis were
to investigate the association between inflammation-based
prognostic indicators and the survival of patients undergoing
curative surgical resection for ICC.
Materials and methods
Patients
For this retrospective cohort study, 221 patients
who were pathologically diagnosed with ICC in the First Affiliated
Hospital of Xi'an Jiaotong University (Xi'an, China) between
September 2008 and July 2017 were retrospectively recruited.
Patients with active hepatitis, parasitic infection, acute
cholangitis or other malignant tumors were excluded. Ultimately,
123 patients following curative resection were enrolled into the
study. The institutional ethics committee at the study center
approved this study. All participants gave consent after being
fully informed of the goal and characteristics of this
research.
Treatment and follow-up
Blood tests and computed tomography (CT) scans were
routinely performed as preoperative tests within 3 days prior to
surgery. The clinical staging was based on The American Joint
Committee on Cancer (AJCC) 8th Edition Cancer Staging System
(22). Each patient was followed-up
at least every 2 months following hospital discharge during the
first year and every 3 months thereafter. The final follow-up date
was September 30th, 2017.
Demographics and clinical
characteristic data
All clinical data were collected from the patients'
medical records in the department of Hepatobiliary Surgery of the
First Affiliated Hospital of Xi'an Jiaotong University. Clinical
data included age, gender, tumor size, number of nodules and
presence or absence of vascular invasion. Furthermore, preoperative
biochemical indices were measured, including white blood cell
counts (WBC), platelet counts (PLT), neutrophil counts, lymphocyte
counts, megakaryocyte counts, RDW, α-fetoprotein (AFP) levels,
alanine transaminase (ALT) levels, aspartate aminotransferase (AST)
levels, total bilirubin (TBIL) levels, indirect bilirubin (IBIL)
levels, alkaline phosphatase (ALP) levels, ALB levels, hepatitis B
surface antigen (HbsAg) levels and hepatitis B virus
deoxyribonucleic acid (HBV-DNA) load. NLR was defined as the
neutrophil count/lymphocyte count ratio; dNLR was calculated by
neutrophil count/(WBC-neutrophil counts) ratio; LMR was defined as
the lymphocyte count/megakaryocyte count ratio; PLR was defined as
the platelet count/lymphocyte count ratio; PNI was defined as ALB +
(5× lymphocyte count). The primary endpoints of this study were
overall survival time (OS) and disease-free survival time (DFS). OS
was defined as the time between radical surgery and mortality. DFS
was defined as the time between radical surgery and tumor
recurrence.
Statistical methods
Statistical analyses were performed using SPSS
version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous data are
presented as the mean ± standard deviation and were compared using
a unpaired Student's t-test or one-way analysis of variance for
normal distribution data with Fisher's LSD post hoc test for the
comparison of among different groups, Kruskal-Wallis test was used
for multi-group comparison of abnormal distribution. The
categorical variables were compared using a χ2 test and
a Fisher's exact test. The diagnostic accuracy of all the
indicators was determined using receiver operating characteristic
(ROC) curve analysis. Indicators that displayed significance ere
chosen for the next part of the study and the Youden's index was
applied to determine the optimal cut-off values (22). Patients were divided into different
groups according to these aforementioned cut-off values. Univariate
analysis of variables associated with survival was performed using
log-rank testing to evaluate clinical factors associated with OS.
Multivariate analysis was performed using Cox proportional hazards
regression modelling using backward elimination and likelihood
ratio testing, and the included variables were those which had
significant associations with OS, determined by the univariate
analysis. The inflammation-based scoring system was defined as
follows: Patients with high NLR and high LMR were assigned a score
of 2; patients with high NLR and low LMR or low NLR and high LMR
were assigned as score of 1; patients who had low NLR and LMR were
assigned a score of 0. Patients were grouped according to this
score. The Kaplan-Meier method was used to analyze the long-term
effect of the different groups and these were compared using the
log-rank test. Finally, nomograms were used to validate the
outcomes. Nomograms for possible prognostic factors associated with
OS were using R software 3.4.0 (Institute for Statistics and
Mathematics, Vienna, Austria). and the model performance for
predicting outcome was evaluated by Harrell's concordance index
(c-index), as previously described (23). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients' characteristics
A total of 123 patients were recruited to the
present study, who had been pathologically diagnosed with ICC and
undergone radical resection between September 2008 and July 2017,
including 67 males and 56 females. The mean age of the patients was
56.80±10.67 (29–79) years old. The final follow-up date was
September 30th, 2017. The median follow-up time was 29.1 months
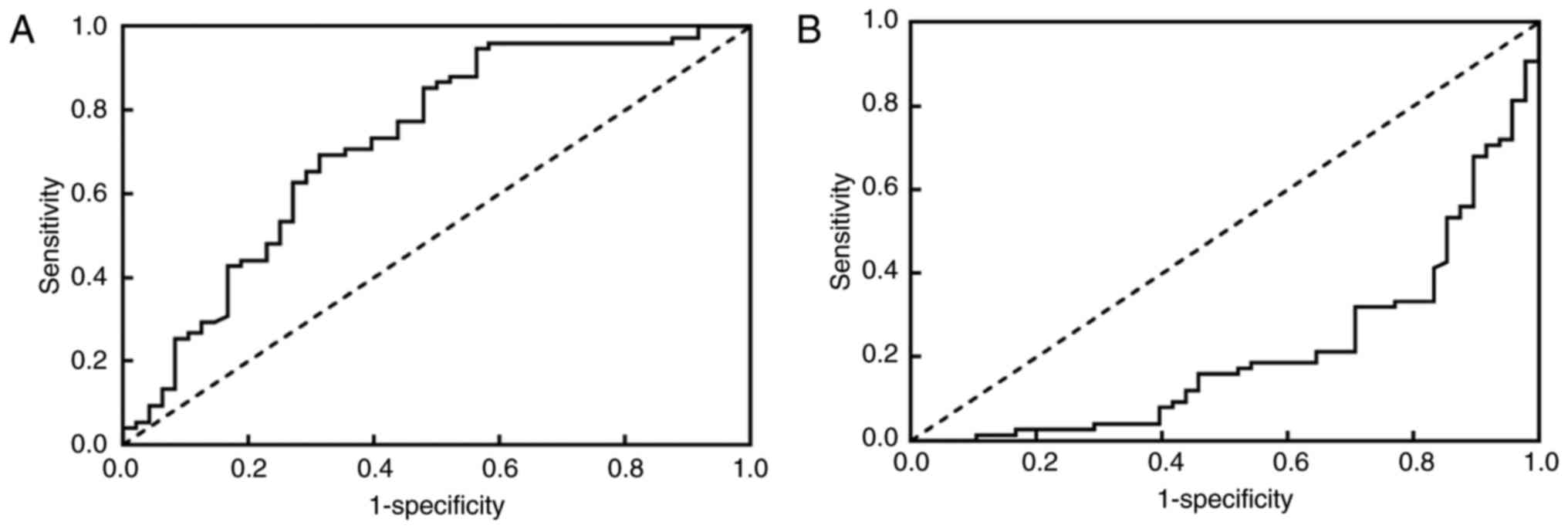
(2–64 months). Following analysis with the Area Under the ROC curve
(AUROC), NLR and LMR only were found to be significantly associated
with the OS of patients (Fig. 1, data
for indictors which were not associated with OS not presented). The
optimal cut-off value of OS for LMR and NLR was 3.42 and 2.05,
respectively.
