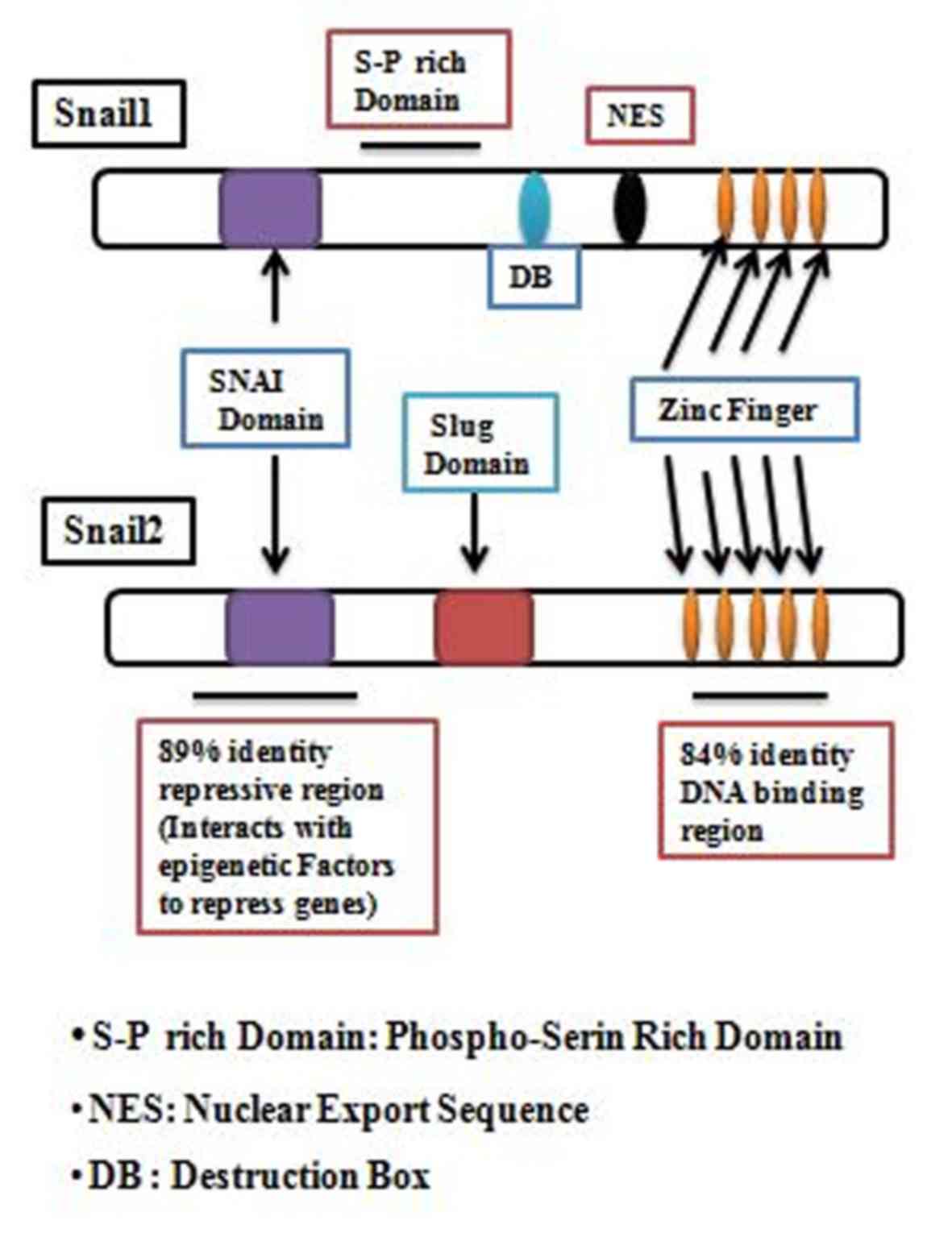
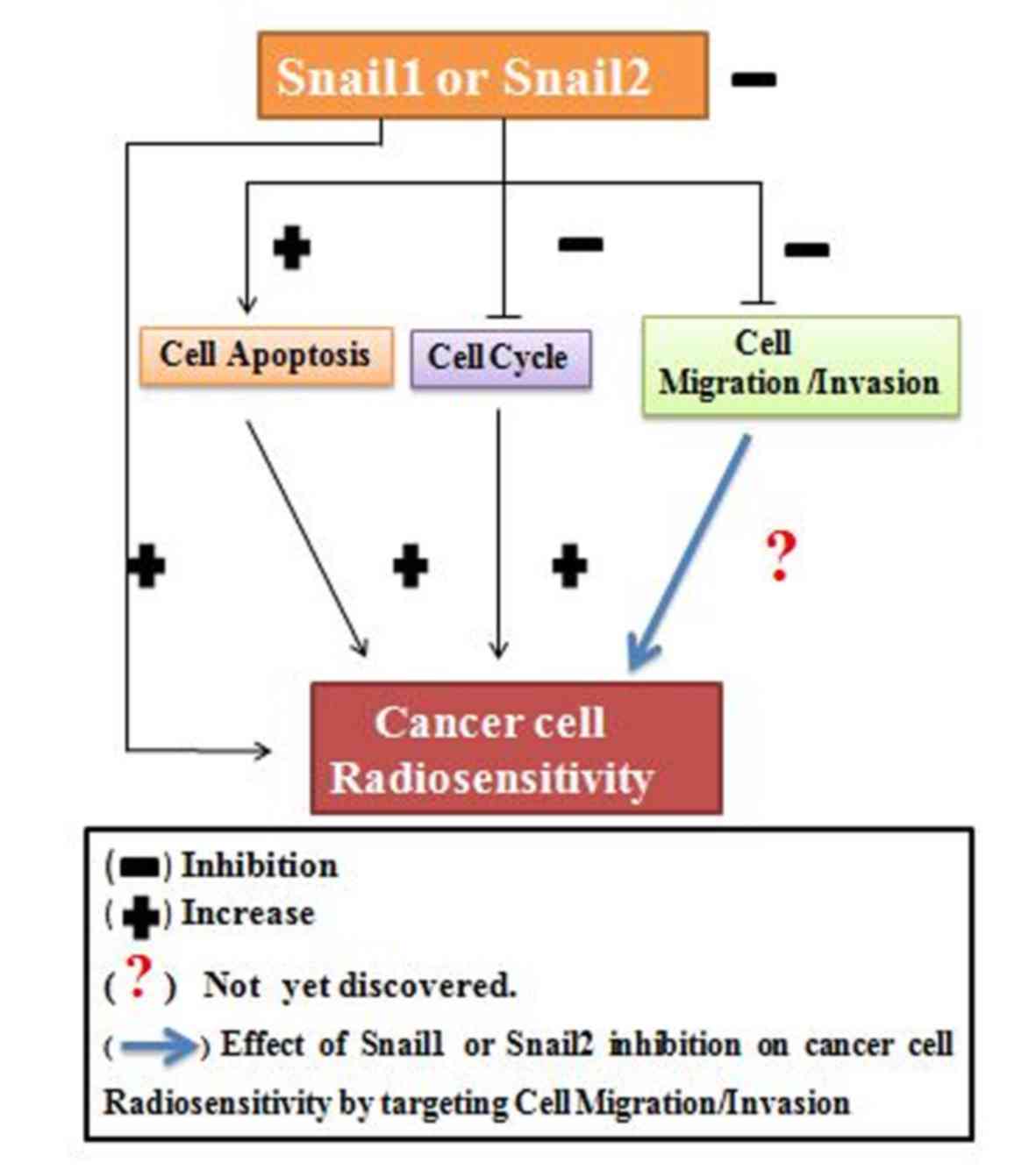
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: International Agency for Research on Cancer. GLOBOCAN 2012
v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase
No.11.Globocan.iarc.fr. December. 12. 2013
|
|
2
|
World Health Organization. Health
Statistics and Information Systems. WHO Mortality. simpleDatabase.who.int/healthinfo/mortality_data/en/November
6–2014
|
|
3
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clifton K, Gutierrez-Barrera A, Ma J,
Bassett R Jr, Litton J, Kuerer H, Moulder S, Albarracin C,
Hortobagyi G and Arun B: Adjuvant versus neoadjuvant chemotherapy
in triple-negative breast cancer patients with BRCA mutations.
Breast Cancer Res Treat. 170:101–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lui F, Xu K, Yang H, Li Y, Liu J, Wang J
and Guan Z: A novel approach to glioma therapy an oncolytic
adenovirus with two specific promoters. Oncol Lett. 15:3362–3368.
