Introduction
Esophageal cancer is one of the most common cancer
types with the 11th highest morbidity rate and 6th highest
mortality rate globally, and caused ~439,000 mortalities in 2015
(1). The incidence of esophageal
cancer notably varies among different regions, with eastern Asia
and eastern and southern Africa exhibiting the highest rates of
incidence and western Africa exhibiting the lowest rates in 2012
(1,2).
In China, the estimated esophageal cancer cases and mortalities
were 477,000 and 375,000, respectively, in 2015 (3). Although the morbidity rate of esophageal
cancer has decreased in middle and high-middle sociodemographic
index countries between 2005 and 2015, the mortality rate remains
high due to the poor prognosis (1,4). The
combination of surgical resection with chemotherapy or radiotherapy
has been used to treat esophageal cancer, however, it has been
reported that between 2003 and 2014 the 5-year survival rate
remained <20% in China, USA and Europe (4,5). For these
reasons, it is urgent to develop novel therapeutic agents to treat
esophageal cancer.
Traditional Chinese herbal medicine (CHM) has been
used to treat various cancer types, including non-small cell lung
cancer (6), colorectal cancer
(7), hepatocellular carcinoma
(8). Recently, the clinical trials
reported that the combination of CHM with chemotherapy or
radiotherapy not only demonstrated a number of benefits on the
quality of life and alleviating side effects induced by
chemotherapy or radiotherapy (9,10), but
also improved the survival rate of patients with non-small cell
lung, liver, gastric, colorectal, nasopharyngeal or cervical cancer
(9). However, there is conflicting
evidence regarding the efficacy of CHM treatment on esophageal
cancer (10,11). Numerous studies determined that a
number of herbal medicines or components could inhibit the growth
of esophageal cancer cells in vitro and in vivo,
including Andrographis paniculata (12,13),
Daikenchuto (14), icariin (15), Rosa Roxburghii Tratt and
Fagopyrum Cymosum (16),
Jaridonin (17), Marsdenia
tenacissima (18), OP16 (a novel
ent-kaurene diterpenoid) (19), Qigesan (20) and Tonglian decoction (21). Cistanche is a type of CHM and exerts
various biological functions, including anti-oxidation,
anti-inflammation and neuroprotection (22,23). Our
previous study demonstrated that Cistanche tubulosa
phenylethanoid glycosides (CTPG) could suppress the growth of
melanoma B16-F10 cells in vitro and in vivo (24). However, the poor water solubility of
CTPG previously used limits the drug development (24). Therefore, water-soluble CTPG (CTPG-W)
was used and the antitumor effect on esophageal cancer cells
(Eca-109) was investigated. It was determined that CTPG-W could
dose-dependently inhibit the viability of Eca-109 cells through the
induction of apoptosis via a mitochondrial-dependent pathway.
Materials and methods
Animals
Female C57BL/6 mice (6–8 weeks, ~25 g) were
purchased from the Beijing Laboratory Animal Research Center
(Beijing, China) and housed in the temperature-controlled (25°C),
light-cycled (12/12) Animal Facility of Xinjiang University
(Urumqi, China). All animals received pathogen-free water and
food.
Cell line and culture
The human esophageal carcinoma cell line (Eca-109)
was preserved by the Xinjiang Key Laboratory of Biological
Resources and Genetic Engineering (College of Life Science and
Technology, Xinjiang University, Urumqi, China) and cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; MRC, EN MOASAI Biological Technology Co., Ltd, Jiangsu,
China), 1% L-glutamine (100 mM), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
High performance liquid chromatography
(HPLC)
CTPG-W (cat. no. SGJG20170410) was purchased from
Shanghai Upbio Tech Co., Ltd. (Shanghai, China). The major
compounds of CTPG were qualified and quantified by HPLC according
to our previous study (24). Briefly,
HPLC was conducted on a ZORBAX SB-C18 Column (250×4.6 mm; 5 µm) at
30°C and eluted with 0.2% formic acid solution and a gradient of
methanol starting at 23%, as 1 ml was added every min for 45 min
until reaching 31%. A total of 10 µl sample was injected and
detected at 330 nm. The echinacoside standard was purchased from
Shanghai Baoban Biotech Co., Ltd. (Shanghai, China), and acteoside
standard was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). The standards were used to analyze the components of
CTPG-W.
MTT assay
Cell proliferation was measured with an MTT assay.
Eca-109 cells were inoculated into 96-well plates at a density of
5×103 cells in 100 µl RPMI-1640 medium/well and cultured
at 37°C for 24 h, then treated by different concentrations (0, 200,
400, 600 and 800 µg/ml) of CTPG-W or 0.4% dimethyl sulfoxide (DMSO)
for 24, 48 and 72 h. DMSO was used as solvent control (800 µg/ml
CTPG-W containing 0.4% DMSO). Cisplatin (20 µg/ml) was used as the
positive control. Subsequently, the supernatant was discarded
following centrifugation at 225 x g for 5 min at room
temperature and 100 µl MTT solution (0.5 mg/ml in RPMI-1640 medium
without FBS) was added to each well and incubated at 37°C for 3 h.
The formed formazan crystals were dissolved in 200 µl DMSO. The
optical density (OD) values were measured at a wavelength of 490 nm
by a 96-well microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The relative cell viability was calculated
according to the formula: Cell viability
(%)=(ODtreated/ODuntreated)×100%. The
morphology of Eca-109 cells was observed with an inverted
fluorescence microscope (magnification, ×200) (Nikon Eclipse Ti-E;
Nikon Corporation, Tokyo, Japan).
For the proliferation of splenocytes, C57BL/6 mice
were euthanized by cervical dislocation and spleens were isolated.
The single cell suspension was made and splenocytes were seeded
into 96-well plates at a density of 1×105 cells/well in
100 µl RPMI-1640 medium, and then treated with different
concentrations (0, 200, 400, 600 and 800 µg/ml) of CTPG-W for 24,
48 and 72 h at 37°C with 5% CO2. The proliferation of
splenocytes was measured by MTT assay, according to the
aforementioned protocol. Stimulatory
index=ODtreated/ODuntreated.
Measurement of apoptosis and the cell
cycle
Eca-109 cells were cultured in 60 mm dishes at a
density of 2.5×105 cells/dish for 24 h and treated with
different concentrations (0, 200, 400, 600 and 800 µg/ml) of CTPG-W
or 0.4% DMSO for 24 h at 37°C with 5% CO2. Cells were
collected and stained with an Annexin V-fluorescein isothiocyanate
(FITC)/Propidium iodide (PI) Apoptosis Detection kit (Shanghai
Shengsheng Biotechnology Co., Ltd., Shanghai, China), according to
the manufacturer's protocols. Samples were collected by flow
cytometry (BD FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA)
and analyzed by FlowJo 7.6 (Tree Star, Inc., Ashland, OR, USA). To
analyze the effect of CTPG-W on the cell cycle, 2.5×105
Eca-109 cells were seeded in 60 mm culture dishes and treated with
CTPG-W (0, 100, 200 and 400 µg/ml) or 0.4% DMSO for 24 h at 37°C
with 5% CO2. All cells were harvested and washed twice
with ice-cold PBS (Gibco; Thermo Fisher Scientific, Inc.), then
fixed in 70% ice-cold ethanol at 4°C for 30 min. Following washing
twice with ice-cold PBS, cells were resuspended in 300 µl PI/RNase
staining buffer (BD Biosciences, San Jose, CA, USA) for 10 min at
room temperature. The cell cycle distribution was analyzed with the
ModFit LT 3.0 software by flow cytometry (BD FACSCalibur).