According to the cut-off value of NLR and LMR, the
cohort was divided into lower and higher groups as presented in
Table I. Higher NLR was observed to
be associated with a higher ratio of male patients, larger tumor
diameter, higher lymph node metastasis rate and increased white
blood cell and megakaryocyte counts. Lower LMR was associated with
larger tumor diameter, higher lymph node metastasis rate, increased
numbers of patients at tumor stage 3–4 (T3-4) and Tumor, Node,
Metastasis (TNM) stage 3–4, lower ALB levels and increased
megakaryocyte counts.
 | Table I.Associations between clinical
characteristics of NLR and LMR. |
Table I.
Associations between clinical
characteristics of NLR and LMR.
| Parameter | NLR≤2.05
(n=25) | NLR>2.05
(n=98) | P-value | LMR≤3.42
(n=57) | LMR>3.42
(n=66) | P-value |
|---|
| Age,
yearb | 56.08±11.81 | 56.99±10.42 | 0.705 | 56.58±11.06 | 57.00±10.41 | 0.828 |
| Sex
(male/female) | 8/17 | 59/39 | 0.011a | 32/25 | 35/31 | 0.730 |
| BMI, kg/m2
b | 23.00±4.24 | 23.13±3.70 | 0.878 | 22.50±3.91 | 23.63±3.64 | 0.101 |
| Tumor diameter,
cmb | 4.81±2.63 | 6.46±3.18 | 0.018a | 6.86±3.18 | 5.48±2.98 | 0.015a |
| Differentiated
(well/poorly) | 17/8 | 53/45 | 0.152 | 28/29 | 42/24 | 0.105 |
| Incisal margin
(negative/positive) | 13/12 | 63/35 | 0.259 | 34/23 | 42/24 | 0.650 |
| N (−/+) | 22/3 | 53/45 | 0.002a | 25/32 | 50/16 | 0.000a |
| T (1–2/3-4) | 17/8 | 51/47 | 0.152 | 25/32 | 43/23 | 0.018a |
| TNM stage
(I–II/III–IV) | 11/14 | 27/71 | 0.112 | 10/47 | 28/38 | 0.003a |
| Vascular invasion
(absent/present) | 18/7 | 63/35 | 0.468 | 34/23 | 47/19 | 0.177 |
| WBC count,
×103/mlb | 5.75±2.23 | 7.19±2.71 | 0.015a | 7.37±2.86 | 6.49±2.47 | 0.071 |
| Platelet count,
×103/mlb | 178.96±83.70 | 198.01±93.65 | 0.356 | 201.40±82.81 | 187.86±98.96 | 0.416 |
| Albumin,
g/dlb | 39.05±5.14 | 38.07±5.83 | 0.455 | 36.58±5.21 | 39.73±5.72 | 0.002a |
| Neutrophil count,
×103/mlb | 6.51±14.33 | 4.82±2.18 | 0.562 | 5.13±2.46 | 5.20±8.86 | 0.952 |
| Lymphocyte count,
×103/mlb | 2.38±5.23 | 1.47±0.52 | 0.391 | 1.36±0.45 | 1.91±3.23 | 0.203 |
| Megakaryocyte
count, ×103/mlb | 0.33±0.17 | 0.48±0.21 | 0.001a | 0.54±0.22 | 0.37±0.17 | 0.000a |
| TBIL,
mmol/lb | 32.92±57.42 | 38.12±68.62 | 0.728 | 37.57±65.67 | 36.62±67.36 | 0.937 |
| AST,
U/lb | 66.62±61.00 | 133.21±659.63 | 0.616 | 163.25±798.85 | 69.23±106.15 | 0.380 |
| ALT,