2018.PubMed/NCBI
|
|
6
|
Bykov IM, Izhnina EV, Kochurova EV and
Lapina NV: Radiation-associated changes in salivation of patients
with cancer of maxillofacial region. Stomatologia (Mosk). 97:67–70.
2018.(In Russian). View Article : Google Scholar
|
|
7
|
Ochoa CE and Joseph RW: Nivolumab in renal
cell carcinoma: Current trends and future perspectives. J Kidney
Cancer VHL. 5:15–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delaney G, Jacob S, Featherstone C and
Barton M: The role of radiotherapy in cancer treatment: Estimating
optimal utilization from a review of evidence-based clinical
guidelines. Cancer. 104:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delaney G, Jacob S and Barton M:
Estimating the optimal external-beam radiotherapy utilization rate
for genitourinary malignancies. Cancer. 103:462–473. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedroza-Torres A, Campos-Parra AD,
Millan-Catalan O, Loissell-Baltazar YA, Zamudio-Meza H, Cantú de
León D, Montalvo-Esquivel G, Isla-Ortiz D, Herrera LA,
Ángeles-Zaragoza Ó, et al: MicroRNA-125 modulates radioresistance
through targeting p21 in cervical cancer. Oncol Rep. 39:1532–1540.
2018.PubMed/NCBI
|
|
11
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tetzlaff MT, Teh BS, Timme TL, Fujita T,
Satoh T, Tabata K, Mai WY, Vlachaki MT, Amato RJ, Kadmon D, et al:
Expanding the therapeutic index of radiation therapy by combining
in situ gene therapy in the treatment of prostate cancer. Technol
Cancer Res Treat. 5:23–36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng X, Zhang Y, Liu Y, Fang L, Li L, Sun
J, Pan Z, Xin W and Huang P: HIF-2α activated lncRNA NEAT1 promotes
hepatocellular carcinoma cell invasion and metastasis by affecting
the epithelial-mesenchymal transition. J Cell Biochem.
119:3247–3256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang F, Gu Y, Zhao Z, Huang J, Jiang WG
and Cheng S: NHERF1 suppresses lung cancer cell migration by
regulation of epithelial-mesenchymal transition. Anticancer Res.
37:4405–4414. 2017.PubMed/NCBI
|
|
15
|
Robson EJ, Khaled WT, Abell K and Watson
CJ: Epithelial-to-mesenchymal transition confers resistance to
apoptosis in three murine mammary epithelial cell lines.
Differentiation. 74:254–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lovisa S, LeBleu VS, Tampe B, Sugimoto H,
Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC,
Pentcheva-Hoang T, et al: Epithelial-to-mesenchymal transition
induces cell cycle arrest and parenchymal damage in renal fibrosis.
Nat Med. 21:998–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du B and Shim JS: Targeting
Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance
in Cancer. Molecules. 21(pii): E9652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Desai S, Barai A, Bukhari AB, De A and Sen
S: α-Actinin-4 confers radioresistance coupled invasiveness in
breast cancer cells through AKT pathway. Biochim Biophys Acta Mol
Cell Res. 1865:196–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martínez-Alvarez C, Blanco MJ, Pérez R,
Rabadán MA, Aparicio M, Resel E, Martínez T and Nieto MA: Snail
family members and cell survival in physiological and pathological
cleft palates. Dev Biol. 265:207–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Côme C, Arnoux V, Bibeau F and Savagner P:
Roles of the transcription factors snail and slug during mammary
morphogenesis and breast carcinoma progression. J Mammary Gland
Biol Neoplasia. 9:183–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP: Epithelial-mesenchymal
transitions in tumor progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savanger P: Leaving the neighbourhood:
Molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Storci G, Sansone P, Trere D, Tavolari S,
Taffurelli M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M,
Montanaro L, et al: The basal-like breast carcinoma phenotype is
regulated by SLUG gene expression. J Pathol. 214:25–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hajra KM, Chen DY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 62:1613–1618. 2002.PubMed/NCBI
|
|
29
|
Zhou W, Lv R, Qi W, Wu D, Xu Y, Liu W, Mou
Y and Wang L: Snail contributes to the maintenance of stem
cell-like phenotype cells in human pancreatic cancer. PLoS One.