Analysis of mitochondrial membrane
potential (Δψm) and reactive oxygen species (ROS)
To analyze the Δψm, Eca-109 cells were treated with
CTPG-W (0, 400, 600 and 800 µg/ml) or 0.4% DMSO for 24 h at 37°C
with 5% CO2, and stained with the Mitochondrial membrane
potential assay kit with JC-1 (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer's protocol, for 20
min at 37°C. Following washing twice with JC-1 washing buffer
(Beyotime Institute of Biotechnology), samples were resuspended
with 300 µl JC-1 washing buffer and analyzed by flow cytometry (BD
FACSCalibur). The fluorescence of JC-1 dye in Eca-109 cells was
also observed with an inverted fluorescence microscope
(magnification, ×200; Nikon Eclipse Ti-E). For analysis of ROS,
Eca-109 cells were treated with CTPG-W (0, 400, 600 and 800 µg/ml)
for 2, 4, 6, 12 and 24 h, and stained with Reactive Oxygen Species
Assay kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol, for 20 min at 37°C. Following washing
three times with ice-cold PBS, samples were collected by flow
cytometry (BD FACSCalibur) and analyzed by FlowJo 7.6 software.
2,2-diphenyl-1-picrylhydrazyl (DPPH)
radical scavenging activity
The free radical scavenging activity of CTPG-W was
determined with a DPPH free radical assay according to the
published protocol with a minor modification, as methanol was
substituted with ethanol to dissolve DPPH (25,26). For
steady state measurements, 150 µl DPPH (100 mmol/l) in ethanol was
mixed with different concentrations of CTPG-W (25, 50, 75, 100,
250, 300, 400, 500 and 600 µg/ml) in 50 µl PBS, and incubated in
the dark for 30 min at room temperature. The absorbance at 517 nm
was detected in the presence and absence of CTPG-W. A total of 50
µl Vitamin C was used as the positive control. The DPPH radical
scavenging activity was calculated using the formula: Scavenging
(%)=[1-(Asample-Ablank)/A0] ×100,
where Ablank is the absorbance of the control (without
DPPH), Asample is the absorbance of the sample and
A0 is the absorbance of PBS with DPPH.
Western blot analysis
Eca-109 cells were treated with CTPG-W (0, 200, 600
µg/ml) or 0.4% DMSO for 24 h at 37°C with 5% CO2.
Following washing twice with PBS, cells were lysed in
Radioimmunoprecipitation Assay Lysis buffer (Beijing ComWin Biotech
Co., Ltd., Beijing, China) for 20 min on ice. After centrifugation
at 10,000 x g for 10 min at 4°C, the supernatants were
collected, and protein concentrations were detected with a
bicinchoninic acid kit (Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocols. Western blot analysis was
conducted according to our previous description (24). The antibodies against caspase-7 (cat.
no. D120077), caspase-8 (cat. no. D155240), caspase-9 (cat. no.
D220078), B-cell lymphoma-2 (Bcl-2)-associated X (Bax) (cat. no.
D220073) and Bcl-2 (cat. no. D260117), and anti-mouse
IgG-horseradish peroxidase (HRP) (cat. no. D111050) and anti-rabbit
IgG-HRP (cat. no. D110058) were purchased from BBI Life Sciences
(Shanghai, China). The antibodies against caspase-3 (cat. no.
E-AB-10050) and active caspase-3 (cat. no. E-AB-22115) were bought
from Elabscience (Wuhan, China). Other antibodies against caspase-7
(cat. no. 9492), poly (ADP-ribose) polymerase (PARP) (cat. no.
9542), cytochrome c (cat. no. AC909), c-Jun
NH2-terminal kinase (JNK) (cat. no. 9252S) and β-actin
(cat. no. 58169) were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). All primary and secondary antibodies were
diluted at 1:1,000. The primary antibodies were incubated at 4°C
overnight and the secondary antibodies were incubated at 37°C for 1
h. The target proteins were detected using enhanced
chemiluminescence assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer's protocol.
Statistical analysis
Statistical significance was calculated by one-way
analysis of variance with Tukey's post hoc test and results were
analyzed using GraphPad Prism 5.0 software (GraphPad Software, La
Jolla, CA, USA) among the treatment and control groups. All data
were presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
CTPG-W suppresses the growth of
Eca-109 cells
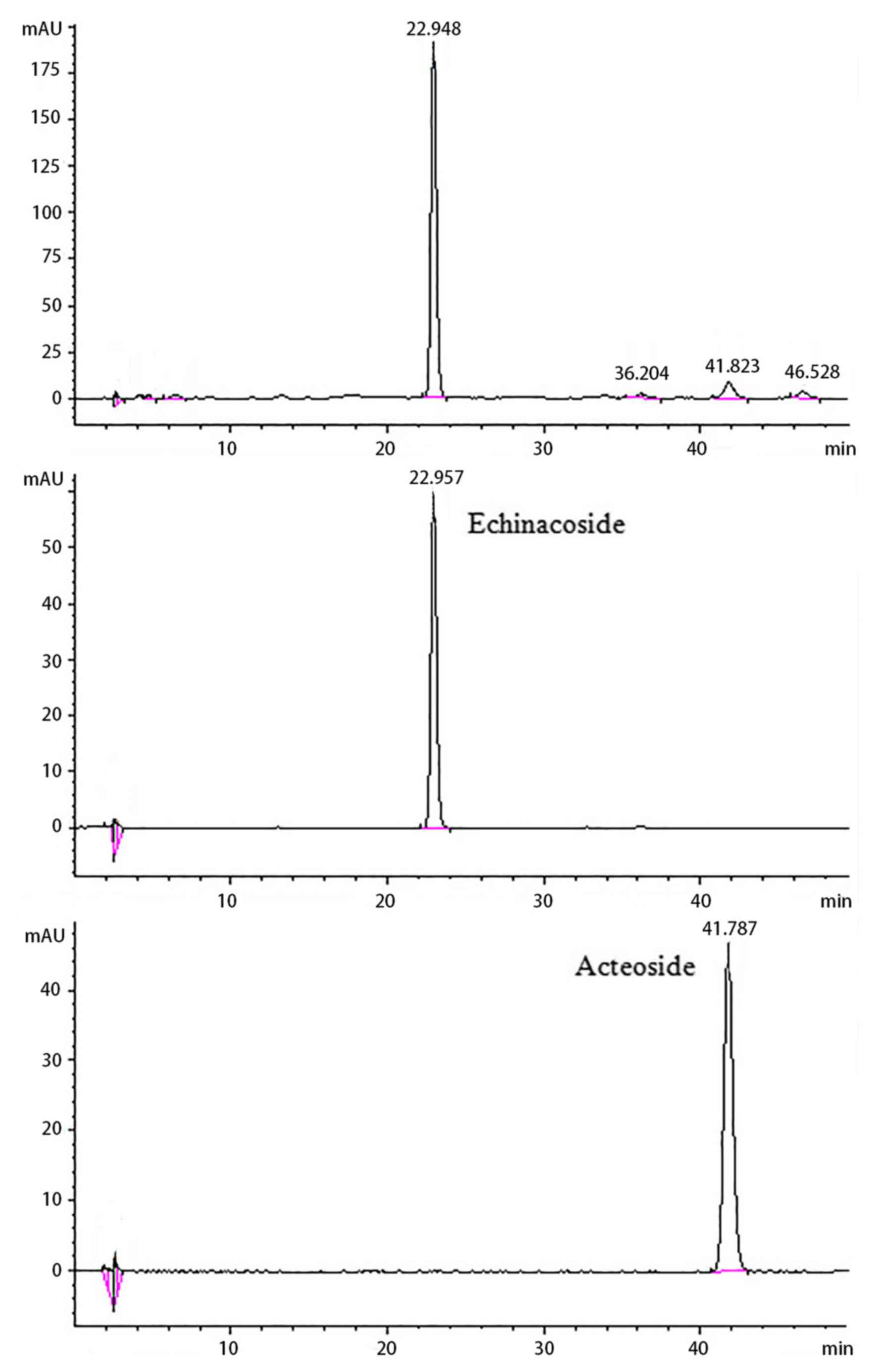
The components of CTPG-W were qualified and
quantified by HPLC (Fig. 1), which
were compared with the standards of echinacoside and acteoside.