U/lb | 68.20±68.92 | 131.17±545.37 | 0.567 | 152.14±652.15 | 79.28±148.61 | 0.411 |
| AFP,
ng/mlb | 12.34±40.56 | 31.37±157.13 | 0.551 | 3.16
(1.21–1,440) | 3.11(1.22–420) | 0.563 |
| CA-199 kU/l
(median) | 34.04
(0.6–10,000) | 185
(7.74–10,000) | 0.065 | 185
(3.55–10,000) | 61(0.6–10,000) | 0.001 |
| GGT,
U/lb | 162.61±187.45 | 241.74±347.26 | 0.275 | 251.52±396.68 | 195.71±204.48 | 0.320 |
| Child-plug score
(A,B/C) | 19/6 | 68/30 | 0.517 | 37/20 | 50/16 | 0.187 |
Survival outcomes
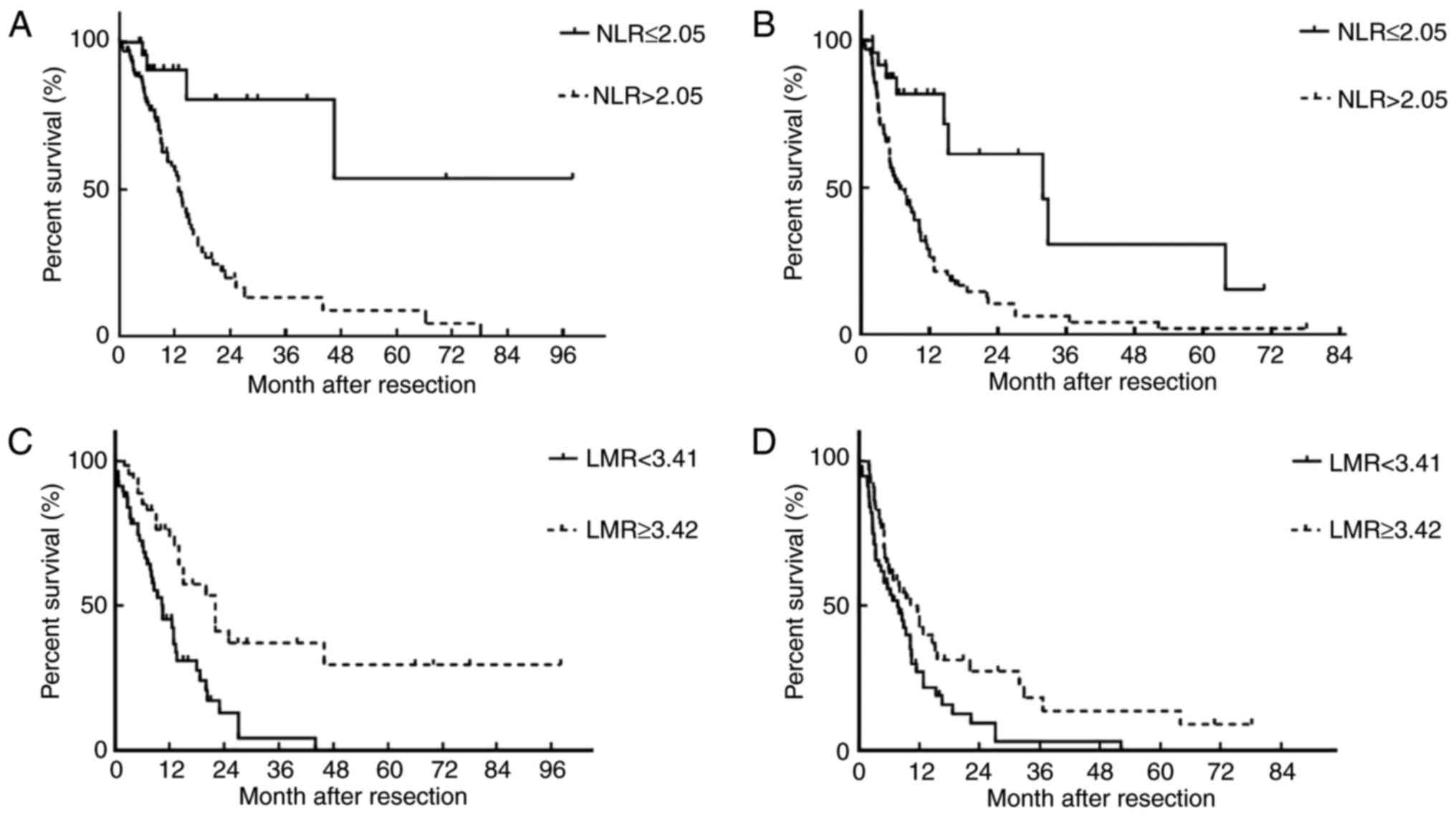
The 1-year and 3-year OS for the whole cohort were
37.40 and 5.69%, respectively. The median OS was 9 months. Higher
NLR was associated with poor OS and decreased DFS. Patients with
higher LMR exhibited higher 3-year OS and 3-year DFS (Fig. 2).
The univariate analysis of OS-associated indicators
are presented in Table II. Tumor
diameter, degree of tumor differentiation, lymph node metastasis,
resection margin, T stages, TNM stage, vascular invasion, NLR, LMR
and macrophage counts were found to be associated with the OS for
the cohort. In order to exclude the colinear regression amongst the
related factors, TNM stages were not included in the multivariate
analysis. The results showed that the T stage, resection margin,
NLR and LMR were significantly associated with the OS of patients
with ICC.
 | Table II.Univariate and Multivariate analysis
of the clinical characteristic factors associated with OS. |
Table II.
Univariate and Multivariate analysis
of the clinical characteristic factors associated with OS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age (years) |
| <60;
≥60 | 0.588 | 1.145 | 0.701–1.872 | – | – | – |
| Sex |
| Male;
female | 0.459 | 0.829 | 0.504–1.362 | – | – | – |
| BMI
(kg/m2) |
| <24;
≥24 | 0.615 | 0.879 | 0.532–1.452 | – | – | – |
| Tumor diameter |
| <5
cm; ≥5 cm | 0.031a | 1.773 | 1.053–2.984 | 0.788 | 1.087 | 0.592–1.996 |
| Differentiated |
| Well;
poorly | 0.036a | 0.589 | 0.359–0.967 | 0.147 | 0.675 | 0.397–1.148 |
| Incisal margin |
|
Negative; positive | 0.010a | 2.036 | 1.184–3.502 | 0.190a | 2.132 | 1.132–4.016 |
| N |
| N-;
N+ | 0.007a | 1.990 | 1.204–3.288 | 0.091 | 1.642 | 0.924–2.918 |
| T |
| T1-2;
T3-4 | 0.006a | 2.027 | 1.228–3.347 | 0.014a | 2.015 | 1.155–3.516 |
| TNM stage |
| 1; 2;
3; 4 | 0.003a | 1.605 | 1.179–2.185 | NA | NA | NAb |
| Vascular
invasion |
| Yes;