9:e874092014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guaita S, Puig I, Franci C, Garrido M,
Dominguez D, Batlle E, Sancho E, Dedhar S, De Herreros AG and
Baulida J: Snail induction of epithelial to mesenchymal transition
in tumor cells is accompanied by MUC1 repression and ZEB1
expression. J Biol Chem. 277:39209–39216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perez-Moreno MA, Locascio A, Rodrigo I,
Dhondt G, Portillo F, Nieto MA and Cano A: A new role for E12/E47
in the repression of E-cadherin expression and
epithelial-mesenchymal transitions. J Biol Chem. 276:27424–27431.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nieto MA, Sargent MG, Wilkinson DG and
Cooke J: Control of cell behavior during vertebrate development by
Slug, a zinc finger gene. Science. 264:835–839. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elloul S, Elstrand MB, Nesland JM, Tropé
CG, Kvalheim G, Goldberg I, Reich R and Davidson B: Snail, Slug,
and Smad-interacting protein 1 as novel parameters of disease
aggressiveness in metastatic ovarian and breast carcinoma. Cancer.
103:1631–1643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Côme C, Magnino F, Bibeau F, De Santa
Barbara P, Becker KF, Theillet C and Savagner P: Snail and slug
play distinct roles during breast carcinoma progression. Clin
Cancer Res. 12:5395–5402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hemavathy K, Ashraf SI and Ip YT:
Snail/slug family of repressors: Slowly going into the fast lane of
development and cancer. Gene. 257:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cobaleda C, Perez-Caro M, Vicente-Dueñas C
and Sánchez-García I: Function of the zinc-finger transcription
factor SNAI2 in cancer and development. Annu Rev Genet. 41:41–61.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor Snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tripathi MK, Misra S and Chaudhuri G:
Negative regulation of the expressions of cytokeratins 8 and 19 by
SLUG repressor protein in human breast cells. Biochem Biophys Res
Commun. 329:508–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kurrey NK, K A and Bapat SA: Snail and
slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu Z, Jiang Y, Steed H, Davidge S and Fu
Y: TGFβ and EGF synergistically induce a more invasive phenotype of
epithelial ovarian cancer cells. Biochem Biophys Res Commun.
401:376–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peiró S, Escrivà M, Puig I, Barberà MJ,
Dave N, Herranz N, Larriba MJ, Takkunen M, Francí C, Muñoz A, et
al: Snail1 transcriptional repressor binds to its own promoter and
controls its expression. Nucleic Acids Res. 34:2077–2084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kumar B, Uppuladinne MV, Jani V, Sonavane
U, Joshi RR and Bapat SA: Auto-regulation of Slug mediates its
activity during epithelial to mesenchymal transition. Biochim
Biophys Acta. 1849:1209–12018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Osorio LA, Farfán NM, Castellón EA and
Contreras HR: SNAIL transcription factor increases the motility and
invasive capacity of prostate cancer cells. Mol Med Rep.
13:778–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Z, Li X, Cai X, Huang C and Zheng M:
miR-497 inhibits epithelial-mesenchymal transition in breast
carcinoma by targeting Slug. Tumour Biol. 37:7939–7950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aletaha M, Mansoori B, Mohammadi A, Fazeli
M and Baradaran B: The Effect of Snail1 Gene Silencing by siRNA in
Metastatic Breast Cancer Cell Lines. Iran J Public Health.
46:659–670. 2017.PubMed/NCBI
|
|
51
|
Kajita M, McClinic KN and Wade PA:
Aberrant expression of the transcription factors snail and slug
alters the response to genotoxic stress. Mol Cell Biol.
24:7559–7566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Franco DL, Mainez J, Vega S, Sancho P,
Murillo MM, de Frutos CA, Del Castillo G, López-Blau C, Fabregat I
and Nieto MA: Snail1 suppresses TGF-beta-induced apoptosis and is
sufficient to trigger EMT in hepatocytes. J Cell Sci.
