Introduction
Colorectal cancer (CRC) is one of the most commonly
diagnosed cancer types, and is a leading cause of cancer-related
mortality. Although surgical resection is an optimal therapeutic
strategy during the early stages of CRC, chemotherapy remains an
important treatment option for patients diagnosed at an advanced
stage. Indeed, platinum-based chemotherapy, including cisplatin
(DDP), is usually adopted for treating CRC (1). However, a large proportion of patients
with cancer eventually relapse and develop drug resistance, despite
an initial response to DDP (2).
Recently, the co-administration of DDP along with other anticancer
agents has been proven to be a more effective treatment strategy
(3,4).
Therefore, understanding the potential mechanisms underlying the
development of DDP resistance may aid in the design of combined
chemotherapy regimens.
Chemotherapy usually induces tumor microenvironment
remodeling to sustain the cellular hierarchy of the tumor via
secreting cytokines. The tumor microenvironment is characterized by
proinflammatory mediator accumulation and cell infiltration. A
significant correlation has been demonstrated between chronic
inflammation and tumor invasion, metastasis and chemoresistance
(5). Interleukin (IL)-17 is a
proinflammatory cytokine that is secreted by T-helper 17 (Th17)
cells and serves an important role in the host defense in
inflammatory conditions and in cancer development, including
inflammatory bowel disease (6),
rheumatoid arthritis (7), lung cancer
(8) and thyroid cancer (9). Indeed, interleukin-17 (IL-17) has a
pleiotropic function in tumor initiation, development and
metastasis (10–15). Notably, IL-17 has been suggested to
serve a crucial role in promoting chemoresistance in tumor cells.
In breast cancer, tumor-infiltrating T lymphocytes were shown to
produce significant amounts of IL-17, further upregulating
phosphorylated extracellular signal-regulated protein kinases 1 and
2 (ERK1/2) and activating the mitogen-activated protein kinase
(MAPK) pathway. Furthermore, IL-17 was found to be able to promote
the migration and invasion of breast cancer cells (16).
Similarly, IL-17 is key to the initiation and
development of CRC. Exogenous IL-17 may facilitate the self-renewal
and invasion of cancer-initiating cells. However, the role of IL-17
in DDP resistance in CRC has not been fully elucidated. The present
study evaluated IL-17 production among clinical CRC samples and its
association with chemoresistance. Furthermore, the CRC HCT116 line
was used to investigate the biological function of IL-17 in
facilitating the development of DDP resistance in CRC.
Materials and methods
Specimens
A total of 37 pairs of colon tumor tissue samples
and corresponding para-carcinoma tissue samples were collected from
37 patients (mean age, 55.82±13.61 years; age range, 49–71 years)
during radical resection of colorectal cancer in Weihai Central
Hospital (Weihai, Shandong, China) between November 2015 and
December 2016. All the patients in this cohort were pathologically
diagnosed with colon cancer, and had not received any preoperative
radiotherapy and/or chemotherapy. Detailed clinical characteristics
and pathological parameters were collected, and all the patients
provided written informed consent for their data to be used in the
present study. The study was approved by the Ethical Committee of
Weihai Central Hospital.
Tissue sections and
immunohistochemical staining
Formalin-fixed (10%) paraffin-embedded tissue blocks
(fixed at room temperature for 24 h) were cut into 3-µm sections.
Following dewaxing in xylene and hydration through graded ethanol,
the sections were immersed in 3% H2O2 at room
temperature for 15 min to block endogenous peroxidase binding,
followed by boiling in ethylenediaminetetraacetic acid-alkaline
solution for antigen retrieval. The sections were then blocked at
room temperature for 10 min using 5% animal normal serum (cat. no.
C0265; Beyotime Institute of Biotechnology, Shanghai, China). A
monoclonal rabbit anti-human IL-17 primary antibody (cat. no.
ab79056; 1:50; Abcam, Cambridge, MA, USA) was incubated with the
slides overnight at 4°C. The following day, the sections were
washed and incubated with horse peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibodies (cat. no. ab6721; 1:100; Abcam) at
room temperature for 30 min. The slides were then rinsed with 0.05%
Tween-phosphate-buffered saline (PBS), and treated with diluted
streptavidin-horseradish peroxidase for 30 min at room temperature.
The slides were washed with 0.05% Tween-PBS buffer. Finally, the
sections were stained with diaminobenzidine (DAB) for 90 sec at
room temperature, and stained with hematoxylin for 60 sec at room
temperature and fixed in 60% neutral balata for 12 h. The
expression of IL-17 in the tissue samples was determined by the
intensity score of the IHC image: A score of 0 was defined as
negative expression, and 1, 2 and 3 as weak expression, moderate
expression and strong expression, respectively, by visually
analyzing the intensity of the staining. Five randomly selected
fields of each sample were picked, and the sum of the intensity
scores was calculated. A total score of between 0 and 2 was defined
as negative expression, 3 and 4 were defined as weak expression,
and a score of >5 was defined as strong expression.
