Cancer is one of the leading causes of mortality in
more and less economically developed countries (1). It was estimated that 14.9 million new
cancer cases and 8.2 million cancer-associated mortalities occurred
in 2012 globally, among which lung and breast cancer were the most
frequently diagnosed cancer types in overall and less developed
countries, respectively (2). It is
predicted that 22 million new cancer cases will occur annually
within two decades (3). Based on
these data, cancer can be considered as a significant public health
issue worldwide, and thus requires intense research into the
improvement of prevention strategies and enhancing the
effectiveness of treatment. Numerous types of curative therapy,
including chemotherapy (4), gene
therapy (5), radiotherapy and
immunotherapy (6,7), are used to treat cancer. Radiotherapy is
an effective and commonly employed treatment in the management of
>50% of human malignancies and remains a standard therapeutic
modality for cancer patients (8,9). However,
intrinsic or acquired resistance, such as genetic abnormalities,
which lead to the promotion of angiogenesis and tissue progression,
limit the efficacy of radiotherapy (10,11).
Cancer gene therapy, which represents one ideal therapeutic tool,
can be combined with radiotherapy to enhance the radiotherapy
effect (12). Epithelial-mesenchymal
transition (EMT) is involved in cancer cell invasion (13), migration (14), resistance to apoptosis (15), the cell cycle (16) and therapy resistance (17). Modulation of EMT could change the
behaviors of cancer cells against therapies, particularly
radiotherapy (18). The SNAIL
transcription factor family has been associated with EMT (19). Snail1 and Slug are key SNAIL family
transcription factors that regulate EMT, and are also involved in
cancer progression (20) and
resistance to treatment such as radiotherapy (21). The present study reviews the
literature wherein the modulation of Snail1 and Snail2 (Slug)
expression was shown to influence cancer cell apoptosis, the cell
cycle and cell invasion/migration, and in which Snail1 and Slug
modulation enhanced the efficacy of radiotherapy by targeting
cancer cell apoptosis, the cell cycle and cell
invasion/migration.
EMT is recognized as a phenotypic conversion that
occurs during embryo development, as gastrulation, and during
neural crest formation in nervous system development (22). EMT has also been described in the
process of re-epithelialization during wound healing and in the
generation of tissue fibroblasts during the process of organ
fibrosis. EMT is a key step in the metastasis and invasion of
tumors (23), in the development of
tumor resistance to apoptosis and in cancer radiotherapy resistance
(24). A central group of EMT
regulators is the SNAIL superfamily of transcription factors, which
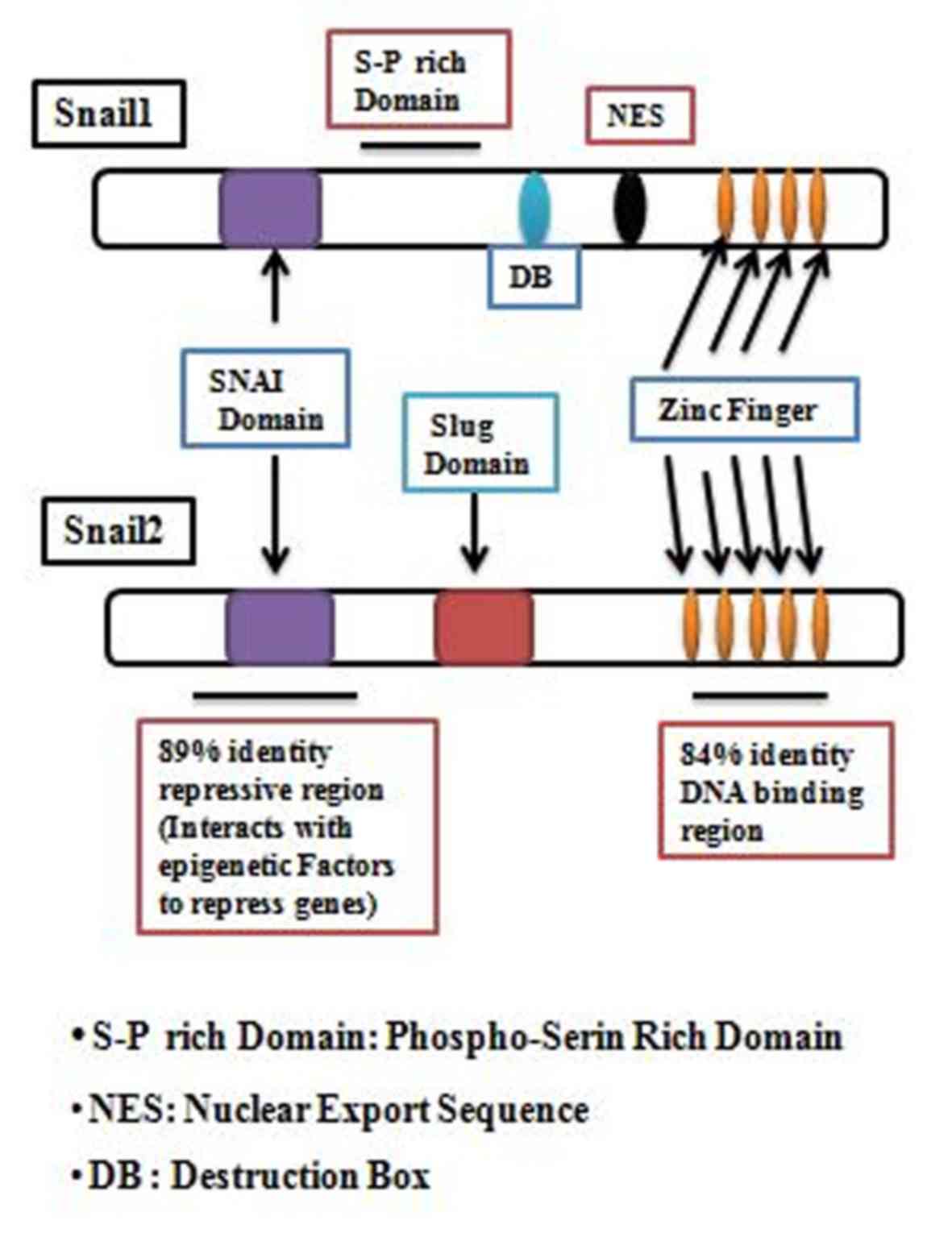
includes Snail1 and Slug (Fig. 1),
the most highly studied members of this family (25–28). SNAIL
family members are highly expressed in a variety of cancer types
and have been implicated in the regulation of tumor invasion,
metastasis, cell survival and cell proliferation (29–36).