No | 0.017a | 1.863 | 1.117–3.105 | 0.362 | 1.316 | 0.729–2.374 |
| NLR |
| ≤2.05:
>2.05 | 0.031a | 1.029 | 1.003–1.055 | 0.046a | 1.033 | 1.001–1.067 |
| LMR |
| ≤3.42;
>3.42 | 0.000a | 0.686 | 0.547–0.819 | 0.023a | 0.789 | 0.643–0.968 |
| WBC count
(×103/ml) | 0.056 | 1.093 | 0.998–1.197 | – | – | – |
| Platelet count
(×103/ml) |
|
<100; ≥100 | 0.253 | 1.494 | 0.751–2.973 | – | – | – |
| Albumin (g/dl) |
| <35;
≥35 | 0.363 | 0.778 | 0.453–1.337 | – | – | – |
| Neutrophil count
(×103/ml) | 0.685 | 1.011 | 0.964–1.060 | – | – | – |
| Lymphocyte count
(×103/ml) | 0.769 | 1.020 | 0.896–1.161 | – | – | – |
| Macrophages count
(×103/ml) | 0.009a | 4.173 | 1.435–12.133 | 0.279 | 2.064 | 0.556–7.665 |
| TBIL (mmol/l) |
|
<20.5; ≥20.5 | 0.441 | 1.223 | 0.733–2.038 | – | – | – |
| AST (U/l) |
| <45;
≥45 | 0.580 | 0.866 | 0.520–1.433 | – | – | – |
| ALT (U/l) |
| <45;
≥45 | 0.296 | 0.756 | 0.447–1.278 | – | – | – |
| AFP (ng/ml) |
|
<400; ≥400 | 0.403 | 1.297 | 0.705–2.386 | – | – | – |
| CA-199 (kU/l) |
| <35;
≥35 | 0.789 | 0.928 | 0.537–1.603 | – | – | – |
| Child-plug
score |
| A; B;
C | 0.422 | 1.207 | 0.763–1.911 | – | – | – |
| ALP (U/l) |
|
<100; ≥100 | 0.249 | 1.357 | 0.808–2.278 | – | – |
|
| GGT (U/l) |
| <50;
≥50 | 0.277 | 1.370 | 0.777–2.414 | – | – | – |
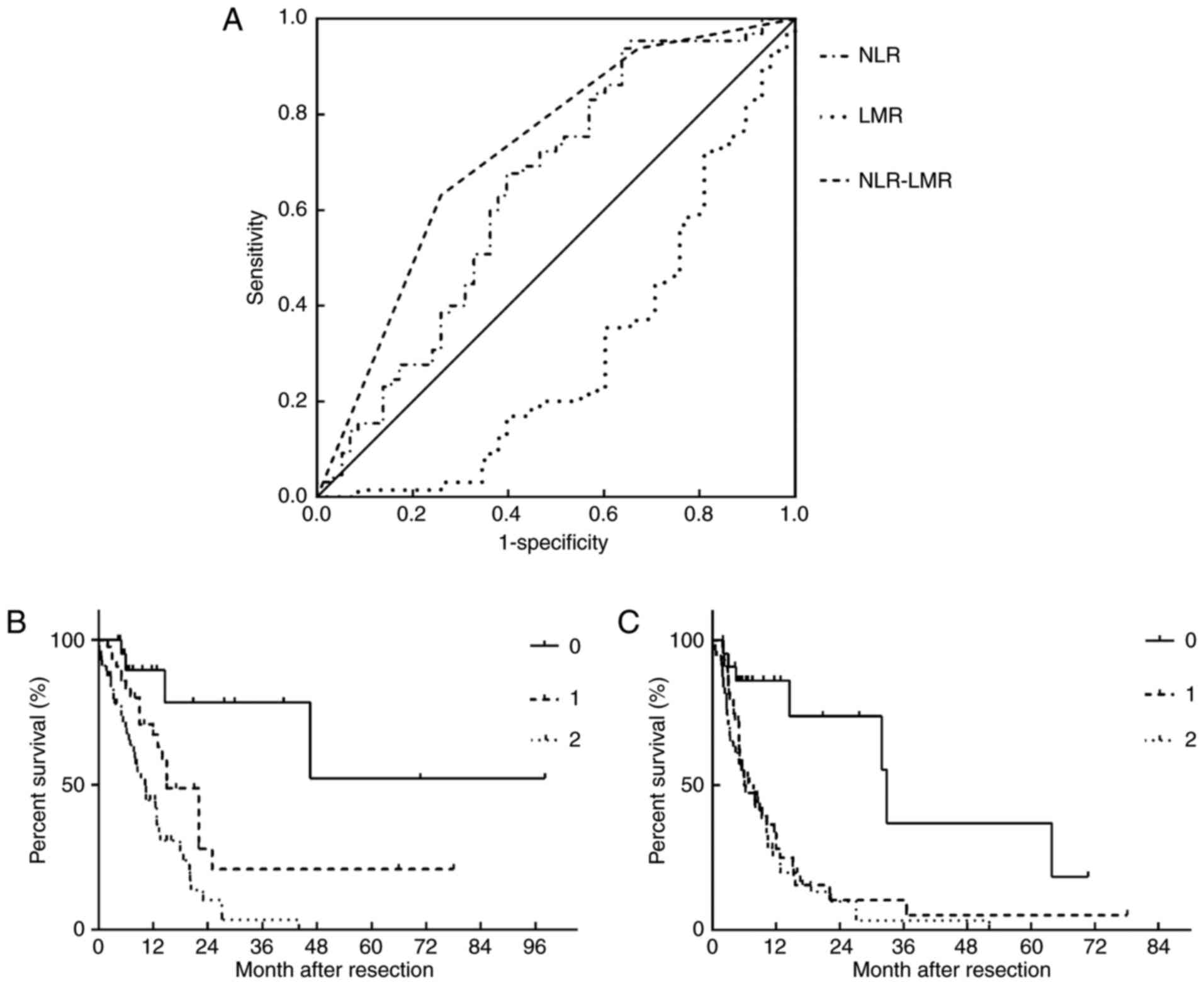
To comprehensively evaluate the association between
the inflammation-based score and the OS, patients were divided into
three groups: Score 0 group (n=23); score 1 group (n=44); and score
2 group (n=56). The ROC analysis was used to determine the
discriminatory capacity of the NLR, LMR and inflammation-based
score system, as presented in Fig.
3A. The AUROC of NLR, LMR and the inflammation-based scoring
system was 0.645, 0.299 and 0.724, respectively. When comparing the
OS and DFS rates of different groups based on the
inflammation-based score system, patients in the higher score group
had worse prognosis, as presented in Fig.
3B and C.
The clinical characteristics of different
inflammatory-based score groups were further compared. The three
groups exhibited differences in the tumor diameter, incisal margin,
lymph node metastasis, T stage, TNM stage, serum ALB level, white
blood cell count, and lymphocyte and megakaryocyte counts (Table III).
 | Table III.Association between clinical
characteristics and the inflammation-based scoring system. |
Table III.