Cell culture
The human CRC HCT116 cell line (purchased from
Chinese Academy of Sciences Cell Bank, Shanghai, China) was
cultured with RPMI-1640 (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) plus 10% fetal bovine serum (Invitrogen;
Thermo Fisher Scientific, Inc.) in a humidified incubator at 37°C
(5% CO2). To examine the inhibitory effects of DDP on
HCT116 cells, cells were seeded on 96 well plates with the density
of 5×104 and treated with RPMI-1640 containing 100, 200,
400 or 800 nM DDP. To determine the effect of IL-17 on HCT116
cells, cells were seeded on 12 well plates with the density of
5×105, and the treatments included DDP alone (400 nM),
or a combination of DDP and IL-17 (50 ng/ml) or IL-17 inhibitors
(IL-17a and IL-17 monoclonal antibody Secukinumab; cat. no.
NDC:0078-0639; Novartis International AG, Basel, Switzerland; 4
mg/ml).
Assay for cell viability
The MTT assay (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to monitor the cell viability of each group.
Briefly, HCT116 cells were first washed with buffer (PBS; pH 7.4),
trypsinized, washed, counted and then re-seeded into a 96-well
plate. Next, 10 µl MTT reagent was added and the plate was cultured
at 37°C in a humidified incubator (5% CO2) until a
purple precipitate appeared. Thereafter, 100 µl detergent solution
was added and the plate was incubated for 2 h in the dark.
Absorbance was detected at 570 nm using a microplate reader.
Western blot analysis
Treated cells were washed in PBS, immediately
followed by cell use of extraction buffer (Thermo Fisher
Scientific, Inc.) with a protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland) for cell lysis, according to the
manufacturer's protocol. The quantification of protein was further
determined using a Bradford protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proteins (20 µg for each
sample) were separated by 10% SDS-PAGE and then transferred to a
polyvinylidene difluoride membrane, which was blocked by 5% skimmed
milk (suspended in TBS with 0.05% Tween-20) at room temperature for
1 h, and then incubated with the primary antibodies against
phosphorylated protein kinase B (p-Akt; cat. no. ab38449; 1:1,000
dilution), apoptosis regulator BAX (Bax; cat. no. ab32503; 1:1,000
dilution), apoptosis regulator Bcl-2 (Bcl-2; cat. no. ab59348;
1:1,000 dilution) and serine/threonine-protein kinase mTOR (mTOR;
cat. no. ab2732; 1:2,000 dilution; all Abcam) proteins at 4°C
overnight. β-actin (cat. no. ab8227; 1:1,000; Abcam) served as the
internal control. The following day, the membrane was washed and
chemiluminescence was measured following incubation with the goat
anti-rabbit HRP-conjugated (IgG H&L) secondary antibodies (cat.
no. ab6721; 1:5,000; Abcam). The membranes were then incubated with
Enhanced Chemiluminescence Detection kit (Vazyme Biotech Co., Ltd.,
Nanjing, China) The relative expression of p-Akt, Bax, Bcl-2 and
mTOR proteins to the expression of β-actin was statistically
evaluated by ImageJ (version 1.45f; National Institutes of Health,
Bethesda, MD, USA).
Cell apoptosis analysis
Once the cells had been treated with DPP with or
without IL-17 for 72 h, flow cytometry with the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
method was employed to monitor cell apoptosis in each group.
Briefly, the cells were washed, trypsinized and resuspended in the
staining solution provided with the Annexin V-FITC Apoptosis
Detection kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Subsequent to incubation
for 1 h, cell apoptosis was measured by a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). Cells with a positive
Annexin V-FITC signal and a negative PI signal were considered to
be apoptotic.
Statistical analysis
All experiments were verified with repetition in
triplicate. Final data were statistically analyzed using SPSS 22.0
software (IBM Corp., Armonk, NY, USA). All data are presented as
the mean ± standard deviation. Differences among multiple groups
were analyzed by one-way analysis of variance followed by Dunnett's
post-hoc test. Results with a P-value of <0.05 were considered
to be statistically significant.