According to the peak retention times and the peak areas, CTPG-W
contained 39.16% of echinacoside and 2.44% of acteoside. Firstly,
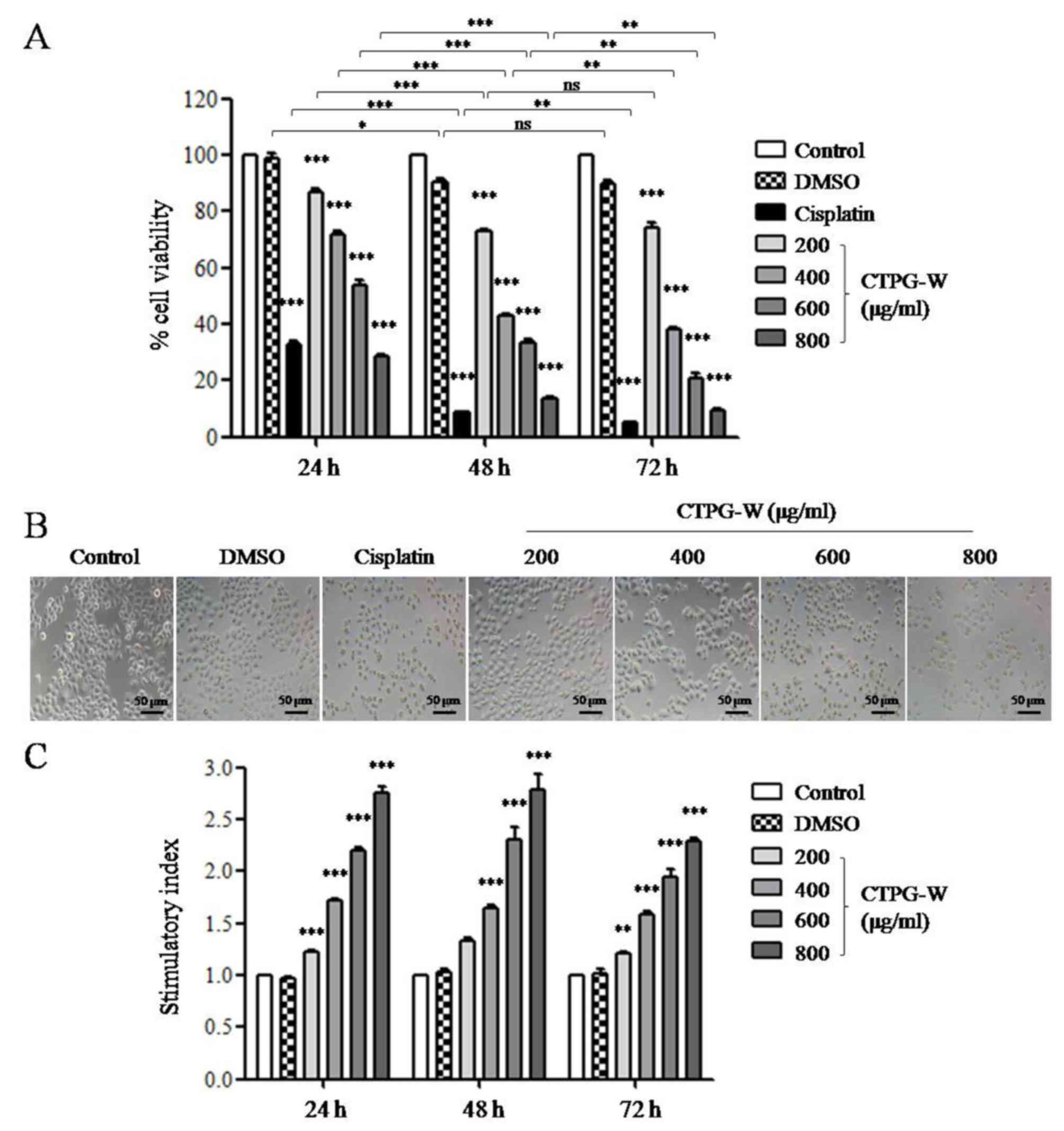
the effect of CTPG-W on the viability of Eca-109 cells was
determined with an MTT assay. CTPG-W was dissolved in DMSO at 200
mg/ml and diluted with RPMI-1640 medium containing 10%
heat-inactivated FBS to indicated concentrations. Eca-109 cells
were treated with CTPG-W and cell viability was analyzed with an
MTT assay at the indicated time points. CTPG-W treatment
significantly reduced the viability of Eca-109 cells in a dose- and
time-dependent manner (P<0.001; Fig.
2A). The morphology of Eca-109 cells was observed with an
inverted fluorescence microscope (magnification, ×200) following
CTPG-W treatment for 24 h, which changed notably in a
dose-dependent manner, with the cells shrinking and becoming round
following CTPG-W treatment (Fig. 2B).
These results indicate that CTPG-W suppresses the growth of Eca-109
cells. The effect of CTPG-W on the proliferation of splenocytes was
also detected with an MTT assay. CTPG-W significantly promoted the
proliferation of splenocytes in a dose-dependent manner (Fig. 2C), indicating that it has no cytotoxic
effect on splenocytes.