Association between clinical
characteristics and the inflammation-based scoring system.
|
| Inflammation
score |
|
|---|
|
|
|
|
|---|
| Parameter | 0 N=23 | 1 N=44 | 2 N=56 | P-value |
|---|
| Ages,
yearsf | 55.65±12.21 | 58.05±9.25 | 56.30±11.15 | 0.615 |
| Sex
(male/female) | 8/15 | 26/18 | 33/23 | 0.110 |
| BMI,
kg/m2 f | 23.12±4.41 | 23.87±3.16 | 22.51±3.94 | 0.208 |
| Tumor diameter,
cmf |
4.83±2.70d | 5.83±3.05 |
6.88±3.21d | 0.023a |
| Differentiated
(well/poorly) | 15/8 | 27/17 | 28/28 | 0.351 |
| Incisal margin
(negative/positive) | 20/3 | 29/15 | 27/29 | 0.004a |
| N (−/+) | 19/4 | 31/13 | 25/31 | 0.002a |
| T (1–2/3-4) | 15/8 | 29/15 | 24/32 | 0.040a |
| TNM stage
(I–II/III–IV) | 11/12 | 19/25 | 8/48 | 0.001a |
| Vascular invasion
(absent/present) | 17/6 | 30/14 | 34/22 | 0.490 |
| WBC count,
×103/mlf |
5.57±2.22c,d |
6.99±2.44c |
7.38±2.89d | 0.022a |
| Platelet count,
×103/mlf | 178.17±87.22 | 191.00±103.41 | 203.16±83.99 | 0.528 |
| Albumin,
g/dlf | 39.87±4.02 | 39.06±6.83 | 36.99±5.07 | 0.064 |
| Neutrophil count,
×103/mlf |
2.45±5.46d | 4.40±1.57 |
5.17±2.49d | 0.007a |
| Lymphocyte count,
×103/mlf |
2.85±6.35c,d |
1.64±0.55c |
1.34±0.44d | 0.012a |
| Megakaryocyte,
×103/mlf |
0.31±0.17d |
0.40±0.16e |
0.54±0.22d,e | 0.000a |
| TBIL,
mmol/lf | 25.57±43.09 | 45.99±85.16 | 34.77±61.13 | 0.463 |
| AST,
U/lf | 59.97±57.28 | 80.69±123.95 | 67.69±106.11 | 0.716 |
| ALT,
U/lf | 64.86±69.91 | 196.06±796.56 | 79.31±149.86 | 0.420 |
| AFP,
ng/mlf | 3.06
(1.41–206.00) |
3.12(1.22–420.66) |
3.16(1.21–1,440) | 0.555b |
| CA-199, kU/l
(median) | 44.08
(0.6–10,000) |
95.11(0.8–10,000) |
185(3.55–10,000) | 0.697b |
| GGT,
U/lf | 154.49±182.39 | 290.97±458.74 | 203.54±215.42 | 0.204 |
| Child-plug score
(A, B/C) | 20/3 | 30/14 | 37/19 | 0.161 |
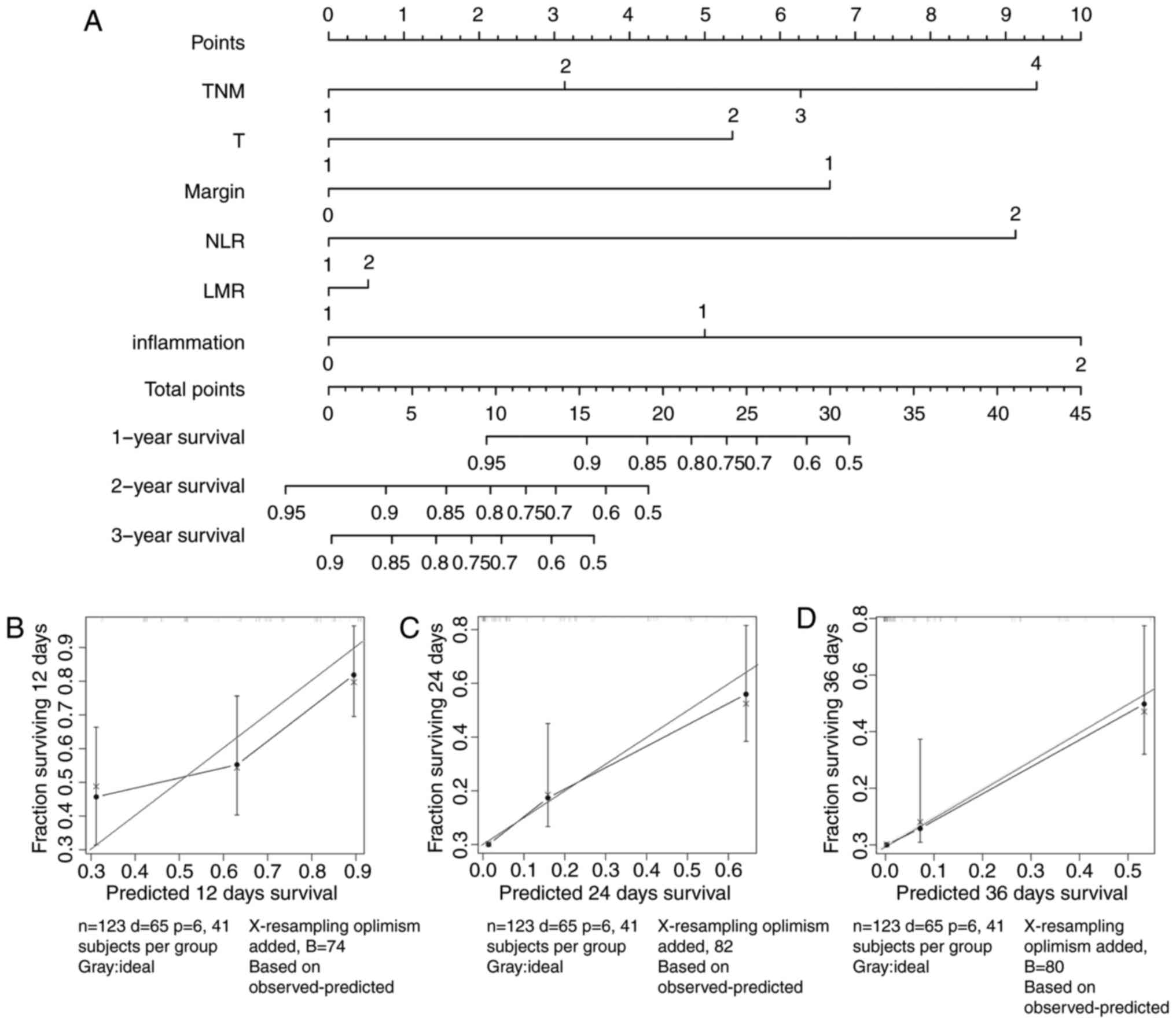
To verify the results, a nomogram was established,
using the indicators that were significantly associated with the
OS. The results were identified to be the same as those for the Cox
regression analysis (Fig. 4A). The
Harrell's c-indexes of the nomograms for prediction of the OS of
patients with ICC were 0.74 (95% CI: 0.677–0.803). Calibration
curves for 1-year, 2-year and 3-year nomograms (Fig 4B-D) revealed no deviations from the
reference line and no need for recalibration. Thus, the nomogram
verified that the NLR and LMR may serve as effective indicators for
the prognosis of ICC.