Results
Association of IL-17 with clinical and
pathological parameters in patients with CRC
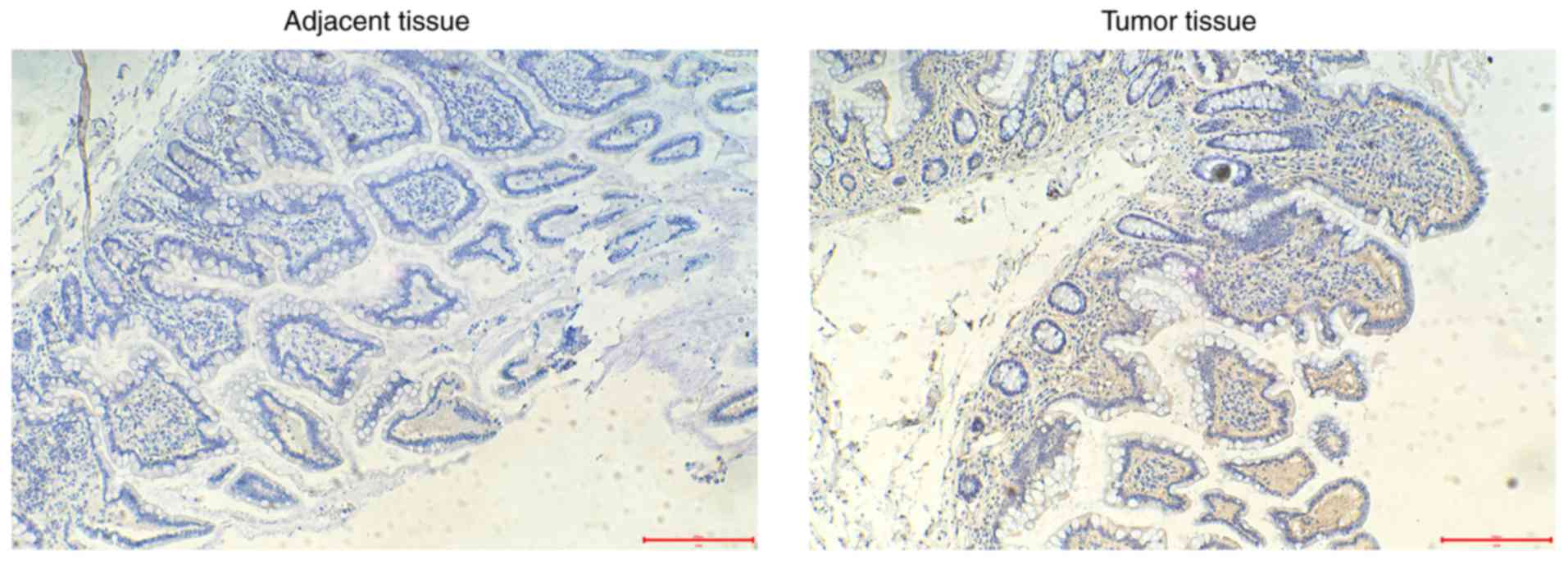
To determine IL-17 expression in CRC tissue, CRC
samples from 37 patients collected during surgery were included in
the present study. Immunohistochemical staining was used to
identify the positivity of IL-17 (Fig.
1). The clinical and pathological characteristics, and the
IL-17 expression data, are summarized in Table I. The expression levels of IL-17,
stratified by sex, age, tumor size, tumor differentiation, invasion
depth, lymph node metastasis and distant metastasis, were
determined. Notably, IL-17 expression was positively associated
with the tumor size (P=0.011), poor tumor cell differentiation
(P=0.025), a greater depth of invasion (T3-T4; P=0.007) and the
presence of lymph node metastasis (N1-N3; P=0.034). These results
indicated a positive association between IL-17 expression and CRC
development.
 | Table I.Association of IL-17 expression with
clinical and pathological parameters in patients with colorectal
cancer. |
Table I.
Association of IL-17 expression with
clinical and pathological parameters in patients with colorectal
cancer.
|
|
| IL-17 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Factors | Cases, n | Negative | Weak | Strong | P-value |
|---|
| Total patients |
| 11 | 14 | 12 |
|
| Sex |
|
|
|
| 0.818 |
| Male | 23 | 6 | 9 | 8 |
|
|
Female | 14 | 5 | 5 | 4 |
|
| Age, years |
|
|
|
| 0.177 |
|
<60 | 25 | 8 | 7 | 10 |
|
| ≥60 | 12 | 3 | 7 | 2 |
|
| Tumor size,
cm3 |
|
|
|
| 0.011 |