CTPG-W induces apoptosis in Eca-109
cells
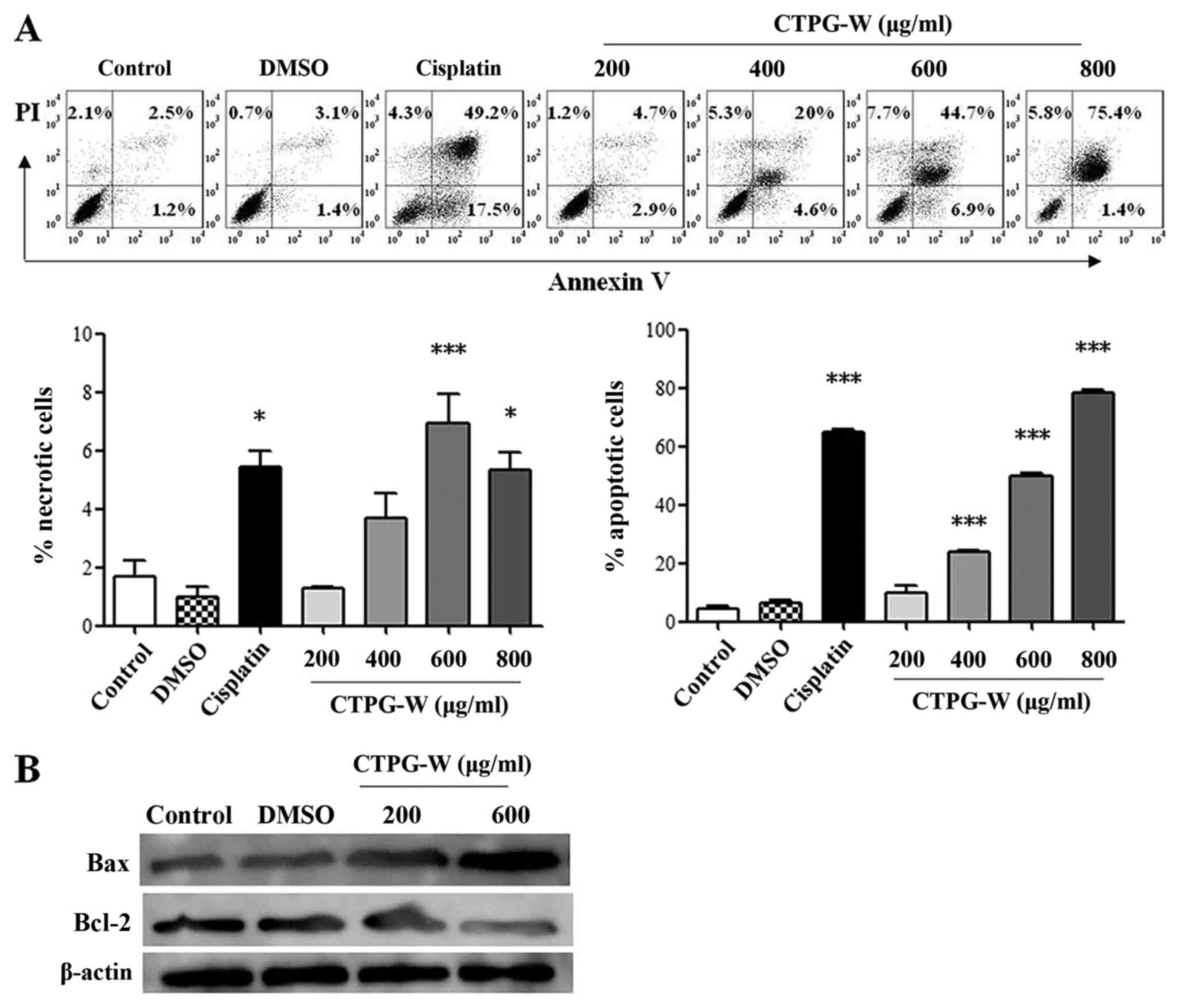
To investigate whether CTPG-W suppressed the growth
of Eca-109 cells through the induction of apoptosis or necrosis,
cells were treated with different concentrations of CTPG-W. After
24 h, the apoptosis and necrosis of Eca-109 cells were detected
with Annexin V/PI staining. As depicted in Fig. 3A, Annexin V−/PI+
cells were gated as necrotic cells, and Annexin
V+/PI+ and Annexin
V+/PI− cells were gated as apoptotic cells.
CTPG-W primarily induced the apoptosis of Eca-109 cells in a
dose-dependent manner, although the necrotic Eca-109 cells also
increased significantly under the treatment of 600 and 800 µg/ml
CTPG-W (P<0.001 at 600 µg/ml and P<0.05 at 800 µg/ml).
Consistently, the levels of pro-apoptotic Bax and anti-apoptotic
Bcl-2 in Eca-109 cells were upregulated and downregulated,
respectively, upon CTPG-W treatment (Fig.
3B). The results indicated that CTPG-W primarily inhibited the
growth of Eca-109 cells through the induction of apoptosis.
CTPG-W induces cell cycle arrest at
the S phase in Eca-109 cells
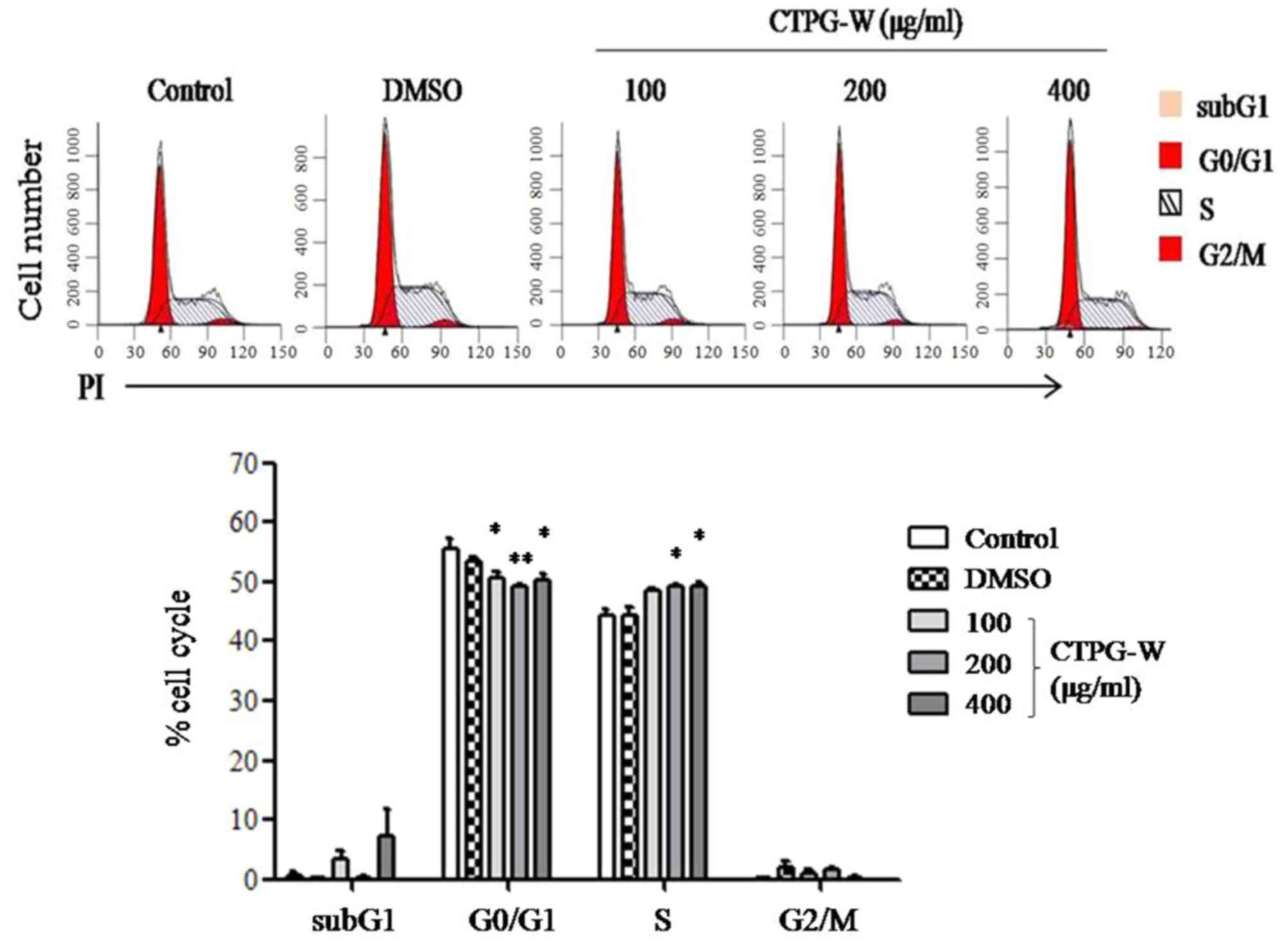
Disturbance of the cancer cell cycle will suppress
cell growth and promote apoptosis (27). The distribution of the cell cycle in
Eca-109 cells was detected with PI staining following CTPG-W
treatment for 24 h. It was observed that cells in the S phase
increased and cells in the G0/G1 phases
indicated an overall significant decrease upon CTPG-W treatment
(P<0.05; Fig. 4), indicating that
CTPG-W arrests the Eca-109 cell cycle at the S phase.