Proteins of the SNAIL family have a similar structural
organization. The carboxyl terminus contains (4 to 6 motifs in the
terminus) C2H2 zinc-finger motifs that facilitate the direct
binding of the protein to DNA. The CAGGTG sequence is the consensus
DNA binding site for SNAIL family proteins and this motif is a
subset of the E-box sequence to which a number of basic
helix-loop-helix transcription factors bind (37,38).
The SNAIL gene (Snail1/Snail2) is implicated in EMT
via the suppression of epithelial markers (E-cadherin, vascular
endothelial cadherin, claudin, occludin, desmoplakin, cytokeratin
and mucin) associated with an epithelial phenotype (39–42) and
via upregulation of mesenchymal markers (fibronectin and
vitronectin) associated with the mesenchymal phenotype (31,39).
Snail-mediated EMT (Snail1/Snail2) associated with the suppression
of E-cadherin causes inhibition of cancer cell adhesion and
promotes the migratory capacity (43). At the molecular level, EMT regulation
by Slug is often associated with its ability to transcriptionally
repress the expression of epithelial gene E-cadherin (26). In epithelial cells, Snail
transcription is low and E-cadherin expression is high, which
prevents the stimulation of NK-κB and other signaling pathways.
Moreover, external stimuli, such as transforming growth factor-β
(TGF-β) expression, can induce Snail1/Snail2 protein activation
(44), which then binds to the SNAIL
gene (Snail1 protein binds to Snail1 gene and Snail2 protein binds
to Snail2 gene) (45,46). When E-cadherin expression is
inhibited, SNAIL (SNAI1/SNAI2) expression is amplified by a
self-stimulation loop due to the suppression of nuclear factor-κB
(NF-κB). Thus, the activity of the self-stimulation loop is
enhanced by the downregulation of E-cadherin via SNAIL
(Snail1/Snail2). Additionally, the induction of mesenchymal genes
and other suppressors, including zinc finger E-box-binding homeobox
1 (Zeb1), by NF-κB activation, leads to SNAIL inhibition by Zeb1
without a phenotype reversion. This could explain why the SNAIL
(Snail1/Snail2) gene is required for triggering EMT (47).
Snail1- or Slug-mediated EMT in cancer cells
generates a phenotype closely associated with the resistance of
cancer cells to apoptosis (48,49).
However, little research has been performed on cancer cells with
regard to the link between the direct or indirect modulation of
Snail1/Slug and cancer cell apoptosis.
It has been reported that the direct or indirect
modulation of Snail1 expression affects cancer cell apoptosis.
Through use of terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) assays to assess apoptosis, Aletaha et al
(50) showed that knockdown of Snail1
enhanced breast cancer cell apoptosis. In another study, Kajita
et al (51) reported that,
following induction of DNA damage by exposing breast cancer cells
to topoisomerase inhibitor Adriamycin (ADR), the relative apoptotic
activity of parental breast cancer cells was substantially
increased relative to that of adeno-Snail1 MCF-7 cells
(overexpressing Snail1), suggesting that Snail1 acts to prevent
ADR-induced cell death in breast cancer cell lines. In a prostate
cancer cell line, following the evaluation of caspase 3 and caspase
7 activities by fluorescence detection as a marker of apoptosis,
Osorio et al (48) showed that
Snail1 overexpression decreased the rate of cell apoptosis and that
prostate cancer cells with Snail1 silencing (shRNA-Snail1)
exhibited increased apoptosis (48).
Franco et al (52) also found
that Snail1 downregulation enhanced the apoptosis in murine hepatic
cells, and that activation of its expression blocked the apoptotic
effect of TGF-β in adult hepatocytes. Wan et al (53) reported that the inhibition of Snail1
enhanced TRAIL-induced apoptosis by upregulating cellular tumor
antigen p53 expression following combined hepatocarcinoma cell
transfection with lentiviral short hairpin (sh)Snail1 and
adenovirus type 5-TRAIL. However, in contrast to the aforementioned
reports, the study by Olmeda et al (54) showed that there was no significant
difference in the apoptotic index of the tumors caused by
sh-Snail1-derived cells and their corresponding controls, and that
there was also no change in the apoptotic response to serum
deprivation in HaCa4 shSnail1 and CarB-ShSnail1 cells compared with
that in their corresponding parental or control cells. Taken
together, these data suggest that Snail1 acts as an inhibitor of
apoptosis and that this function is dependent on the type of cell
line or tissue.
It has also been reported that the modulation of
Slug can affect cancer cell apoptosis. By analyzing the expression
levels of the B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X
(Bax) apoptosis markers, Wu et al (49) revealed that silencing of Slug using
Slug-shRNA or microRNA-497 (miR-497) in a non-metastatic breast
cancer cell line (MCF-7) enhanced the apoptotic index. Kajita et
al (51) also reported that
following transfection of MCF-7 cells with Slug adenovirus to
induce slug overexpression (MCF-7adSlug) and treatment with ADR as
a cell apoptosis inducer, there was a notable reduction in the
apoptotic abilities of treated cells (MCF-7adSlug) relative to that
of untreated cells, suggesting that Slug acted as an apoptosis
inhibitor in the breast cancer cell line. In another cancer cell
line (PyMT-N-cad), Kim et al (55) showed that Slug attenuation by shRNA or
fibroblast growth factor receptor inhibitor in a mammary tumor cell
line increased caspase3 activity and poly(adenosine diphosphate
ribose) polymerase levels, which are markers of apoptosis. It was
previously shown that Slug silencing in human alveolar epithelial
A549 cells and treatment with apoptosis-inducer tumor necrosis
factor-α increased the apoptotic index in Slug-silenced cells
(56). Mancini et al (57) also demonstrated that Slug
overexpression contributes to apoptosis resistance in leukemic
progenitors. In contrast to this study, and in confirmation of
other aforementioned studies, Zhang et al (58) assessed apoptosis by measuring caspase
3 activity, TUNEL assay and Hoechst 33258 staining, and showed that
slug overexpression does not have a significant effect on the
apoptotic index in the TE-7 cell line, but that the inhibition of
Slug expression in the esophageal cancer OE33 cell line leads to a
marked increase in apoptosis in vitro and in vivo.