| ≥5 | 15 | 2 | 4 | 9 |
|
|
<5 | 22 | 9 | 10 | 3 |
|
| Tumor
differentiation |
|
|
|
| 0.025 |
| Well,
moderate | 16 | 8 | 6 | 2 |
|
| Poor | 21 | 3 | 8 | 10 |
|
| Invasion depth |
|
|
|
| 0.007 |
|
T1-T2 | 19 | 6 | 11 | 2 |
|
|
T3-T4 | 18 | 5 | 3 | 10 |
|
| Lymph node
metastasis |
|
|
|
| 0.034 |
| N0 | 15 | 8 | 4 | 3 |
|
|
N1-N3 | 22 | 3 | 10 | 9 |
|
| Distant
metastasis |
|
|
|
| 0.332 |
| No | 17 | 7 | 6 | 4 |
|
| Yes | 20 | 4 | 8 | 8 |
|
DDP inhibits the viability of CRC
cells
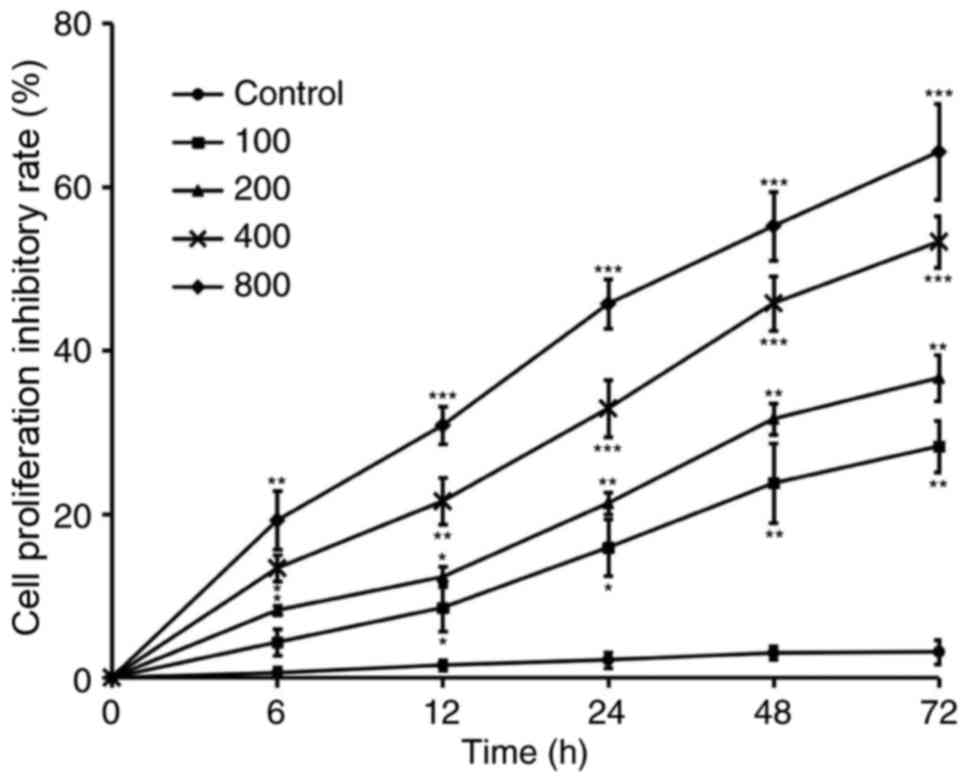
DDP was reported to exert a potent inhibitory effect
against the growth of CRC. To further determine the optimal half
maximal inhibitory concentration of DDP for HCT116 cells, a panel
of serial 2-fold diluted concentration (100–800 nM) of DDP was
added to the cell culture for 72 h. The MTT assay was used to
evaluate cell viability at 0, 6, 12, 24, 48 and 72 h. As shown in
Fig. 2, 100 nM DDP was sufficient to
inhibit >20% of cell proliferation by 72 h, while 800 nM DDP
successfully inhibited 60% of cell proliferation by 72 h. Based on
these results, 400 nM inhibited ~50% of cell proliferation and it
was selected as the optimal concentration to further determine the
role of IL-17 in the DDP resistance of CRC cells.
IL-17 facilitates DDP resistance in
CRC cells
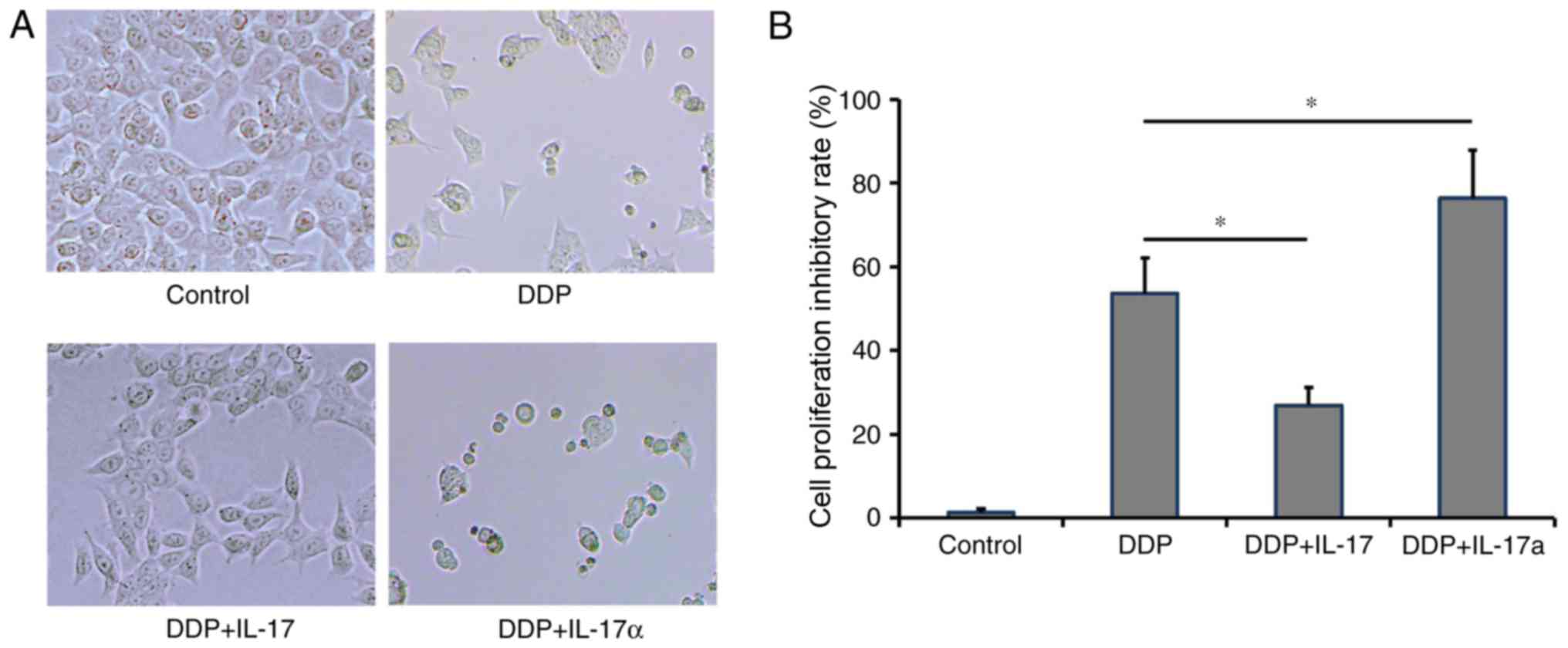
To determine whether IL-17 facilitated the
development of CRC resistance to DDP, HCT116 cells were treated
with DDP, DDP plus IL-17 or DDP plus IL-17a. An additional group of
HCT116 cells without any treatment was used as a negative control.