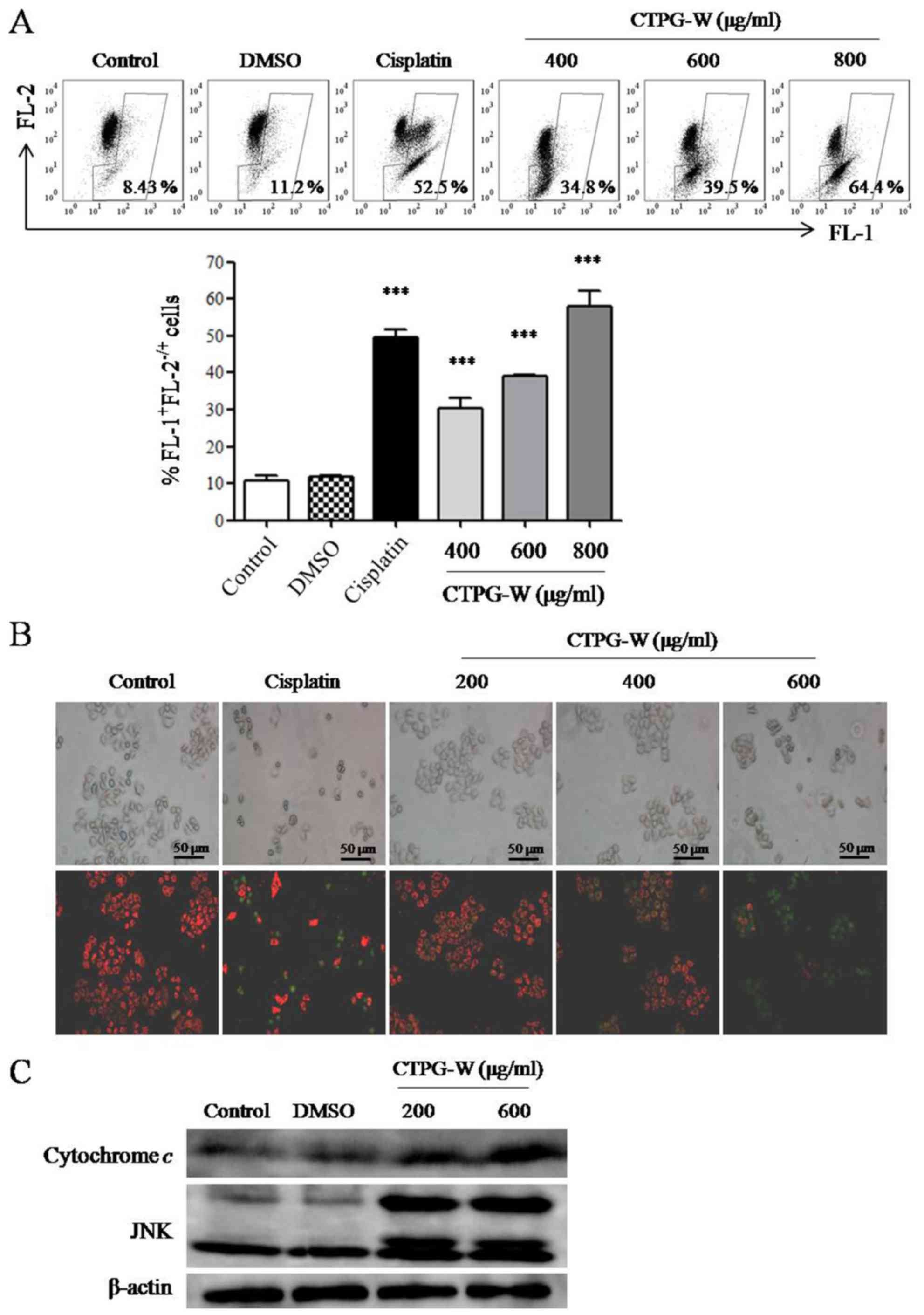
CTPG-W decreases Δψm and induces the release of
cytochrome c. Apoptosis can be induced by a
mitochondrial-dependent pathway (28,29). The
pro- and anti-apoptotic members of the BCL-2 protein family serve
important roles in the regulation of mitochondrial membrane
integrity (30,31). Following CTPG-W treatment for 24 h,
the Δψm was assessed using JC-1 staining. JC-1 aggregate (red
fluorescence detected in FL-2) will disintegrate into monomer
(green fluorescence detected in FL-1) when Δψm is reducing
(32). As depicted in Fig. 5A, the frequencies of
FL-1+FL-2−/+ cells increased significantly in
a dose-dependent manner, indicating that the Δψm of Eca-109 cells
decreased. The fluorescence changes in Eca-109 cells were also
observed with an inverted fluorescence microscope (Fig. 5B). With the increasing concentrations
of CTPG-W, the red fluorescence decreased and the green
fluorescence increased, which is consistent with the data from flow
cytometry. It was also observed that the level of cytochrome
c in cytosol was notably increased (Fig. 5C), which is a result of a reduction of
Δψm. This reinforces the conclusion drawn from the increased count
of FL-1+FL-2−/+ cells that Δψm decreased as a
result of CTPG-W treatment. It has reported that JNK can regulate
the activation of the BCL-2 protein family causing the release of
cytochrome c (33–35). It was also determined that the level
of JNK was notably upregulated following CTPG-W treatment (Fig. 5C). The results indicated that CTPG-W
may induce the apoptosis of Eca-109 cells through a
mitochondrial-dependent pathway.
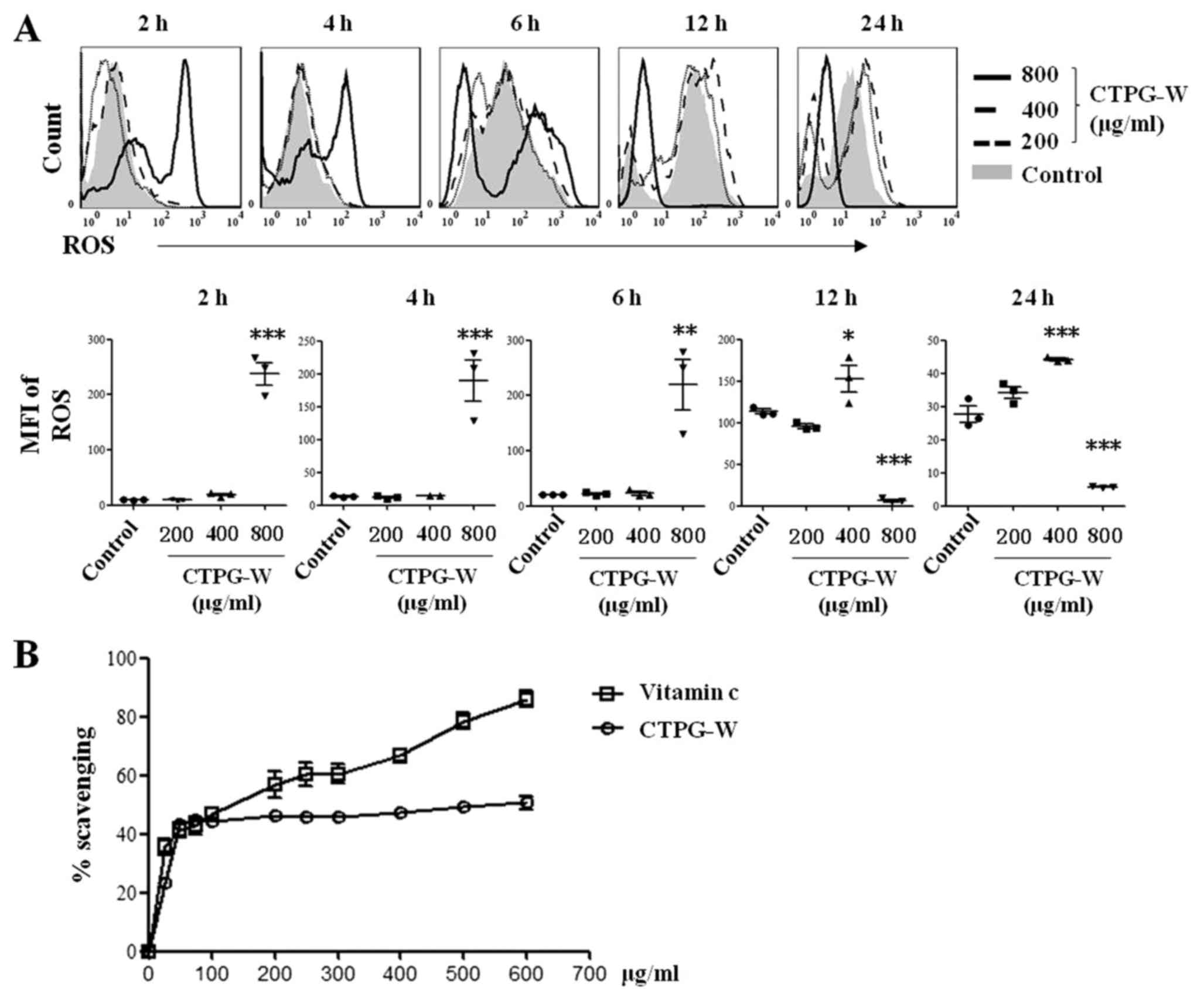