123:3467–3477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wan Z, Pan H, Liu S, Zhu J, Qi W, Fu K,
Zhao T and Liang J: Downregulation of SNAIL sensitizes
hepatocellular carcinoma cells to TRAIL-induced apoptosis by
regulating the NF-κB pathway. Oncol Rep. 33:1560–1566. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Olmeda D, Jordá M, Peinado H, Fabra A and
Cano A: Snail silencing effectively suppresses tumour growth and
invasiveness. Oncogene. 26:1862–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim S, Yao J, Suyama K, Qian X, Qian BZ,
Bandyopadhyay S, Loudig O, De Leon-Rodriguez C, Zhou ZN, Segall J,
et al: Slug promotes survival during metastasis through suppression
of Puma-mediated apoptosis. Cancer Res. 74:3695–3706. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Yue B, Yu X, Wang Z and Wang M:
SLUG is activated by nuclear factor kappa B and confers human
alveolar epithelial A549 cells resistance to tumor necrosis
factor-alpha-induced apoptosis. World J Surg Oncol. 11:122013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mancini M, Petta S, Iacobucci I,
Salvestrini V, Barbieri E and Santucci MA: Zinc-finger
transcription factor slug contributes to the survival advantage of
chronic myeloid leukemia cells. Cell Signal. 22:1247–1253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang K, Zhang S, Jiao X, Wang H, Zhang D,
Niu Z, Shen Y, Lv L and Zhou Y: Slug regulates proliferation and
invasiveness of esophageal adenocarcinoma cells in vitro and in
vivo. Med Oncol. 28:1089–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mezencev R, Matyunina lV, Jabbari N and
McDonald JF: Snail-induced epithelial-to-mesenchymal transition of
MCF-7 breast cancer cells: Systems analysis of molecular changes
and their effect on radiation and drug sensitivity. BMC Cancer.
16:2362016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Escrivà M, Peiró S, Herranz N, Villagrasa
P, Dave N, Montserrat-Sentís B, Murray SA, Francí C, Gridley T,
Virtanen I and García de Herreros A: Repression of PTEN phosphatase
by Snail1 transcriptional factor during gamma radiation-induced
apoptosis. Mol Cell Biol. 28:1528–1540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang K, Jiao X, Liu X, Zhang B, Wang J,
Wang Q, Tao Y and Zhang D: Knockdown of snail sensitizes pancreatic
cancer cells to chemotherapeutic agents and irradiation. Int J Mol
Sci. 11:4891–4892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang K, Zhang B, Lu Y, Sun C, Zhao W,
Jiao X, Hu J, Mu P, Lu H and Zhou C: Slug inhibition upregulates
radiation-induced PUMA activity leading to apoptosis in
cholangiocarcinomas. Med Oncol. 28 (Suppl 1):S301–S309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jiang F, Zhou L, Wei C, Zhao W and Yu D:
Slug inhibition increases radiosensitivity of oral squamous cell
carcinoma cells by upregulating PUMA. Int J Oncol. 49:709–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Inoue A, Seidel MG, Wu W, Kamizono S,
Ferrando AA, Bronson RT, Iwasaki H, Akashi K, Morimoto A, Hitzler
JK, et al: Slug, a highly conserved zinc finger transcriptional
repressor, protects hematopoietic progenitor cells from
radiation-induced apoptosis in vivo. Cancer Cell. 2:279–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arienti C, Tesei A, Carloni S, Ulivi P,
Romeo A, Ghigi G, Menghi E, Sarnelli A, Parisi E, Silvestrini R and
Zoli W: SLUG silencing increases radiosensitivity of melanoma cells
in vitro. Cell Oncol (Dordr). 36:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vega S, Morales AV, Ocaña OH, Valdés F,
Fabregat I and Nieto MA: Snail blocks the cell cycle and confers
resistance to cell death. Genes Dev. 18:1131–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mittal MK, Singh K, Misra S and Chaudhuri
GJ: SLUG-induced elevation of D1 cyclin in breast cancer cells
through the inhibition of its ubiquitination. Biol Chem.
286:469–479. 2011. View Article : Google Scholar
|
|
68
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu J, Uygur B, Zhang Z, Shao L, Romero D,
Vary C, Ding Q and Wu WS: Slug inhibits proliferation of human
prostate cancer cells via downregulation of cyclin D1 expression.
Prostate. 70:1768–1777. 2010.PubMed/NCBI
|
|
70
|
Emadi Baygi M, Soheili ZS, Essmann F,
Deezagi A, Engers R, Goering W and Schulz WA: Slug/SNAI2 regulates
cell proliferation and invasiveness of metastatic prostate cancer
cell lines. Tumour Biol. 31:297–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Biade S, Stobbe CC and Chapman JD: The
intrinsic radiosensitivity of some human tumor cells throughout
their cell cycles. Radiat Res. 147:416–421. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Neal CL, Mckeithen D and Odero-Marah VA:
Snail negatively regulates cell adhesion to extracellular matrix
and integrin expression via the MAPK pathway in prostate cancer
cells. Cell Adh Migr. 5:249–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia
L, Wu Y, Zhou BP and Feng Y: Snail is critical for tumor growth and
metastasis of ovarian carcinoma. Int J Cancer. 126:2102–2111.