At 72 h post-treatment, compared with the negative control group,
the DDP-treatment group exhibited 53.6±8.6% inhibition of
proliferation. The addition of IL-17 was able to reduce inhibition
of cellular proliferation to 26.9±4.29%, while IL-17a treatment
further promoted the inhibition of cell proliferation to
76.35±11.43% (Fig. 3A and B).
IL-17 inhibits the apoptosis of CRC
cells
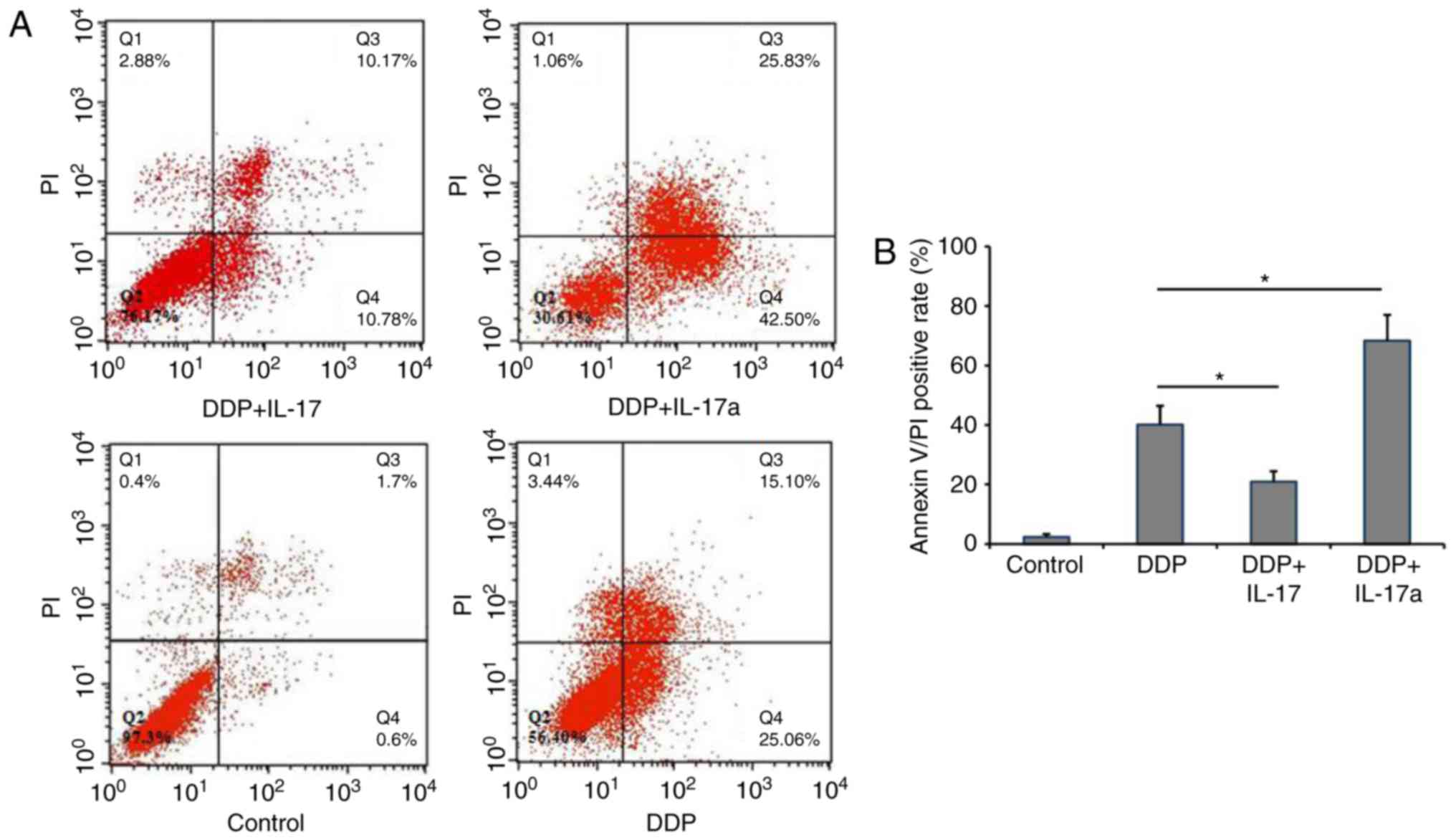
Given the observation that IL-17 was able to inhibit
the proliferation of HCT116 cells, whether IL-17 could inhibit the
apoptosis of CRC cells was further determined. Using Annexin
V-FITC/PI staining, the percentage of apoptotic HCT116 cells was
determined. As shown in Fig. 4A and
B, the apoptosis rate in the negative control group (HCT166
cells only) was 2.03±1.03%. DDP treatment increased the apoptosis
rate to 40.16±6.26%, while adding IL-17 reduced the apoptosis rate
to 20.95±3.49% and inhibiting IL-17 signaling in DDP-treated HCT116
cells increased the apoptosis rate to 68.33±8.68%. These results
suggested that IL-17 signaling facilitated DDP resistance in CRC
cells, while inhibiting IL-17 signaling via treatment with an
anti-IL-17 antibody was able to markedly improve the efficacy of
DDP.