The effect of CTPG-W on intracellular
ROS generation
ROS could reduce Δψm to induce apoptosis (36). To investigate whether CTPG-W can
increase ROS production, Eca-109 cells were treated with different
concentrations of CTPG-W. Cells were collected at the indicated
time points and stained with DCFH-DA. The production of
intracellular ROS in Eca-109 cells was determined by flow
cytometry. As depicted in Fig. 6A,
800 µg/ml CTPG-W significantly increased ROS production from 2–6 h,
and decreased from 12–24 h. Additionally, 400 µg/ml CTPG-W
significantly increased ROS production from 12–24 h. Furthermore,
200 µg/ml CTPG-W did not notably alter ROS production. The dynamic
changes of ROS production may be associated with Eca-109 cell
apoptosis. It was also determined that CTPG-W had free radical
scavenging activity (Fig. 6B), which
may be associated with the decreased ROS production in Eca-109
cells treated with 800 µg/ml CTPG-W after 12 h.
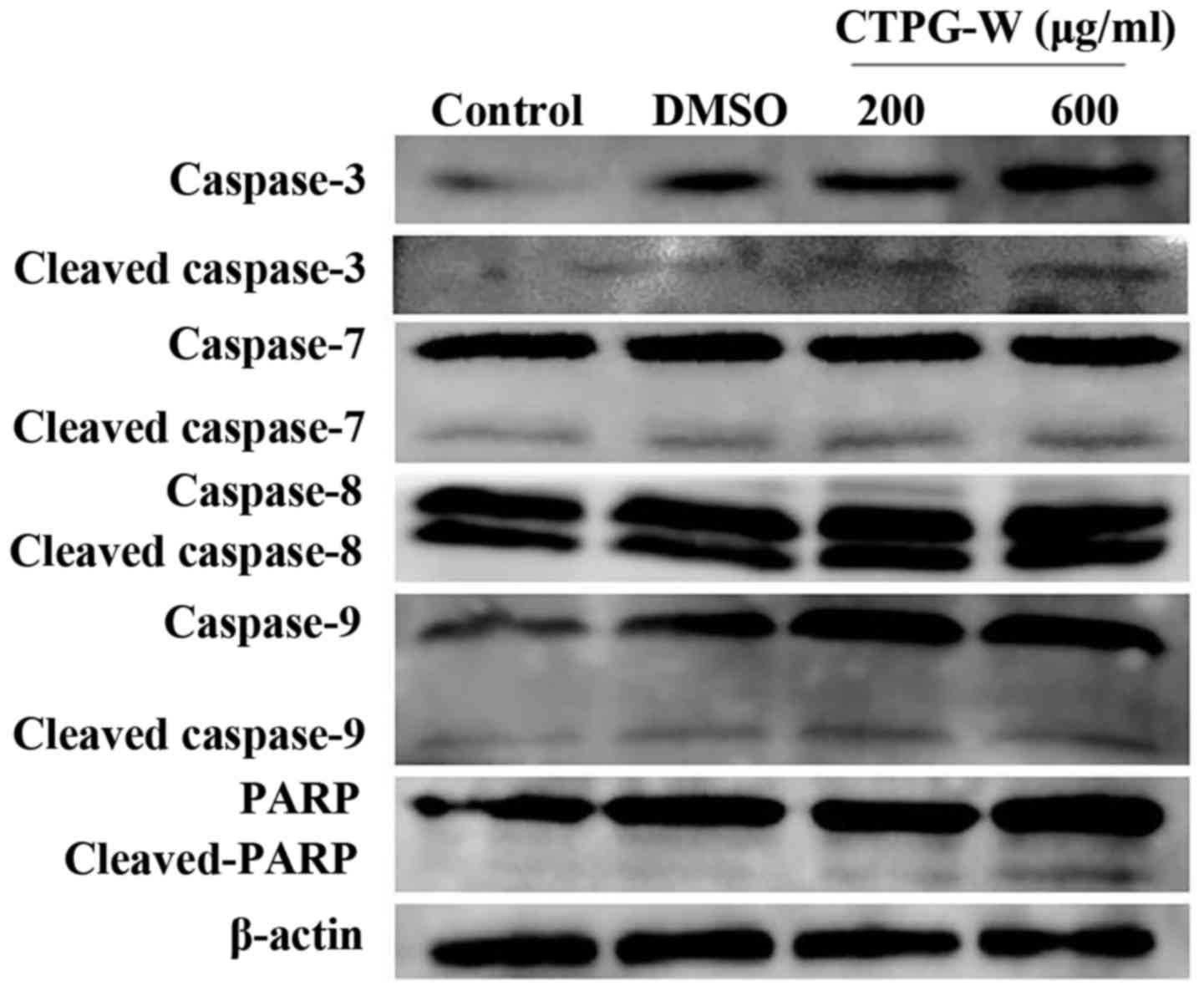
CTPG-W upregulates the activity of
caspase-3, caspase-7, caspase-9 and PARP
The release of cytochrome c due to Δψm
reduction could activate the caspase proteases to induce apoptosis
(29,30,37).
Following CTPG-W treatment for 24 h, the activation of caspase-3,
7, 8, 9 and PARP was detected by western blot analysis. Compared
with the control, the levels of cleaved-caspase-9,
cleaved-caspase-7, cleaved-caspase-3 and cleaved-PARP, but not the
levels of cleaved-caspase-8, were upregulated by CTPG treatment in
a dose-dependent manner (Fig. 7).
These results indicated that CTPG-W reduced Δψm and promoted
cytochrome c release to activate caspases that induce the
apoptosis of Eca-109 cells.