This suggests that Slug silencing can effectively inhibit tumor
growth in vitro and in vivo through the induction of
apoptosis. According to these data, Snail1 and Slug modulation
could have a significant role in cancer therapy and improve cancer
therapy effectiveness when the modulation is combined with another
cancer therapeutic strategy such as radiotherapy. However, further
studies are required regarding the link between Snail1/Slug
inhibition or overexpression and the apoptosis in different cancer
cell lines.
Apoptosis, also known as programmed cell death,
serves an important role in cancer cell radiation sensitivity. To
date, there have been few studies concerning the roles of EMT
transcription factors Snail1 and Slug in cancer radiosensitivity,
specifically by targeting cell apoptosis. According to the
aforementioned description of the association between Snail1 and
cancer apoptosis, the modulation of Snail1 could impair cancer
radiosensitivity by targeting cell apoptosis. Mezencev et al
(59) found that MCF-7 cells with
ectopic expression of Snail1 displayed increased radiosensitivity,
but the association with apoptosis has yet to be studied. This
study does not correlate with the study by Escriva et al
(60), which showed that ectopic
expression of Snail1 in the MDCK cell line induced cancer cell
radioresistance by diminishing the apoptosis caused by irradiation
where only 8–10% of cells that ectopically expressed Snail1
underwent apoptosis 48 h after γ-irradiation (60). However, cells with decreased Snail1
expression displayed a higher sensitivity to irradiation-induced
apoptosis, as described in the study by Zhang et al
(61), which stated that the
combination of γ-irradiation (6 Gy) and type 2 recombinant
adeno-associated virus (rAAV2)-small interfering (si)RNA-Snail lead
to a markedly enhanced apoptotic response and radiosensitivity in
pancreatic PANC-1 cells and to decreased radiosensitivity in MDCK
cells via the targeting of apoptosis due to Snail1 overexpression
(60). The data suggest that the
modulatory role served by Snail1 in cancer cell radiosensitivity
via the targeting of apoptosis is dependent on the type on cells.
As with Snail1, it has been reported that Slug modulation also
impairs cancer cell radiosensitivity by targeting cell apoptosis.
Zhang et al (62) used the
TUNEL assay to show that transfection with Slug siRNA and
adenovirus rAAV2 for Slug silencing combined with 6 Gy irradiation
significantly increased the apoptosis in a human cholangiocarcinoma
cell line compared with that found with Slug silencing or
irradiation alone. In the oral squamous carcinoma HSC3 and HSC6
cell lines, Jiang et al (63)
used caspase 3, Bax and Bcl-2 expression levels as apoptosis
markers and showed that X-ray irradiation improved Slug expression,
and that the inhibition of Slug associated with X-ray irradiation
enhanced the apoptosis induced (63).
Inoue et al (64) also
reported that Slug (−/-) mice are more radiosensitive compared to
the Wild-type mice, and used TUNEL assays to demonstrate that
hematopoietic progenitor of Slug (−/-) mice exhibited increased
apoptosis 6 h after irradiation (6 Gy). Arienti et al
(65) used western blotting to
analyze the expression level of certain apoptotic marker proteins
(caspase 3, p53 upregulated modulation of apoptosis and p21) and
found that the inhibition of slug expression in melanoma cells
enhanced their radiosensitivity by increasing the expression of
these proteins. Therefore, Slug inhibition was shown to improve
radiosensitivity by targeting apoptosis in the melanoma cell line.
Thus, Snail1 and Slug modulation is suggested to modulate cancer
cell radiosensitivity. However, this suggestion should be confirmed
in various types of cancer cell lines.
The cell cycle is one of the important steps to
cancer cell progression and the response to radiotherapy. Moreover,
Snail1 has a major role in certain steps of cancer development,
including cell cycle progression. To date, few studies have been
conducted on the link between Snail1 and the cancer cell cycle.
Using a fluorescence-activated cell sorting (FACS) assay to assess
the cell cycle, Aletaha et al (50) showed that Snail1 inhibition in
MDA-MB-468 cells regulated G1 phase transition (early
and late) and the G1/S checkpoint, which resulted in
cell cycle arrest at the sub-G1 and S phases. Moreover,
Vega et al (66) reported that
in MDCK-Snail1 cells stably overexpressing Snail1 following
transfection, the majority (93%) were in the
G0/G1 phase of the cell cycle under basal
conditions after 72 h in culture. These data suggested that Snail1
modulation can impair cell cycle progression by causing cell cycle
arrest and that the phase of the cell cycle in which the cells are
arrested is dependent on the type of cancer cell.
As with Snail1, Slug is also involved in the control
of cell cycle progression. Mittal et al (67) showed that Slug is positively
correlated with cyclin D1, which serves a pivotal role in cell
cycle control in normal and cancer cells, particularly in the
G1 phase of the cell cycle (68). Downregulation of Slug could lead to
the inhibition of cyclin D1 expression and cell cycle arrest in the
G1 phase (67). This
hypothesis does not correlate with the findings of the study by Liu
et al (69), in which Slug
expression was negatively correlated with cyclin D1 expression in a
prostate cancer cell line. According to this study, the induced
expression of Slug can lead to the inhibition of cyclin D1 and cell
cycle arrest in the G0/G1 phase; therefore,
the effect of Slug modulation on the cancer cell cycle is also
dependent on the type of cancer cell line. However, Essmann et
al (70), through use of FACS
assays, reported that the treatment of the PC3-16 cells line with
Slug siRNA to downregulate its expression resulted in
G0/G1 cell cycle phase arrest in the majority
(84.2±2.6 vs. 69.6±0.62%) of cells at 72 h post-treatment, meaning
that Slug modulation has an impact on G1 phase
transition.