IL-17 promotes the expression of
p-Akt, Bcl-2 and mTOR, and inhibits Bax expression in CRC
cells
To investigate the molecular mechanisms underlying
the inhibitory effect of IL-17 in terms of the DDP resistance of
CRC cells, western blot analysis was used to determine the
expression of key apoptosis-related proteins (Fig. 5A and B). As shown in Fig. 5A and B, Bax was significantly
downregulated, and p-Akt, mTOR and Bcl-2 were significantly
upregulated in the tumor tissues compared with the adjacent normal
tissues. Moreover, compared with the negative control group, DDP
treatment induced a significant reduction in the expression of
p-Akt, Bcl-2 and mTOR, along with significantly increased Bax
expression (Fig. 5C and D). The
addition of IL-17 to the DPP-treated cells was able to restore the
expression of p-Akt, Bcl-2 and mTOR, and to inhibit Bax expression.
Inhibition of IL-17 signaling (DDP + IL-17a) in the HCT116 cells
caused further suppression of the expression of p-Akt, Bcl-2 and
mTOR, and promoted the expression of Bax, compared with that in the
group treated with DDP and IL-17 (Fig. 5C
and D). These results indicated the mechanism that underlies
IL-17 promoting the development of DDP resistance.
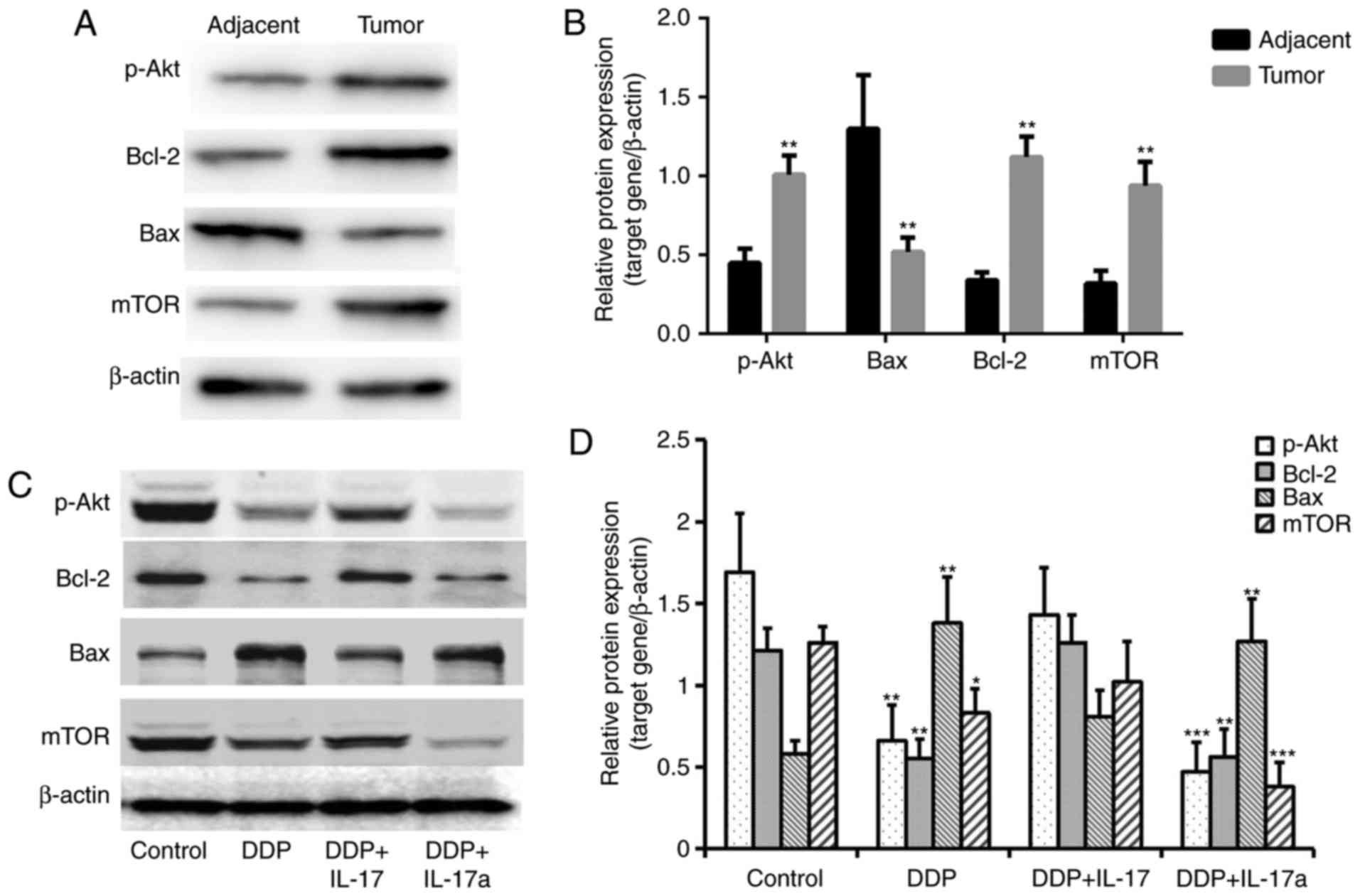 | Figure 5.IL-17 promotes the expression of
p-Akt, Bax and mTOR in colorectal cancer cells. (A) Western blot
analysis revealed elevated levels of p-Akt, Bcl-2 and mTOR, and
decreased levels of Bax in tumor tissues compared with that in the
adjacent tissues. (B) Semi-quantification of each protein
expression level in the different groups tested in panel (A). (C)
Western blot analysis revealed elevated levels of p-Akt, Bcl-2 and
mTOR following the addition of IL-17 to HCT116 cells treated with
DDP. (D) Semi-quantification of each protein expression level in
the different groups tested in panel (A). *P<0.05, **P<0.01
and ***P<0.001 vs. control. DDP, cisplatin; IL-17, interleukin
17; IL-17a, interleukin 17 antibody; p-Akt, phosphorylated protein
kinase B; Bcl-2, apoptosis regulator Bcl-2; Bax, apoptosis
regulator BAX; mTOR, serine/threonine-protein kinase mTOR. |
Discussion
As CRC is one of the most prevalent and lethal
cancer types worldwide, its treatment is challenging. DDP is a
potent anticancer agent and has been widely applied in the
treatment of CRC in clinical practice (17). However, DDP resistance is one of the
most challenging problems encountered during CRC treatment. The
present study observed elevated IL-17 expression in tumor tissues
collected from patients with CRC. Furthermore, the findings
indicated that IL-17 may be one of the key factors mediating DDP
resistance, and inhibition of IL-17 signaling in CRC cell culture
was able to reverse resistance to DDP, at the biological functional
and molecular levels. First, the addition of IL-17 to DPP-treated
cells was able to inhibit apoptosis and promote HCT116 cell growth,