Discussion
Traditional CHM could induce apoptosis of esophageal
cancer cells through different pathways, including the extrinsic
death receptor, intrinsic mitochondrial and endoplasmic reticulum
stress pathways (29). Our previous
study demonstrated that CTPG, as the major component of C.
tubulosa, inhibited the growth of melanoma B16-F10 cells
through the induction of apoptosis via a mitochondrial-dependent
pathway (24). In the present study,
the antitumor effect of CTPG-W on Eca-109 cells was investigated
and it was determined that CTPG-W suppressed the growth of Eca-109
cells, induced apoptosis and cell cycle arrest, reduced Δψm,
increased the release of cytochrome c and activated
caspases. CTPG and CTPG-W could induce the apoptosis and cell cycle
arrest in cancer cells. However, the accurate mechanisms are
different due to the different components of CTPG (26.64%
echinacoside, 10.19% acteoside and 1.71% isoacteoside) and CTPG-W
(39.16% echinacoside and 2.44% acteoside). CTPG arrested B16-F10
cells at the G0/G1 phases, but CTPG-W
arrested Eca-109 cells at the S phase (24). ROS production was dose-dependently
increased by CTPG, but it indicated a change in a time-dependent
manner by high dose of CTPG-W, which increased significantly at the
beginning of CTPG-W treatment (2–6 h) and decreased significantly
after 12 h, compared with the control. A possible reason is that
the major component of CTPG-W is echinacoside. A number of studies
reported that echinacoside could inhibit ROS production and
ROS-induced apoptosis to exert its neuroprotective and anti-aging
effects (38–40). Similarly, the free radical scavenging
activity was observed in the present study. Therefore, it was
speculated that some components, including verbascoside,
iso-verbascoside and salidroside in a high dose of CTPG-W might
immediately induce ROS generation to cause apoptosis of Eca-109
cells (41,42), and then ROS was scavenged by
echinacoside. Another possible reason for the differences in ROS
production by CTPG and CTPG-W is that different cell lines were
used in this study and previous study (24). Dong et al (43) reported that echinacoside could induce
the apoptosis of human SW480 colorectal cancer cells through the
generation of oxidative DNA damages without increased ROS
levels.
CTPG-W treatment reduced Δψm and caused the release
of cytochrome c, which promotes the cleavage of caspase-9
(28). Consistently, the levels of
cleaved-caspase-9 were upregulated by CTPG-W treatment.
Subsequently, the active caspase-9 can activate caspase-3 to induce
apoptosis (44). The levels of
cleaved-caspase-3 were also upregulated by CTPG-W treatment.
However, caspase-8 was not activated by CTPG-W, indicating that the
extrinsic death receptor pathway was not involved in the apoptosis
induced by CTPG-W. These observations indicate that CTPG-W induces
apoptosis of Eca-109 cells through the activation of a
mitochondrial-dependent pathway.
PARP serves important roles in the genomic stability
and can be cleaved by the active caspases, particularly caspase-3
and −7 (45). It was determined that
CTPG-W treatment activated caspase-3 and −7, which may cleave PARP
to inhibit DNA repair and cause apoptosis.
CTPG-W also dose- and time-dependently suppresses
the growth of human hepatocellular carcinoma BEL-7404 cells
(unpublished data). Although CTPG-W inhibits the growth of Eca-109
and BEL-7404 cells, it promotes the proliferation of splenocytes,
which may be due to the content of polysaccharides (~50%) in CTPG-W
(46). Similarly, a number of studies
have reported that polysaccharides can promote the proliferation of
splenocytes (46–49). In the mouse model, it was determined
that CTPG-W significantly increased the spleen index, compared with
the control group, but did not affect the body weight and the other
organ indexes including heart, liver, kidney and lung (unpublished
data), indicating that CTPG-W has no cytotoxic effect on normal
cells.
Collectively, the data indicated that CTPG-W
inhibits the growth of Eca-109 cells by induction of apoptosis via
a mitochondrial-dependent pathway.
Acknowledgements
The authors would like to thank Dr Jianhua Yang
(Baylor College of Medicine) for polishing the manuscript.
Funding
This study was supported by the 13th Five-Year Plan
for Key Discipline Biology Bidding Project (grant no. 17SDKD0202),
Xinjiang Normal University and Key Laboratory of Special
Environment Biodiversity Application and Regulation in Xinjiang
(grant no. XJTSWZ-2017-04) to JL and the Chinese National Natural
Science Foundation Grant (grant no. 31760260) to XW.
Availability of data and materials
Data and materials used and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
CF, AA, YY and QC performed the experiments. LX, JLv
and XW analyzed the data and the prepared figures. JinyuL and
JinyaoL designed the project and wrote the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Committee on the Ethics of Animal Experiments of Xinjiang Key
Laboratory of Biological Resources and Genetic Engineering
(approval, no. BRGE-AE001; Xinjiang University).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
CHM
|
Chinese herbal medicine
|
|
CTPG-W
|
water-soluble phenylethanoid
glycosides of C. tubulosa
|
|
Δψm
|
mitochondrial membrane potential
|
|
JNK
|
c-Jun NH2-terminal
kinase
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R,
Dandona L, et al: Global, regional, and national cancer incidence,
mortality, years of life lost, years lived with disability, and
disability-adjusted life-years for 32 cancer groups, 1990 to 2015:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3:170482017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samson P and Lockhart AC: Biologic therapy
in esophageal and gastric malignancies: Current therapies and
future directions. J Gastrointest Oncol. 8:418–429. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XW, Liu W, Jiang HL and Mao B:
Chinese herbal medicine for advanced non-small-cell lung cancer: A
systematic review and meta-analysis. Am J Chin Med. 46:923–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu H, Hao J, Niu Y, Liu D, Chen D and Wu
X: Molecular targets of Chinese herbs: A clinical study of
metastatic colorectal cancer based on network pharmacology. Sci
Rep. 8:72382018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun L, Fahey P, Zhu X, Ng W, Chen ZP, Qiu
Y, Lai H, Lin J and Lin L: A cohort study to examine the use of
chinese herbal medicine in combination with conventional therapies
for patients with hepatocellular carcinoma in China. Integr Cancer
Ther. 17:902–911. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung VC, Wu X, Hui EP, Ziea ET, Ng BF, Ho
RS, Tsoi KK, Wong SY and Wu JC: Effectiveness of chinese herbal
medicine for cancer palliative care: Overview of systematic reviews
with meta-analyses. Sci Rep. 5:181112015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Deng L, Jiang X and Wu T: Chinese
herbal medicine for oesophageal cancer. Cochrane Database Syst Rev.
22:CD0045202016.
|
|
11
|
Cai YM, Zhu H, Niu JX, Bing L, Sun Z,
Zhang WH, Ying JZ, Yin XD, Li J, Pang Y and Li JL: Identification
of herb pairs in esophageal cancer. Complement Med Res. 24:40–45.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yue GG, Lee JK, Li L, Chan KM, Wong EC,
Chan JY, Fung KP, Lui VW, Chiu PW and Lau CB: Andrographis
paniculata elicits anti-invasion activities by suppressing
TM4SF3 gene expression and by anoikis-sensitization in esophageal
cancer cells. Am J Cancer Res. 5:3570–3587. 2015.PubMed/NCBI
|
|
13
|
Li L, Yue GG, Lee JK, Wong EC, Fung KP, Yu
J, Lau CB and Chiu PW: The adjuvant value of Andrographis
paniculata in metastatic esophageal cancer treatment-from
preclinical perspectives. Sci Rep. 7:8542017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagata T, Toume K, Long LX, Hirano K,
Watanabe T, Sekine S, Okumura T, Komatsu K and Tsukada K:
Anticancer effect of a kampo preparation daikenchuto. J Nat Med.
70:627–633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan C, Yang Y, Liu Y, Jiang S, Di S, Hu W,
Ma Z, Li T, Zhu Y, Xin Z, et al: Icariin displays anticancer
activity against human esophageal cancer cells via regulating
endoplasmic reticulum stress-mediated apoptotic signaling. Sci Rep.