Since radiosensitivity has previously been shown to
be dependent on the phase of the cell cycle (71,72) it is
hypothesized that targeting the cell cycle by modulating Snail1 or
Slug could enhance the effect of radiotherapy. Mezencev et
al (59) used a FACS assay to
show that the ectopic expression of Snail1 increased the proportion
of MCF-7 cells in the G2/M phase. This suggested that
this proportion of G2/M phase cells could be increased
following irradiation, which can be implicated in high
radiosensitivity, as cells are more sensitive to irradiation during
the M and G2 phases (72).
In the MDCK cell line, it was reported that MDCK-Snail1 clones
presented with a high proportion of cells in the G1
phase relative to the control (40 vs. 20%), during and at 48 h
post-irradiation. Additionally, an increase in G2/M
MDCK-Snail1 cell cycle arrest (56% of MDCK-Snail1 cells in the
G2/M phase) was noted (66). As aforementioned, Slug also can
modulate the radiosensitivity of cancer cells by targeting the cell
cycle. In melanoma cancer cell lines, Arienti et al
(65) showed that Slug silencing with
or without irradiation impaired cell cycle progression. Radiation
treatment enhanced the percentage of G2/M phase cells in
the M14 and M19 cell lines, an effect that was greater with 5 Gy
than with 2.4 Gy of treatment (65).
Slug silencing further increased the proportion of G2/M
phase M14 cells following irradiation with 5 Gy, confirming the
results of the study by Mezencev et al (59), and increased the percentage of M19
cells in the S phase. Concordant with the results by Arienti et
al (65) using M19 cells, the
study by Jiang et al (63)
found that X-ray treatment combined with Slug silencing increased
the proportion of cells arrested in the S phase, as compared with
Slug silencing or X-ray alone, in the HSC3 and HSC6 cell lines.
Therefore, the modulation of Snail1 and Slug expression can impair
cell cycle arrest and modulate cancer cell radiosensitivity by
targeting the cell cycle. However, the efficacy of this approach is
dependent on the type of cancer.
Several studies have implicated the modulation of
Snail1 and Slug expression in cancer cell migration and invasion
(48,73–75). A
study using a scratch-wound assay to assess the directional
migration of the breast cancer MDA-MB-468 cell line reported that
silencing Snail1 significantly reduced the cell migration and
invasion at 24 and 48 h post-transfection (50). Zhang et al (76) showed that parental MDA-MB-231 cells
(overexpressing Snail1) exhibited high mobility compared with MDCK
cells (used as a good cell line for invasion/migration studies and
with no Snail1 expression). Following transfection with AdvSnail1,
MDCK-Snail1 cells started to migrate faster into the wound region
relative to the control, and Adv-antisense-Snail1-transfected
MDA-MB-231 cells colonized ~50% of the wound region at 24 h
post-wounding, while the mock infected cells occupied almost 95% of
the wound region. Following assessment of invasion ability, the
percentage of invasive Adv-Snail1-transfected MDCK cells increased
(0.236±0.022%), whereas the percentage of invasive non-transfected
parental cells was 0.126±0.015%. The invasive ability of the
MDA-MB-231 cells transfected with Adv-antisense-Snail1 was markedly
decreased compared with that of the non-transfected parental cells
(0.215±0.0140 vs. 0.392±0.021%) (76). Smith et al (77), using migration and invasion assays
performed on collagen I and fibronectin matrices, reported that
MCF-7-Snail1 displayed decreased cell adhesion and increased cell
migration compared with mock MCF-7-Neo cells (77). These data suggested that Snail1
expression is positively correlated with cell migration and
invasion abilities.
As with Snail1, the modulation of Slug expression
has also been implicated in the impairment of cell invasion and
migration capacities (78–84). Bai et al (85) used Transwell and wound-healing assays
to show that the relative migrated distance at 24 h
post-transfection compared with the corresponding control was ~38.5
and 23.1% in the control and MDA-MB-231 siRNA-Slug groups,
respectively; similar results were found at 60 h post-transfection.
The study by Chen et al (86)
indicated that the inhibition of slug in MDA-MB-231 and MDA-MB-436
cells can lead to the inhibition of migration to ~40% of the rate
observed in control cells. This result corroborates that of the
study by Liang et al (87), in
which Slug silencing using miR-124 reduced the migration and
invasion abilities of the MDA-MB-231 cell line, whereas activation
of Slug by overexpression of Slug in MDA-MB-231 miR-124 cells
abrogated this reduction of migration and invasion capacities.
Contrary to the hypothesis that Slug-knockdown reduces cell
migration/invasion, Kim et al (55) showed that Slug-knockdown did not
inhibit cell migration and invasion in the PyMT-N-cad metastatic
cell line, meaning that Snail1 and Slug inhibition-mediated
reduction of cell migration and/or invasion depends on the type of
the cancer cell line.
The malignant progression of cancer depends on
various cell properties, including mobility, invasiveness and
metastatic potential, among others. It has been demonstrated that
radiation can enhance the invasiveness and migratory capacity in a
number of cancer cell lines (88).
Young et al (89) also
reported that a 2.3 Gy dose of irradiation was sufficient to
increase the migration of metastatic MDA-MB-231 cells. Conversely,
Rodman et al (90) reported
that 2 Gy of irradiation can reduce the migratory ability of
MDA-MB-231 cells. Based on these reports, the effect of radiation
on cell migration and invasion is dependent on the cell type.