while inhibition of IL-17 signaling exerted a synergistic
inhibitory effect on CRC cell proliferation.
The molecular mechanism through which IL-17 promotes
the development of DDP resistance was also investigated. It was
found that IL-17 promotes cell proliferation and inhibits apoptosis
by enhancing the expression of p-Akt, mTOR and Bcl-2, and
suppressing the expression of Bax. The phosphoinositide 3-kinase
(PI3K)-Akt-mTOR pathway is often found to be constitutively
activated in human tumor cells, integrating environmental and
intracellular signals to support cell growth (18). Also referred to as a Bcl-2-like
protein, Bax is a key pro-apoptotic member of the Bcl-2 protein
family. Bcl-2 is localized in the outer mitochondrial membrane,
where it serves a key role in promoting cell survival and
inhibiting the function of pro-apoptotic proteins (19). These findings were also consistent
with those of the present study, where IL-17 was revealed to
enhance the proliferation and inhibit the apoptosis of DPP-treated
HCT166 cells. The present findings further confirmed that the
inhibition of IL-17 is a promising therapeutic target for DDP
resistance in CRC.
The role of IL-17 in promoting chemoresistance and
cancer cell proliferation was also investigated in other types of
cancer. In breast cancer, IL-17 levels and IL-17-secreting cells
were found to be increased in tumor tissues and were correlated
with a poor prognosis (20).
Recombinant IL-17 was shown to trigger the MAPK pathway by
activation of ERK1/2 signaling in breast cancer, therefore
promoting cancer cell proliferation and chemotherapy resistance
(16). In non-small-cell lung cancer,
IL-17 was able to promote lung cancer growth by promoting
inflammation, which contributes to resistance to programmed cell
death protein 1 blockade, and tumor sensitivity to cytokine
depletion (8). The present study
identified a novel mechanism mediated by IL-17 to promote DDP
resistance via promoting PI3K-Akt-mTOR signaling and suppressing
Bcl-2 expression, which may assist in the design of novel
therapeutic strategies to overcome DDP resistance.
A notable observation from the clinical samples was
that elevated IL-17 was present in CRC tissues, consistent with
previous findings. Lereclus et al (21) suggested that the baseline level of
serum IL-17 concentration is an important factor that is
significantly associated with the response of patients with
metastatic CRC to bevacizumab, a monoclonal antibody that targets
vascular endothelial growth factor (21). However, the mechanism underlying the
elevated IL-17 levels observed in the tumor remains unclear.
Al-Samadi et al (22) reported
that the numbers of Th17 cells secreting IL-17 were increased in
the stroma, as well as in the adenomatous/cancerous epithelium
(22). However, additional studies
are required to elucidate the mechanism underlying the
significantly increased numbers of Th17 cells and IL-17 levels
present in CRC.