2010.PubMed/NCBI
|
|
75
|
De Craene B, Gilbert B, Stove C, Bruyneel
E, van Roy F and Berx G: The transcription factor snail induces
tumor cell invasion through modulation of the epithelial cell
differentiation program. Cancer Res. 65:6237–6244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang A, Chen G, Meng L, Wang Q, Hu W, Xi
L, Gao Q, Wang S, Zhou J, Xu G, Meng L and Ma D: Antisense-Snail
transfer inhibits tumor metastasis by inducing E-cadherin
expression. Anticancer Res. 28:621–628. 2008.PubMed/NCBI
|
|
77
|
Smith BN, Burton LJ, Henderson V, Randle
DD, Morton DJ, Smith BA, Taliaferro-Smith L, Nagappan P, Yates C,
Zayzafoon M, et al: Snail promotes epithelial mesenchymal
transition in breast cancer cells in part via activation of nuclear
ERK2. PLoS One. 9:e1049872014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qian J, Liu H, Chen W, Wen K, Lu W, Huang
C and Fu Z: Knockdown of Slug by RNAi inhibits the proliferation
and invasion of HCT116 colorectal cancer cells. Mol Med Rep.
8:1055–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gu A, Jie Y, Yao Q, Zhang Y and Mingyan E:
Slug is associated with tumor metastasis and angiogenesis in
ovarian cancer. Reprod Sci. 24:291–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhao X, Sun B, Sun D, Liu T, Che N, Gu Q,
Dong X, Li R, Liu Y and Li J: Slug promotes hepatocellular cancer
cell progression by increasing sox2 and nanog expression. Oncol
Rep. 33:149–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu Y, Li L, Zheng Z, Chen S, Chen E and Hu
Y: Long non-coding RNA linc00261 suppresses gastric cancer
progression via promoting Slug degradation. J Cell Mol Med.
21:955–967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang YP, Wang MZ, Luo YR, Shen Y and Wei
ZX: Lentivirus-mediated shRNA interference targeting SLUG inhibits
lung cancer growth and metastasis. Asian Pac J Cancer Prev.
13:4947–4951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sun Y, Song GD, Sun N, Chen JQ and Yang
SS: Slug overexpression induces stemness and promotes
hepatocellular carcinoma cell invasion and metastasis. Oncol Lett.
7:1936–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bai JW, Chen MN, Wei XL, Li YC, Lin HY,
Chen M, Li JW, Du CW, Man K and Zhang GJ: The zinc-finger
transcriptional factor Slug transcriptionally downregulates ERα by
recruiting lysine-specific demethylase 1 in human breast cancer.
Oncogenesis. 6:e3302017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan
ZK, Liu K and Huang S: Extracellular signal-regulated kinase
signaling pathway regulates breast cancer cell migration by
maintaining slug expression. Cancer Res. 69:9228–9235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: MiR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Paquette B, Baptiste C, Therriault H,
Arguin G, Plouffe B and Lemay R: In vitro irradiation of basement
membrane enhances the invasiveness of breast cancer cells. Br J
Cancer. 97:1505–1512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Young AGH and Bennewith KL: Ionizing
radiation enhances breast tumor cell migration in vitro. Radiat
Res. 188:381–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rodman SN, Spence JM, Ronnfeldt TJ, Zhu Y,
Solst SR, O'Neill RA, Allen BG, Guan X, Spitz DR and Fath MA:
Enhancement of radiation response in breast cancer stem cells by
inhibition of thioredoxin- and glutathione-dependent metabolism.
Radiat Res. 186:385–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Du XL, Jiang T, Wen ZQ, Gao R, Cui M and
Wang F: Silencing of heat shock protein 70 expression enhances
radiotherapy efficacy and inhibits cell invasion in endometrial
cancer cell line. Croat Med J. 50:143–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yanamandra N, Kondraganti S, Srinivasula
SM, Gujrati M, Olivero WC, Dinh DH and Rao JS: Activation of
caspase-9 with irradiation inhibits invasion and angiogenesis in
SNB19 human glioma cells. Oncogene. 23:2339–2344. 2004. View Article : Google Scholar : PubMed/NCBI
|