Discussion
Inflammation has long been reported to be associated
with the development and progression of liver cancer (24). Inflammation may contribute to the
cancer microenvironment and promote the proliferation of cancer
cells (25,26). The cell-mediated componenet of the
immune system serves an important role in the immune response to a
tumor. Levels of peripheral blood cells, such as WBCs, neutrophils
granulocytes and lymphocytes, may reflect the inflammatory status
of patients (27). It has previously
been reported that high numbers of tumor-infiltrating lymphocytes
correlate with better prognosis in patients with breast cancer
(28). Neutrophils are capable of
producing cytokines and chemokines, including vascular endothelial
growth factor (VEGF), which may promote tumor angiogenesis and
cancer cell proliferation, whilst acting to suppress
lymphocyte-mediated cytolysis (29,30).
Furthermore, megakaryocyte and platelet numbers have been reported
to be associated with a cancer-promoting environment. Increasing
evidence has demonstrated that serum inflammatory indicators, such
as NLR, PLR, LMR, RDW and PNI, are associated with the prognosis of
various cancer types (3,19,31,32).
Although the association between inflammatory-based
indicators and HCC has been extensively studied, little is known
about the usefulness of these indicators in ICC. In the present
study, it was revealed that NLR, LMR and the inflammation-based
score based on these may serve as useful indicators in the
prognosis of patients with ICC. Patients with lower LMR, higher NLR
or higher inflammation scores may have worse pathological and
clinical outcomes.
NLR as a prognostic factor for liver cancer has been
widely reported (33–35). It was demonstrated to be associated
with worse clinicopathological characteristics and it is also been
reported to be an independent predictor of long-term survival for
various malignant tumors (32,33,35,36).
In the present study, the optimal cut-off value of NLR was 2.05. In
the multivariate analysis, the hazard ratio was 1.033 (95% CI,
1.001–1.067; P<0.05), which is concordant with previous studies
(17,37). Patients with higher NLR exhibited
tumors of larger diameters and at more advanced stages, which is
consistent with previous studies (3,38,39).
LMR is a favorable prognostic factor for clinical
outcomes in patients with HCC (24).
In the present study it has been identified that LMR is also
associated with the prognosis of ICC. Lymphocytes and monocytes are
vital for the development and prognosis of various cancer types and
are involved in the development of tumors through the release of
various soluble factors, which may be essential for tumor
angiogenesis, invasion and metastasis (40,41). In
the present study, lower LMR was demonstrated to be associated with
worse prognostic and clinical outcomes. Although studies have
previously reported LMR to be an independent factor for HCC, this
is, to the best of our knowledge, the first evidence to suggest
that lower LMR correlates with worse prognosis, therefore may be a
potential clinical indicator for patients with ICC (24,42).
The current study identified NLR and LMR to be
better predictors compared with other inflammatory indicators for
patients with ICC. By combining the two indicators together, it was
discovered that the prognostic significance of the
inflammatory-based system was improved compared with the simple use
of a single index. With AUROC analysis, this inflammatory model had
a stronger predictive ability compared with NLR or LMR alone. On
the basis of this inflammatory score model, it was also discovered
that the higher scoring groups had worse prognostic and
clinicopathological outcomes.
Many studies have reported γ-glutamyl transferase
(GGT)-associated enzymes, including AGR, GPR and GAR, along with
other indicators such as PNI, to be associated with the prognosis
of ICC (20,43,44).
However, the present study demonstrated that these parameters were
not significantly associated with the prognosis of ICC. This may be
due to the fact that the tumors of the enrolled patients were at a
later stage compared with previous studies. The patients in this
cohort displaced high serum GGT levels and low serum PNI levels.
High GGT levels may reflect disorders of the bile tract whilst low
PNI may reflect the nutrition state of the patient, however these
indicators may not be suitable as OS predictors for patients in the
later tumor stage.
Currently, a lot of studies have established various
nomograms using the risk factors for the survival and recurrence of
the ICC patients (9). In 2013, Wang
et al (45) established a
nomogram using preoperative carcinoembryonic antigen levels and
carbohydrate antigen19-9 levels, tumor size, presence or absence of
vascular invasion, nodal status and direct invasion or local
metastasis in a cohort of 367 ICC Asian patients. Furthermore, in
2014, Hyder et al (46) built
a nomogram from a cohort of 514 patients from 13 Western and
Eastern centers, in which the patients' age, tumor size, number of
lesions, nodal status, vascular invasion status and presence of
absence of underlying cirrhotic liver parenchyma were included.
These two studies may predict the prognosis of patients with ICC
and Doussot et al (47)
verified this in 2015. This present study focused chiefly on the
inflammation indicators for the prognosis of ICC and nomograms were
used to verify the results. However, internal validation using the
calibration curves and c-index demonstrated that the nomogram
established in the present study was comparable with previous
studies.
The present study poses a number of limitations.
Firstly, the results were based on a single center retrospective
study, which may generate biases in the data analysis. Secondly,
two systems were established: The nomograms and the systemic
inflammation-based system. Whilst these may effectively predict the
prognosis of patients with ICC, the present study was unable to
establish which one was superior. Thirdly, owing to the limited
number of patients, further indicators, including GPS were not
measured; hence, a full assessment of inflammatory indicators for
ICC was not able to be made.
In the present study, it was demonstrated that low
LMR and high NLR are associated with poor prognosis and worse
clinical outcomes for patients with ICC undergoing curative
surgery. A combined inflammation-based score system based on LMR
and NLR may effectively predict the outcomes and serve as a novel
prognostic predictor for these patients.
Acknowledgements
Not applicable.
Funding
This study was supported by the Project of
Development and Innovation Team of Ministry of Education (grant no.