Combining gene therapy techniques (modulating Snail1 or Slug
expression) with radiotherapy could enhance the radiotherapy
efficacy by reducing the migration and invasion potential caused by
irradiation or by enhancing the reduction of the migration and
invasion effect of radiotherapy or of the modulation of Snail1
and/or Slug. In the study by Du et al (91), it was demonstrated that heat shock
protein 70 silencing significantly inhibited the cell invasion
prior to and following irradiation in the human endometrial cancer
ISK cell line. Moreover, activation of caspase 9 combined with
irradiation enhanced the human glioma SNB19 cell invasion ability
compared with the use of caspase 9 activation or irradiation alone
(92). Taken together, radiotherapy
and gene therapy may have a greater benefit on cell invasion or
migration compared with irradiation or gene therapy alone. However,
more studies should be performed in future confirming the
aforementioned data and hypotheses, particularly that regarding
Snail1 or Slug inhibition enhancing cancer radiosensitivity by
targeting cell migration and invasion, in various types of cancer
cell line.
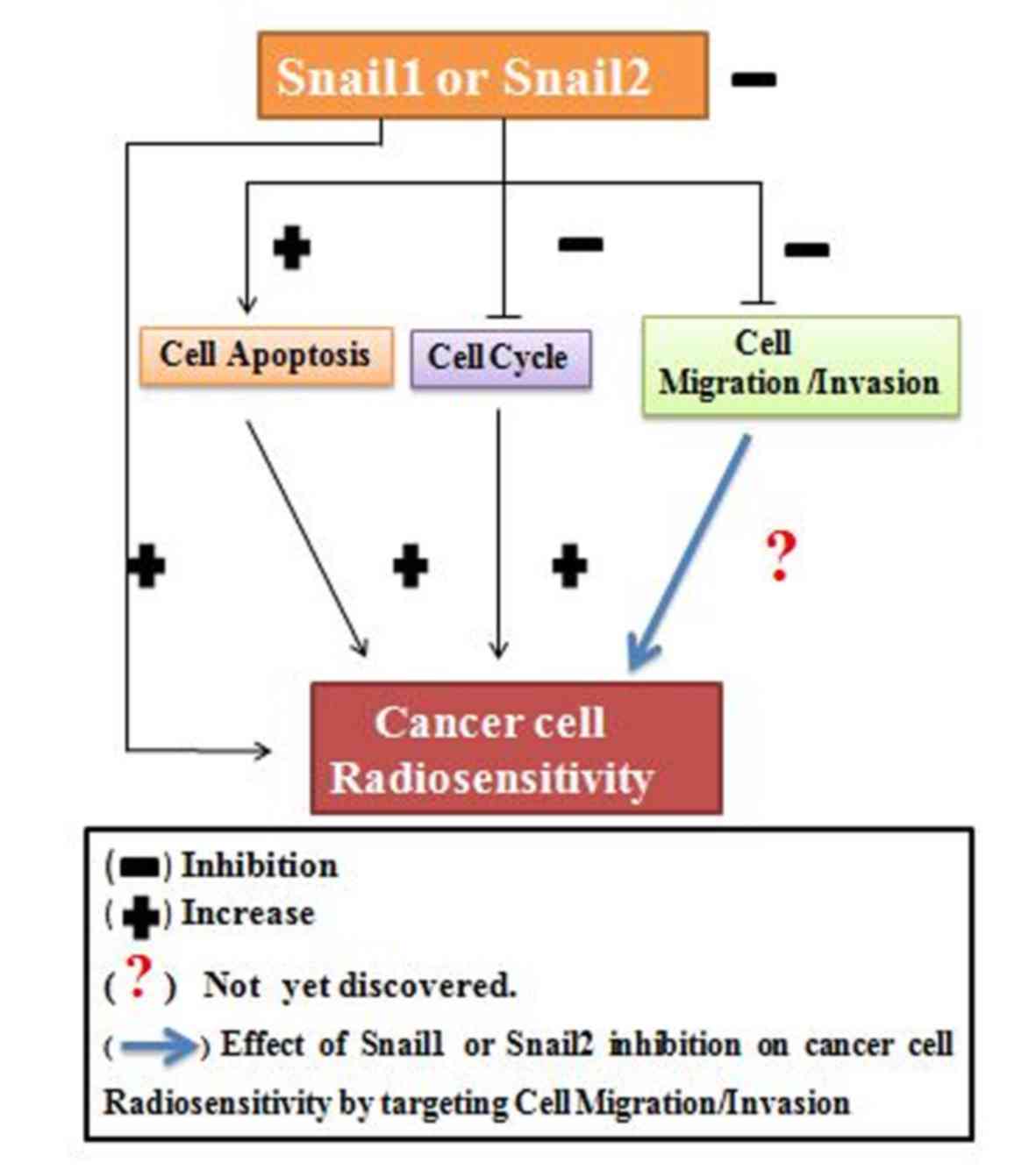
Based on these data, Snail1 and Slug inhibition can
be considered to have an ability to improve cancer radiosensitivity
by increasing cancer cell apoptosis, acting by arresting the cancer
cell cycle, while the association between Snail1 and Slug
inhibition and cancer radiosensitivity by targeting cell migration
or invasion remains to be elucidated (Fig. 2).
Snail1 and Slug are SNAIL family transcription
factors that have been studied in a number of cancer cell types. As
EMT regulators, Snail1 and Slug are highly expressed in numerous
cancer cell types and are implicated in cancer cell cycle
progression, cell apoptosis and cell migration/invasion. Modulation
of the expression of these genes is implicated in the impairment of
cancer progression and cancer cell radiosensitivity by targeting of
cell apoptosis, the cell cycle and migration/invasion. Due to the
limitations of radiotherapy or gene therapy alone, the combined use
of gene therapy, for inhibiting the expression of Snail1 or Slug,
and radiotherapy, enhances the cancer cell radiosensitivity.
However, more research is required concerning the effect of the
modulation (inhibition or overexpression) of expression of Snail1
and Slug on cancer radiosensitivity, particularly in different
types of cancer cell line. This could aid radio-oncologists in
mastering how to manipulate these genes prior to or following
radiotherapy for the enhancement of radiotherapeutic efficacy.
Not applicable.
The present study was supported by the China
Scholarship Council, (serial no: 351569).
Not applicable.