In conclusion, the present study identified an
important role for IL-17 in the DDP resistance of CRC. The addition
of IL-17 inhibited the apoptosis and promoted the proliferation of
a CRC cell line treated with DDP, while the inhibition of IL-17
signaling alongside DDP treatment synergistically increased cell
apoptosis. Finally, IL-17 may facilitate chemotherapy resistance
via promoting PI3K-Akt-mTOR and suppressing Bcl-2 signaling. The
findings of the present study provide solid experimental evidence
for the design of novel combined chemotherapy regimens.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ designed the experiments, and wrote and revised
the paper. GS conducted the majority of the experiments, analyzed
the data and also wrote a portion of the manuscript. YQ, HY and QK
conducted a number of the experiments and analyzed a portion of the
data.
Ethics approval and consent to
participate
All the patients provided written informed consent
for their data to be used in the present study. The study was
approved by the Ethical Committee of Weihai Central Hospital.
Patient consent for publication
Informed consent was obtained from each patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McQuade RM, Stojanovska V, Bornstein JC
and Nurgali K: Colorectal cancer chemotherapy: The evolution of
treatment and new approaches. Curr Med Chem. 24:1537–1557. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dise. 5:e12572014. View Article : Google Scholar
|
|
3
|
Valsecchi ME: Combined nivolumab and
ipilimumab or monotherapy in untreated melanoma. N Eng J Med.
373:12702015. View Article : Google Scholar
|
|
4
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujino S, Andoh A, Bamba S, Ogawa A, Hata
K, Araki Y, Bamba T and Fujiyama Y: Increased expression of
interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chabaud M, Garnero P, Dayer JM, Guerne PA,
Fossiez F and Miossec P: Contribution of interleukin 17 to synovium
matrix destruction in rheumatoid arthritis. Cytokine. 12:1092–1099.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akbay EA, Koyama S, Liu Y, Dries R, Bufe
LE, Silkes M, Alam MM, Magee DM, Jones R, Jinushi M, et al:
Interleukin-17A Promotes lung tumor progression through neutrophil
attraction to tumor sites and mediating resistance to PD-1
blockade. J Thorac Oncol. 12:1268–1279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carvalho DFG, Zanetti BR, Miranda L,
Hassumi-Fukasawa MK, Miranda-Camargo F, Crispim JCO and Soares EG:
High IL-17 expression is associated with an unfavorable prognosis
in thyroid cancer. Oncol Lett. 13:1925–1931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilke CM, Kryczek I, Wei S, Zhao E, Wu K,
Wang G and Zou W: Th17 cells in cancer: Help or hindrance?
Carcinogenesis. 32:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu J, Ye H, Zhang D, Liu W, Li M, Mao Y
and Lu Y: U87MG glioma cells overexpressing IL-17 acclerate
early-stage growth in vivo and cause a higher level of CD31 mRNA
expression in tumor tissues. Oncol Lett. 6:993–999. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ernst M and Putoczki T: IL-17 cuts to the
chase in colon cancer. Immunity. 41:880–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Kim MK, Di Caro G, Wong J,
Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, et
al: Interleukin-17 receptor a signaling in transformed enterocytes
promotes early colorectal tumorigenesis. Immunity. 41:1052–1063.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang Y, Al-Alwan L, Risse PA, Halayko AJ,
Martin JG, Baglole CJ, Eidelman DH and Hamid Q: Th17-associated
cytokines promote human airway smooth muscle cell proliferation.
FASEB J. 26:5152–5160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang B, Kang H, Fung A, Zhao H, Wang T and
Ma D: The role of interleukin 17 in tumour proliferation,
angiogenesis, and metastasis. Mediators Inflamm. 2014:6237592014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cochaud S, Giustiniani J, Thomas C,
Laprevotte E, Garbar C, Savoye AM, Curé H, Mascaux C, Alberici G,
Bonnefoy N, et al: IL-17A is produced by breast cancer TILs and
promotes chemoresistance and proliferation through ERK1/2. Sci Rep.
3:34562013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boulikas T and Vougiouka M: Cisplatin and
platinum drugs at the molecular level. (Review). Oncol Rep.
10:1663–1682. 2003.
|
|
18
|
Zhang HB, Lu P, Guo QY, Zhang ZH and Meng
XY: Baicalein induces apoptosis in esophageal squamous cell
carcinoma cells through modulation of the PI3K/Akt pathway. Oncol
Lett. 5:722–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Fatlawi AA, Al-Fatlawi AA, Irshad M,
Zafaryab M, Rizvi MM and Ahmad A: Rice bran phytic acid induced
apoptosis through regulation of Bcl-2/Bax and p53 genes in HepG2
human hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
15:3731–3736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji Y and Zhang W: Th17 cells: Positive or
negative role in tumor? Cancer Immunol Immunother. 59:979–987.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lereclus E, Tout M, Girault A, Baroukh N,
Caulet M, Borg C, Bouché O, Ternant D, Paintaud G, Lecomte T and
Raoul W: A possible association of baseline serum IL-17A
concentrations with progression-free survival of metastatic
colorectal cancer patients treated with a bevacizumab-based
regimen. BMC Cancer. 17:2202017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Samadi A, Moossavi S, Salem A, Sotoudeh
M, Tuovinen SM, Konttinen YT, Salo T and Bishehsari F: Distinctive
expression pattern of interleukin-17 cytokine family members in
colorectal cancer. Tumor Bio. 37:1609–1615. 2016. View Article : Google Scholar
|