IRT_16R57).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, YL and LH conceived and designed the
experiments. YW, FR and ZX acquired data and performed statistical
analysis. FR, YL, YC and CS interpreted the data. YW, LH, XZ and CS
wrote the manuscript. XZ and YL revised the manuscript. XZ was
involved in the acquisition of data. All authors approved the final
version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The institutional ethics committee at the study center
approved this study. All participants gave consent after being
fully informed of the goal and characteristics of this
research.
Patient consent for publication
Written informed consent was obtained from all
participants prior to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nautsch F, Ludwig JM, Xing M, Johnson KM
and Kim HS: Racial disparities and sociodemographic differences in
incidence and survival among pediatric patients in the United
States with primary liver cancer: A surveillance, epidemiology, and
end results (SEER) population study. J Clin Gastroenterol.
52:262–267. 2018.PubMed/NCBI
|
|
2
|
Xu K, Watanabe-Galloway S, Rochling FA,
Zhang J, Farazi PA, Peng H, Wang H and Luo J: Practice, knowledge,
and barriers for screening of hepatocellular carcinoma among
high-risk chinese patients. Ann Glob Health. 83:281–292. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He CB and Lin XJ: Inflammation scores
predict the survival of patients with hepatocellular carcinoma who
were treated with transarterial chemoembolization and recombinant
human type-5 adenovirus H101. PLoS One. 12:e01747692017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu CS and Kao JH: An update on
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in Asia. Expert Rev Gastroenterol Hepatol. 11:759–772. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erstad DJ and Tanabe KK: Hepatocellular
carcinoma: Early-stage management challenges. J Hepatocell
Carcinoma. 4:81–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SJ and Lim HY: Hepatocellular
carcinoma treatment: A comparative review of emerging growth factor
receptor antagonists. Expert Opin Emerg Drugs. 22:191–200. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rabinel P, Dousse D, Muscari F and Suc B:
Management of liver cancer. The Surgeon's point of view. Rep Pract
Oncol Radiother. 22:176–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rai V, Abdo J, Alsuwaidan AN, Agrawal S,
Sharma P and Agrawal DK: Cellular and molecular targets for the
immunotherapy of hepatocellular carcinoma. Mol Cell Biochem.
437:13–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong S, Cheng Q, Huang L, Wang J, Sha M,
Tong Y, Xia L, Han L, Xi Z, Zhang J, et al: Risk stratification
system to predict recurrence of intrahepatic cholangiocarcinoma
after hepatic resection. BMC Cancer. 17:4642017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Cai F, Zhao J and Liu F: Prognostic
risk factors associated with recurrence and metastasis after
radical resection in patients with hepatolithiasis complicated by
intrahepatic cholangiocarcinoma. Cell Biochem Biophys. 73:455–460.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni Q, Shen W, Zhang M, Yang C, Cai W, Wu M
and Yang J: Prognostic analysis of radical resection for
intrahepatic cholangiocarcinoma: A retrospective cohort study.
Oncotarget. 8:75627–75637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maganty K, Levi D, Moon J, Bejarano PA,
Arosemena L, Tzakis A and Martin P: Combined hepatocellular
carcinoma and intrahepatic cholangiocarcinoma: Outcome after liver
transplantation. Dig Dis Sci. 55:3597–3601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elshamy M, Presser N, Hammad AY, Firl DJ,
Coppa C, Fung J and Aucejo FN: Liver transplantation in patients
with incidental hepatocellular carcinoma/cholangiocarcinoma and
intrahepatic cholangiocarcinoma: A single-center experience.
Hepatobiliary Pancreat Dis Int. 16:264–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chinchilla-Lopez P, Aguilar-Olivos NE,
Garcia-Gomez J, Hernández-Alejandro KK, Chablé-Montero F,
Motola-Kuba D, Patel T and Méndez-Sánchez N: Prevalence, risk
factors, and survival of patients with intrahepatic
cholangiocarcinoma. Ann Hepatol. 16:565–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tarocchi M, Polvani S, Marroncini G and
Galli A: Molecular mechanism of hepatitis B virus-induced
hepatocarcinogenesis. World J Gastroenterol. 20:11630–11640. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song W, Wang K, Zhong FP, Fan YW, Peng L
and Zou SB: Clinicopathological and prognostic significance of
platelet-to-lymphocyte ratio in patients with hepatocellular
carcinoma. Oncotarget. 7:81830–81838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu SJ, Ji F, Han M, Chen MG, Wang XP, Ju
WQ, Zhao Q, Wu LW, Ren QQ, Guo ZY, et al: Prognostic value of
combined preoperative fibrinogen and neutrophil-lymphocyte ratio in
patients with hepatocellular carcinoma after liver transplantation.
Oncotarget. 8:4301–4312. 2017.PubMed/NCBI
|
|
19
|
Howell J, Pinato DJ, Ramaswami R, Arizumi
T, Ferrari C, Gibbin A, Burlone ME, Guaschino G, Toniutto P, Black
J, et al: Integration of the cancer-related inflammatory response
as a stratifying biomarker of survival in hepatocellular carcinoma
treated with sorafenib. Oncotarget. 8:36161–36170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jing CY, Fu YP, Shen HJ, Zheng SS, Lin JJ,
Yi Y, Huang JL, Xu X, Zhang J, Zhou J, et al: Albumin to
gamma-glutamyltransferase ratio as a prognostic indicator in
intrahepatic cholangiocarcinoma after curative resection.
Oncotarget. 8:13293–13303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuno M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Mizuno T, Yamaguchi J and Nagino M: Evaluation of
inflammation-based prognostic scores in patients undergoing
hepatobiliary resection for perihilar cholangiocarcinoma. J
Gastroenterol. 51:153–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng ZW, Pan W, Hong HJ, Chen JZ and Chen
YL: Modified staging classification for intrahepatic
cholangiocarcinoma based on the sixth and seventh editions of the
AJCC/UICC TNM staging systems. Medicine (Baltimore). 96:e78912017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Jia H, Yu W, Xu Y, Li X, Li Q and
Cai S: Nomograms for predicting prognostic value of inflammatory
biomarkers in colorectal cancer patients after radical resection.
Int J Cancer. 139:220–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi S, Chen Q, Ye L, Yin D, Li X, Dai Z
and He J: Prognostic value of systemic inflammation score in
patients with hepatocellular carcinoma after hepatectomy.