GA was in charge of designing and writing the
manuscript. YZ was involved in drafting the manuscript. Both
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: International Agency for Research on Cancer. GLOBOCAN 2012
v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase
No.11.Globocan.iarc.fr. December. 12. 2013
|
|
2
|
World Health Organization. Health
Statistics and Information Systems. WHO Mortality. simpleDatabase.who.int/healthinfo/mortality_data/en/November
6–2014
|
|
3
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clifton K, Gutierrez-Barrera A, Ma J,
Bassett R Jr, Litton J, Kuerer H, Moulder S, Albarracin C,
Hortobagyi G and Arun B: Adjuvant versus neoadjuvant chemotherapy
in triple-negative breast cancer patients with BRCA mutations.
Breast Cancer Res Treat. 170:101–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lui F, Xu K, Yang H, Li Y, Liu J, Wang J
and Guan Z: A novel approach to glioma therapy an oncolytic
adenovirus with two specific promoters. Oncol Lett. 15:3362–3368.
2018.PubMed/NCBI
|
|
6
|
Bykov IM, Izhnina EV, Kochurova EV and
Lapina NV: Radiation-associated changes in salivation of patients
with cancer of maxillofacial region. Stomatologia (Mosk). 97:67–70.
2018.(In Russian). View Article : Google Scholar
|
|
7
|
Ochoa CE and Joseph RW: Nivolumab in renal
cell carcinoma: Current trends and future perspectives. J Kidney
Cancer VHL. 5:15–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delaney G, Jacob S, Featherstone C and
Barton M: The role of radiotherapy in cancer treatment: Estimating
optimal utilization from a review of evidence-based clinical
guidelines. Cancer. 104:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delaney G, Jacob S and Barton M:
Estimating the optimal external-beam radiotherapy utilization rate
for genitourinary malignancies. Cancer. 103:462–473. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedroza-Torres A, Campos-Parra AD,
Millan-Catalan O, Loissell-Baltazar YA, Zamudio-Meza H, Cantú de
León D, Montalvo-Esquivel G, Isla-Ortiz D, Herrera LA,
Ángeles-Zaragoza Ó, et al: MicroRNA-125 modulates radioresistance
through targeting p21 in cervical cancer. Oncol Rep. 39:1532–1540.
2018.PubMed/NCBI
|
|
11
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tetzlaff MT, Teh BS, Timme TL, Fujita T,
Satoh T, Tabata K, Mai WY, Vlachaki MT, Amato RJ, Kadmon D, et al:
Expanding the therapeutic index of radiation therapy by combining
in situ gene therapy in the treatment of prostate cancer. Technol
Cancer Res Treat. 5:23–36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng X, Zhang Y, Liu Y, Fang L, Li L, Sun
J, Pan Z, Xin W and Huang P: HIF-2α activated lncRNA NEAT1 promotes
hepatocellular carcinoma cell invasion and metastasis by affecting
the epithelial-mesenchymal transition. J Cell Biochem.
119:3247–3256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang F, Gu Y, Zhao Z, Huang J, Jiang WG
and Cheng S: NHERF1 suppresses lung cancer cell migration by
regulation of epithelial-mesenchymal transition. Anticancer Res.
37:4405–4414. 2017.PubMed/NCBI
|
|
15
|
Robson EJ, Khaled WT, Abell K and Watson
CJ: Epithelial-to-mesenchymal transition confers resistance to
apoptosis in three murine mammary epithelial cell lines.
Differentiation. 74:254–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lovisa S, LeBleu VS, Tampe B, Sugimoto H,
Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC,
Pentcheva-Hoang T, et al: Epithelial-to-mesenchymal transition
induces cell cycle arrest and parenchymal damage in renal fibrosis.
Nat Med. 21:998–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du B and Shim JS: Targeting
Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance
in Cancer. Molecules. 21(pii): E9652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Desai S, Barai A, Bukhari AB, De A and Sen
S: α-Actinin-4 confers radioresistance coupled invasiveness in
breast cancer cells through AKT pathway. Biochim Biophys Acta Mol
Cell Res. 1865:196–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martínez-Alvarez C, Blanco MJ, Pérez R,
Rabadán MA, Aparicio M, Resel E, Martínez T and Nieto MA: Snail
family members and cell survival in physiological and pathological
cleft palates. Dev Biol. 265:207–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Côme C, Arnoux V, Bibeau F and Savagner P:
Roles of the transcription factors snail and slug during mammary
morphogenesis and breast carcinoma progression. J Mammary Gland
Biol Neoplasia. 9:183–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP: Epithelial-mesenchymal
transitions in tumor progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savanger P: Leaving the neighbourhood:
Molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Storci G, Sansone P, Trere D, Tavolari S,
Taffurelli M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M,
Montanaro L, et al: The basal-like breast carcinoma phenotype is
regulated by SLUG gene expression. J Pathol. 214:25–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hajra KM, Chen DY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 62:1613–1618. 2002.PubMed/NCBI
|
|
29
|
Zhou W, Lv R, Qi W, Wu D, Xu Y, Liu W, Mou
Y and Wang L: Snail contributes to the maintenance of stem
cell-like phenotype cells in human pancreatic cancer. PLoS One.
9:e874092014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guaita S, Puig I, Franci C, Garrido M,
Dominguez D, Batlle E, Sancho E, Dedhar S, De Herreros AG and
Baulida J: Snail induction of epithelial to mesenchymal transition
in tumor cells is accompanied by MUC1 repression and ZEB1
expression. J Biol Chem. 277:39209–39216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perez-Moreno MA, Locascio A, Rodrigo I,
Dhondt G, Portillo F, Nieto MA and Cano A: A new role for E12/E47
in the repression of E-cadherin expression and
epithelial-mesenchymal transitions. J Biol Chem. 276:27424–27431.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nieto MA, Sargent MG, Wilkinson DG and
Cooke J: Control of cell behavior during vertebrate development by
Slug, a zinc finger gene. Science. 264:835–839. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elloul S, Elstrand MB, Nesland JM, Tropé
CG, Kvalheim G, Goldberg I, Reich R and Davidson B: Snail, Slug,
and Smad-interacting protein 1 as novel parameters of disease
aggressiveness in metastatic ovarian and breast carcinoma. Cancer.