6:211452016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu W, Li SY, Huang XE, Cui JJ, Zhao T and
Zhang H: Inhibition of tumor growth in vitro by a combination of
extracts from Rosa roxburghii tratt and Fagopyrum
cymosum. Asian Pac J Cancer Prev. 13:2409–2414. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma YC, Ke Y, Zi X, Zhao W, Shi XJ and Liu
HM: Jaridonin, a novel ent-kaurene diterpenoid from Isodon
rubescens, inducing apoptosis via production of reactive oxygen
species in esophageal cancer cells. Curr Cancer Drug Targets.
13:611–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan W, Sun L, Zhou JQ, Zhang C, Qin S,
Tang Y, Liu Y, Lin SS and Yuan ST: Marsdenia tenacissima
extract induces G0/G1 cell cycle arrest in human esophageal
carcinoma cells by inhibiting mitogen-activated protein kinase
(MAPK) signaling pathway. Chin J Nat Med. 13:428–437.
2015.PubMed/NCBI
|
|
19
|
Peng KZ, Ke Y, Zhao Q, Tian F, Liu HM, Hou
G and Lu Z: OP16, a novel ent-kaurene diterpenoid, potentiates the
antitumor effect of rapamycin by inhibiting rapamycin-induced
feedback activation of Akt signaling in esophageal squamous cell
carcinoma. Biochem Pharmacol. 140:16–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi H, Shi D, Wu Y, Shen Q and Li J:
Qigesan inhibits migration and invasion of esophageal cancer cells
via inducing connexin expression and enhancing gap junction
function. Cancer Lett. 380:184–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia YS, Hu XQ, Li JA, Andras S, Hegyi G
and Han BS: Tonglian Decoction arrests the cell cycle in S-phase by
targeting the nuclear factor-kappa B signal pathway in esophageal
carcinoma Eca109 cells. Chin J Integr Med. 22:384–389. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin LW, Hsieh MT, Tsai FH, Wang WH and Wu
CR: Anti-nociceptive and anti-inflammatory activity caused by
Cistanche deserticola in rodents. J Ethnopharmacol. 83:177–182.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu CR, Lin HC and Su MH: Reversal by
aqueous extracts of Cistanche tubulosa from behavioral
deficits in Alzheimer's disease-like rat model: Relevance for
amyloid deposition and central neurotransmitter function. BMC
Complement Altern Med. 14:2022014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Aipire A, Gao L, Huo S, Luo J and
Zhang F: Phenylethanoid glycosides from Cistanche tubulosa
inhibits the growth of B16-F10 cells both in vitro and in vivo by
induction of apoptosis via mitochondria-dependent Pathway. J
Cancer. 7:1877–1887. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brand-Williams W, Culivier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
LWT-Food Sci Technol. 28:25–30. 1995. View Article : Google Scholar
|
|
26
|
Bansal P, Paul P, Nayak PG, Pannakal ST,
Zou JH, Laatsch H, Priyadarsini KI and Unnikrishnan MK: Phenolic
compounds isolated from Pilea microphylla prevent radiation-induced
cellular DNA damage. Acta Pharm Sin B. 1:226–235. 2011. View Article : Google Scholar
|
|
27
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B Induces G1
arrest, apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YS, Shen Q and Li J: Traditional
chinese medicine targeting apoptotic mechanisms for esophageal
cancer therapy. Acta Pharmacol Sin. 37:295–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Boil. 11:621–632. 2010. View Article : Google Scholar
|
|
31
|
Galluzzi L, Kepp O and Kroemer G:
Mitochondria: Master regulators of danger signalling. Nat Rev Mol
Cell Boil. 13:780–788. 2012. View Article : Google Scholar
|
|
32
|
Chong ZZ, Lin SH, Li F and Maiese K: The
sirtuin inhibitor nicotinamide enhances neuronal cell survival
during acute anoxic injury through AKT, BAD, PARP, and
mitochondrial associated ‘anti-apoptotic’ pathways. Curr Neurovasc
Res. 2:271–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng GZ, Wang Z, Zhao LM, Fan JT and Tan
NH: NF-κB and JNK mediated apoptosis and G0/G1 arrest of HeLa cells
induced by rubiarbonol G, an arborinane-type triterpenoid from
rubia yunnanensis. J Ethnopharmacol. 220:220–227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lei K and Davis RJ: JNK phosphorylation of
Bim-related members of the Bcl2 family induces bax-dependent
apoptosis. Proc Natl Acad Sci USA. 100:2432–2437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tournier C, Hess P, Yang DD, Xu J, Turner
TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA and Davis RJ:
Requirement of JNK for stress-induced activation of the cytochrome
c-mediated death pathway. Science. 288:870–874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. J Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hotchkiss RS and Nicholson DW: Apoptosis
and caspases regulate death and inflammation in sepsis. Nat Rev
Immunol. 6:813–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuang R, Sun Y, Yuan W, Lei L and Zheng X:
Protective effects of echinacoside, one of the phenylethanoid
glycosides, on H(2)O(2)-induced cytotoxicity in PC12 cells. Planta
Med. 75:1499–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao Q, Yang X, Cai D, Ye L, Hou Y, Zhang
L, Cheng J, Shen Y, Wang K and Bai Y: Echinacoside protects against
MPP(+)-induced neuronal apoptosis via ROS/ATF3/CHOP pathway
regulation. Neurosci Bull. 32:349–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang YH, Xuan ZH, Tian S and Du GH:
Echinacoside protects against 6-hydroxydopamine-induced
mitochondrial dysfunction and inflammatory responses in PC12 cells
via reducing ROS production. Evid Based Complement Alternat Med.
2015:1892392015.PubMed/NCBI
|
|
41
|
Lecci RM, Logrieco A and Leone A:
Pro-oxidative action of polyphenols as action mechanism for their
pro-apoptotic activity. Anticancer Agents Med Chem. 14:1363–1375.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng W, Xiao T, Cai A, Cai W, Liu H, Liu
J, Li J, Tan M, Xie L, Liu Y, et al: Inhibiting ROS-TFEB-dependent
autophagy enhances salidroside-induced apoptosis in human
chondrosarcoma cells. Cell Physiol Biochem. 43:1487–1502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong L, Yu D, Wu N, Wang H, Niu J, Wang Y
and Zou Z: Echinacoside induces apoptosis in human SW480 colorectal
cancer cells by induction of oxidative DNA damages. Int J Mol Sci.
16:14655–14668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun SY: Apoptosis induction by
chemopreventive agents. Drug News perspect. 14:75–80.
2001.PubMed/NCBI
|
|
45
|
Herceg Z and Wang ZQ: Functions of
poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity
and cell death. Mutat Res. 477:97–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang L, Yu Z, Lin Y, Cui L, Yao S, Lv L
and Liu J: Low-molecular-weight polysaccharides from agaricus
blazei murrill modulate the Th1 response in cancer immunity. Oncol
Lett. 15:3429–3436. 2018.PubMed/NCBI
|
|
47
|
Yang SF, Zhuang TF, Si YM, Qi KY and Zhao
J: Coriolus versicolor mushroom polysaccharides exert
immunoregulatory effects on mouse B cells via membrane Ig and TLR-4
to activate the MAPK and NF-κB signaling pathways. Mol Immunol.
64:144–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Son YO, Kook SH and Lee JC: Glycoproteins
and polysaccharides are the main class of active constituents
required for lymphocyte stimulation and antigen-specific immune
response induction by traditional medicinal herbal plants. J Med
Food. 20:1011–1021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Swanson-Mungerson M, Incrocci R,
Subramaniam V, Williams P, Hall ML and Mayer AMS: Effects of
cyanobacteria oscillatoria sp. lipopolysaccharide on B cell
activation and Toll-like receptor 4 signaling. Toxicol Lett.
275:101–107. 2017. View Article : Google Scholar : PubMed/NCBI
|