Oncotarget. 8:79366–79375. 2017.PubMed/NCBI
|
|
25
|
Hu D, Lin Y, Liu F, Zeng L, Ouyang X, Wang
K, Zheng X and Huang Q: Elevated preoperative platelet to
lymphocyte ratio indicates poor survival in patients with resected
high-grade serous ovarian carcinoma. Clin Lab. 62:1443–1449. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi L, Qin X, Wang H, Xia Y, Li Y, Chen X,
Shang L, Tai YT, Feng X, Acharya P, et al: Elevated
neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and
decreased platelet-to-lymphocyte ratio are associated with poor
prognosis in multiple myeloma. Oncotarget. 8:18792–18801.
2017.PubMed/NCBI
|
|
27
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen TH, Zhang YC, Tan YT, An X, Xue C,
Deng YF, Yang W, Yuan X and Shi YX: Tumor-infiltrating lymphocytes
predict prognosis of breast cancer patients treated with anti-Her-2
therapy. Oncotarget. 8:5219–5232. 2017.PubMed/NCBI
|
|
29
|
Ji H, Houghton AM, Mariani TJ, Perera S,
Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, et
al: K-ras activation generates an inflammatory response in lung
tumors. Oncogene. 25:2105–2112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shalapour S and Karin M: Immunity,
inflammation, and cancer: An eternal fight between good and evil. J
Clin Invest. 125:3347–3355. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maeda K, Shibutani M, Otani H, Nagahara H,
Ikeya T, Iseki Y, Tanaka H, Muguruma K and Hirakawa K:
Inflammation-based factors and prognosis in patients with
colorectal cancer. World J Gastrointest Oncol. 7:111–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Beltran BE, Aguilar C, Quiñones P, Morales
D, Chavez JC, Sotomayor EM and Castillo JJ: The
neutrophil-to-lymphocyte ratio is an independent prognostic factor
in patients with peripheral T-cell lymphoma, unspecified. Leuk
Lymphoma. 57:58–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Li C, Zhao J, Yang L, Liu F, Zheng
H, Wang Z and Xu Y: Neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratios predict chemotherapy outcomes and
prognosis in patients with colorectal cancer and synchronous liver
metastasis. World J Surg Oncol. 14:2892016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayashi H, Takamura H, Ohbatake Y,
Nakanuma S, Tajima H, Fushida S, Onishi I, Tani T, Shimizu K and
Ohta T: Postoperative changes in neutrophil-to-lymphocyte ratio and
platelet count: A simple prognostic predictor for adult-to-adult
living donor liver transplantation. Asian J Surg. 41:341–348. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, He L, Han J, Wang L, Li M, Jiang Y,
Wang X and Yang Z: Association of neutrophil-lymphocyte ratio and T
lymphocytes with the pathogenesis and progression of HBV-associated
primary liver cancer. PLoS One. 12:e01706052017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li SH, Wang QX, Yang ZY, Jiang W, Li C,
Sun P, Wei W, Shi M and Guo RP: Prognostic value of the
neutrophil-to-lymphocyte ratio for hepatocellular carcinoma
patients with portal/hepatic vein tumor thrombosis. World J
Gastroenterol. 23:3122–3132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Zhang HY, Li J, Shao XY and Zhang
CX: The elevated NLR PLR and PLT may predict the prognosis of
patients with colorectal cancer: A systematic review and
meta-analysis. Oncotarget. 8:68837–68846. 2017.PubMed/NCBI
|
|
38
|
Song X, Zhu H, Pei Q, Tan F, Li C, Zhou Z,
Zhou Y, Yu N, Li Y and Pei H: Significance of inflammation-based
indices in the prognosis of patients with non-metastatic colorectal
cancer. Oncotarget. 8:45178–45189. 2017.PubMed/NCBI
|
|
39
|
Taussig MD, Irene Koran ME, Mouli SK,
Ahmad A, Geevarghese S, Baker JC, Lipnik AJ, Banovac F and Brown
DB: Neutrophil to lymphocyte ratio predicts disease progression
following intra-arterial therapy of hepatocellular carcinoma. HPB
(Oxford). 19:458–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu SJ, Lin YX, Ye H, Li FY, Xiong XZ and
Cheng NS: Lymphocyte to monocyte ratio and prognostic nutritional
index predict survival outcomes of hepatitis B virus-associated
hepatocellular carcinoma patients after curative hepatectomy. J
Surg Oncol. 114:202–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song W, Tian C, Wang K, Zhang RJ and Zou
SB: The pretreatment lymphocyte to monocyte ratio predicts clinical
outcome for patients with hepatocellular carcinoma: A
meta-analysis. Sci Rep. 7:466012017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang C, Wang H, Ning Z, Xu L, Zhuang L,
Wang P and Meng Z: Prognostic nutritional index serves as a
predictive marker of survival and associates with systemic
inflammatory response in metastatic intrahepatic
cholangiocarcinoma. Onco Targets Ther. 9:6417–6423. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang BG, Ge RL, Sun LL, Zong M, Wei GT
and Zhang YJ: Clinical parameters predicting survival duration
after hepatectomy for intrahepatic cholangiocarcinoma. Can J
Gastroenterol. 25:603–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang C, Wang H, Ning Z, Xu L, Zhuang L,
Wang P and Meng Z: Serum liver enzymes serve as prognostic factors
in patients with intrahepatic cholangiocarcinoma. Onco Targets
Ther. 10:1441–1449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan
Z, Wan X, Liu G, Wu D, Shi L, et al: Prognostic nomogram for
intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin
Oncol. 31:1188–1195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hyder O, Marques H, Pulitano C, Marsh JW,
Alexandrescu S, Bauer TW, Gamblin TC, Sotiropoulos GC, Paul A,
Barroso E, et al: A nomogram to predict long-term survival after
resection for intrahepatic cholangiocarcinoma: An Eastern and
Western experience. JAMA Surg. 149:432–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Doussot A, Groot-Koerkamp B, Wiggers JK,
Chou J, Gonen M, DeMatteo RP, Allen PJ, Kingham TP, D'Angelica MI
and Jarnagin WR: Outcomes after resection of intrahepatic
cholangiocarcinoma: External validation and comparison of
prognostic models. J Am Coll Surg. 221:452–461. 2015. View Article : Google Scholar : PubMed/NCBI
|