103:1631–1643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Côme C, Magnino F, Bibeau F, De Santa
Barbara P, Becker KF, Theillet C and Savagner P: Snail and slug
play distinct roles during breast carcinoma progression. Clin
Cancer Res. 12:5395–5402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hemavathy K, Ashraf SI and Ip YT:
Snail/slug family of repressors: Slowly going into the fast lane of
development and cancer. Gene. 257:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cobaleda C, Perez-Caro M, Vicente-Dueñas C
and Sánchez-García I: Function of the zinc-finger transcription
factor SNAI2 in cancer and development. Annu Rev Genet. 41:41–61.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor Snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tripathi MK, Misra S and Chaudhuri G:
Negative regulation of the expressions of cytokeratins 8 and 19 by
SLUG repressor protein in human breast cells. Biochem Biophys Res
Commun. 329:508–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kurrey NK, K A and Bapat SA: Snail and
slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu Z, Jiang Y, Steed H, Davidge S and Fu
Y: TGFβ and EGF synergistically induce a more invasive phenotype of
epithelial ovarian cancer cells. Biochem Biophys Res Commun.
401:376–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peiró S, Escrivà M, Puig I, Barberà MJ,
Dave N, Herranz N, Larriba MJ, Takkunen M, Francí C, Muñoz A, et
al: Snail1 transcriptional repressor binds to its own promoter and
controls its expression. Nucleic Acids Res. 34:2077–2084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kumar B, Uppuladinne MV, Jani V, Sonavane
U, Joshi RR and Bapat SA: Auto-regulation of Slug mediates its
activity during epithelial to mesenchymal transition. Biochim
Biophys Acta. 1849:1209–12018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Osorio LA, Farfán NM, Castellón EA and
Contreras HR: SNAIL transcription factor increases the motility and
invasive capacity of prostate cancer cells. Mol Med Rep.
13:778–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Z, Li X, Cai X, Huang C and Zheng M:
miR-497 inhibits epithelial-mesenchymal transition in breast
carcinoma by targeting Slug. Tumour Biol. 37:7939–7950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aletaha M, Mansoori B, Mohammadi A, Fazeli
M and Baradaran B: The Effect of Snail1 Gene Silencing by siRNA in
Metastatic Breast Cancer Cell Lines. Iran J Public Health.
46:659–670. 2017.PubMed/NCBI
|
|
51
|
Kajita M, McClinic KN and Wade PA:
Aberrant expression of the transcription factors snail and slug
alters the response to genotoxic stress. Mol Cell Biol.
24:7559–7566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Franco DL, Mainez J, Vega S, Sancho P,
Murillo MM, de Frutos CA, Del Castillo G, López-Blau C, Fabregat I
and Nieto MA: Snail1 suppresses TGF-beta-induced apoptosis and is
sufficient to trigger EMT in hepatocytes. J Cell Sci.
123:3467–3477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wan Z, Pan H, Liu S, Zhu J, Qi W, Fu K,
Zhao T and Liang J: Downregulation of SNAIL sensitizes
hepatocellular carcinoma cells to TRAIL-induced apoptosis by
regulating the NF-κB pathway. Oncol Rep. 33:1560–1566. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Olmeda D, Jordá M, Peinado H, Fabra A and
Cano A: Snail silencing effectively suppresses tumour growth and
invasiveness. Oncogene. 26:1862–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim S, Yao J, Suyama K, Qian X, Qian BZ,
Bandyopadhyay S, Loudig O, De Leon-Rodriguez C, Zhou ZN, Segall J,
et al: Slug promotes survival during metastasis through suppression
of Puma-mediated apoptosis. Cancer Res. 74:3695–3706. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Yue B, Yu X, Wang Z and Wang M:
SLUG is activated by nuclear factor kappa B and confers human
alveolar epithelial A549 cells resistance to tumor necrosis
factor-alpha-induced apoptosis. World J Surg Oncol. 11:122013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mancini M, Petta S, Iacobucci I,
Salvestrini V, Barbieri E and Santucci MA: Zinc-finger
transcription factor slug contributes to the survival advantage of
chronic myeloid leukemia cells. Cell Signal. 22:1247–1253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang K, Zhang S, Jiao X, Wang H, Zhang D,
Niu Z, Shen Y, Lv L and Zhou Y: Slug regulates proliferation and
invasiveness of esophageal adenocarcinoma cells in vitro and in
vivo. Med Oncol. 28:1089–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mezencev R, Matyunina lV, Jabbari N and
McDonald JF: Snail-induced epithelial-to-mesenchymal transition of
MCF-7 breast cancer cells: Systems analysis of molecular changes
and their effect on radiation and drug sensitivity. BMC Cancer.
16:2362016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Escrivà M, Peiró S, Herranz N, Villagrasa
P, Dave N, Montserrat-Sentís B, Murray SA, Francí C, Gridley T,
Virtanen I and García de Herreros A: Repression of PTEN phosphatase
by Snail1 transcriptional factor during gamma radiation-induced
apoptosis. Mol Cell Biol. 28:1528–1540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang K, Jiao X, Liu X, Zhang B, Wang J,
Wang Q, Tao Y and Zhang D: Knockdown of snail sensitizes pancreatic
cancer cells to chemotherapeutic agents and irradiation. Int J Mol
Sci. 11:4891–4892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang K, Zhang B, Lu Y, Sun C, Zhao W,
Jiao X, Hu J, Mu P, Lu H and Zhou C: Slug inhibition upregulates
radiation-induced PUMA activity leading to apoptosis in
cholangiocarcinomas. Med Oncol. 28 (Suppl 1):S301–S309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jiang F, Zhou L, Wei C, Zhao W and Yu D:
Slug inhibition increases radiosensitivity of oral squamous cell
carcinoma cells by upregulating PUMA. Int J Oncol. 49:709–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Inoue A, Seidel MG, Wu W, Kamizono S,
Ferrando AA, Bronson RT, Iwasaki H, Akashi K, Morimoto A, Hitzler
JK, et al: Slug, a highly conserved zinc finger transcriptional
repressor, protects hematopoietic progenitor cells from
radiation-induced apoptosis in vivo. Cancer Cell. 2:279–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arienti C, Tesei A, Carloni S, Ulivi P,
Romeo A, Ghigi G, Menghi E, Sarnelli A, Parisi E, Silvestrini R and
Zoli W: SLUG silencing increases radiosensitivity of melanoma cells
in vitro. Cell Oncol (Dordr). 36:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vega S, Morales AV, Ocaña OH, Valdés F,
Fabregat I and Nieto MA: Snail blocks the cell cycle and confers
resistance to cell death. Genes Dev. 18:1131–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mittal MK, Singh K, Misra S and Chaudhuri
GJ: SLUG-induced elevation of D1 cyclin in breast cancer cells
through the inhibition of its ubiquitination. Biol Chem.
286:469–479. 2011. View Article : Google Scholar
|
|
68
|
Sherr CJ: Mammalian G1 cyclins. Cell.
73:1059–1065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu J, Uygur B, Zhang Z, Shao L, Romero D,
Vary C, Ding Q and Wu WS: Slug inhibits proliferation of human
prostate cancer cells via downregulation of cyclin D1 expression.
Prostate. 70:1768–1777. 2010.PubMed/NCBI
|
|
70
|
Emadi Baygi M, Soheili ZS, Essmann F,
Deezagi A, Engers R, Goering W and Schulz WA: Slug/SNAI2 regulates
cell proliferation and invasiveness of metastatic prostate cancer
cell lines. Tumour Biol. 31:297–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Biade S, Stobbe CC and Chapman JD: The
intrinsic radiosensitivity of some human tumor cells throughout
their cell cycles. Radiat Res. 147:416–421. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Neal CL, Mckeithen D and Odero-Marah VA:
Snail negatively regulates cell adhesion to extracellular matrix
and integrin expression via the MAPK pathway in prostate cancer
cells. Cell Adh Migr. 5:249–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia
L, Wu Y, Zhou BP and Feng Y: Snail is critical for tumor growth and
metastasis of ovarian carcinoma. Int J Cancer. 126:2102–2111.
2010.PubMed/NCBI
|
|
75
|
De Craene B, Gilbert B, Stove C, Bruyneel
E, van Roy F and Berx G: The transcription factor snail induces
tumor cell invasion through modulation of the epithelial cell
differentiation program. Cancer Res. 65:6237–6244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang A, Chen G, Meng L, Wang Q, Hu W, Xi
L, Gao Q, Wang S, Zhou J, Xu G, Meng L and Ma D: Antisense-Snail
transfer inhibits tumor metastasis by inducing E-cadherin
expression. Anticancer Res. 28:621–628. 2008.PubMed/NCBI
|
|
77
|
Smith BN, Burton LJ, Henderson V, Randle
DD, Morton DJ, Smith BA, Taliaferro-Smith L, Nagappan P, Yates C,
Zayzafoon M, et al: Snail promotes epithelial mesenchymal
transition in breast cancer cells in part via activation of nuclear
ERK2. PLoS One. 9:e1049872014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qian J, Liu H, Chen W, Wen K, Lu W, Huang
C and Fu Z: Knockdown of Slug by RNAi inhibits the proliferation
and invasion of HCT116 colorectal cancer cells. Mol Med Rep.
8:1055–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gu A, Jie Y, Yao Q, Zhang Y and Mingyan E:
Slug is associated with tumor metastasis and angiogenesis in
ovarian cancer. Reprod Sci. 24:291–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhao X, Sun B, Sun D, Liu T, Che N, Gu Q,
Dong X, Li R, Liu Y and Li J: Slug promotes hepatocellular cancer
cell progression by increasing sox2 and nanog expression. Oncol
Rep. 33:149–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu Y, Li L, Zheng Z, Chen S, Chen E and Hu
Y: Long non-coding RNA linc00261 suppresses gastric cancer
progression via promoting Slug degradation. J Cell Mol Med.
21:955–967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang YP, Wang MZ, Luo YR, Shen Y and Wei
ZX: Lentivirus-mediated shRNA interference targeting SLUG inhibits
lung cancer growth and metastasis. Asian Pac J Cancer Prev.
13:4947–4951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sun Y, Song GD, Sun N, Chen JQ and Yang
SS: Slug overexpression induces stemness and promotes
hepatocellular carcinoma cell invasion and metastasis. Oncol Lett.
7:1936–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bai JW, Chen MN, Wei XL, Li YC, Lin HY,
Chen M, Li JW, Du CW, Man K and Zhang GJ: The zinc-finger
transcriptional factor Slug transcriptionally downregulates ERα by
recruiting lysine-specific demethylase 1 in human breast cancer.
Oncogenesis. 6:e3302017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan
ZK, Liu K and Huang S: Extracellular signal-regulated kinase
signaling pathway regulates breast cancer cell migration by
maintaining slug expression. Cancer Res. 69:9228–9235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: MiR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Paquette B, Baptiste C, Therriault H,
Arguin G, Plouffe B and Lemay R: In vitro irradiation of basement
membrane enhances the invasiveness of breast cancer cells. Br J
Cancer. 97:1505–1512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Young AGH and Bennewith KL: Ionizing
radiation enhances breast tumor cell migration in vitro. Radiat
Res. 188:381–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rodman SN, Spence JM, Ronnfeldt TJ, Zhu Y,
Solst SR, O'Neill RA, Allen BG, Guan X, Spitz DR and Fath MA:
Enhancement of radiation response in breast cancer stem cells by
inhibition of thioredoxin- and glutathione-dependent metabolism.
Radiat Res. 186:385–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Du XL, Jiang T, Wen ZQ, Gao R, Cui M and
Wang F: Silencing of heat shock protein 70 expression enhances
radiotherapy efficacy and inhibits cell invasion in endometrial
cancer cell line. Croat Med J. 50:143–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yanamandra N, Kondraganti S, Srinivasula
SM, Gujrati M, Olivero WC, Dinh DH and Rao JS: Activation of
caspase-9 with irradiation inhibits invasion and angiogenesis in
SNB19 human glioma cells. Oncogene. 23:2339–2344. 2004. View Article : Google Scholar : PubMed/NCBI
|