Introduction
Incidence of nasopharyngeal carcinoma (NPC) in
southern China is high and NPC is a common head and neck malignancy
(1). The etiology of the disease
varies, and EB virus infection, genetic and environmental factors
all contribute to its occurrence (2).
Incidence rate of NPC has reached 10/300,000 (3). More than half of the patients are in
advanced stages at the time of diagnosis, and ~1/3 of patients have
a survival period of <5 years (4).
NPC has the characteristics of rapid growth, high degree of
malignancy, and is prone to early metastasis. Distant metastasis is
the main cause of death after radiotherapy, and ~60–70% of patients
are diagnosed with distant metastases (5). Tumor metastasis is the detachment of the
tumor cells from their primary site to traffic through blood
vessels or lymphatic vessels, reach distant positions and form
tumors of the same nature. In this process, low level of E-cadherin
reduces intercellular adhesion, induces epithelial-mesenchymal
transition, and promotes invasion and metastasis of tumor cells
(6).
MicroRNA is a group of short single-stranded RNAs
identified in eukaryotic cells with pivotal roles in the regulation
of post-transcriptional gene processing (7). Increasing number of studies have shown
that microRNA plays an important role in regulating tumor invasion
and metastasis (8). Studies have also
shown that microRNA-141 is upregulated in endometrial and
colorectal cancers, and the higher the expression, the worse the
prognosis is (9,10). In addition, some studies have
suggested that microRNA-141 is highly expressed in NPC, but some
other studies have reported that microRNA-141 expression is not
observed in NPC and adjacent normal tissues (11). At present, the functional role of
microRNA-183 and microRNA-141 in NPC is still controversial. This
study explored the influence of microRNA-183 and microRNA-141 on
prognosis of patients with NPC with an expectation of providing
guidance for the diagnosis, treatment and prognosis of NPC and
other tumors.
Materials and methods
General information
A total of 317 NPC patients admitted to Zhengzhou
Central Hospital Affiliated to Zhengzhou University (Zhengzhou,
China) between January 2010 and March 2016 were selected for this
research. Patients included 238 males and 79 females. All patients
underwent routine nasopharynx and neck MRI, chest CT, abdominal CT,
and bone scans to determine the recurrence and distant metastases.
Inclusion criteria: i) age ≥18 years, ≤65 years; ⅱ) patients who
had received no radiotherapy or chemotherapy prior to treatment; ⅲ)
there were no other malignant tumors or other diseases severely
affecting survival; and ⅳ) there were no immediate relatives among
the patients. Precautionary and lactating women were excluded. All
patients or their relatives signed an informed consent. The study
was approved by the Ethics Committee of Zhengzhou Central Hospital
Affiliated to Zhengzhou University.
Main reagents and instruments
TRIzol RNA extraction kit and miRNA first-strand
cDNA synthesis kit (both from Sangon Biotech Co., Ltd., Shanghai,
China); SYBR® PrimeScript™ miRNA RT-PCR kit (Takara Bio,
Inc., Otsu, Japan); NanoDrop 2000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); CFX96 fluorescence quantitative PCR instrument
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Specimen collection and RNA
extraction
NPC tumor and adjacent healthy tissues were
collected from NPC patients and were stored in a refrigerator at
−80°C. Total RNA was extracted from the tissues by using TRIzol RNA
extraction kit. The specific extraction steps were as follows:
TRIzol was added to lysate cells, followed by centrifugation at 4°C
(12,300 × g) for 10 min; supernatant was transferred to a new
RNase-free tube, and equal volume of chloroform was added, followed
by centrifugation at 4°C (12,300 × g) for 10 min; supernatant was
transferred to a new RNase-free tube, and equal volume of
isopropanol was added and kept at room temperature for 1 h,
followed by centrifugation at 4°C (12,300 × g) for 10 min;
supernatant was discarded, and the pellet was washed twice with 75%
ethanol; after air dry, DEPC water was added. NanoDrop 2000 was
used to test RNA quality and concentration. RNA with a A260/280
ratio >1.8 was subjected to 1.5% agarose gel electrophoresis to
check RNA integrity.
First-strand cDNA synthesis
First-strand cDNA was synthesized according to the
instructions of cDNA first strand synthesis kit. The reaction
system consisted of 1 µl of 2.5 U/µl poly(A) polymerase, 1 µl of
RTase Mix, 5 µl of 5X PAP/RT buffer, 2 µg of total RNA, and RNase
free dH2O was added to reach a final volume of 25 µl.
Reaction conditions: 37°C for 1 h and 85°C for 5 min.
Detection of microRNA-183 and
microRNA-141 expression in lesion and adjacent tissues of patients
by reverse transcription-quantitative PCR (RT-qPCR)
Synthesized cDNA was used as template and U6 as an
endogenous control. MicroRNA-183 and microRNA-141 expression levels
were detected by RT-qPCR. Reaction system: 10 µl of SYBR-Green
Master Mix, 0.5 µl of upstream and downstream primers, 1 µl of
cDNA, and ddH2O was added to reach a final volume of 20
µl. MicroRNA-141-F and microRNA-183-F were used as upstream
primers, and Uni-miR qPCR Primer in SYBR® PrimeScript™
miRNA RT-PCR kit was used as downstream primer (Table I). The RT-qPCR reaction conditions
were: 95°C for 3 min, followed by 40 cycles of 95°C for 30 sec and
58°C for 1 min. Data were processed using 2−ΔΔCq method
(12).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primers (5′-3′) | Sequences |
|---|
| Uni-miR qPCR
primer | Takara kit |
| MicroRNA-141-F |
CGTAACACTGTCTGGTAAAGATGGA |
| MicroRNA-183-F |
ACACTCCAGCTGGGTATGGCACTGGTAGAATT |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
Statistical analysis
SPSS 17.0 (Beijing Xinmei Jiahong Technology Co.,
Ltd., Beijing, China) was used for statistical analysis.
Measurement data were expressed as mean ± SD, and t-test was used
for the comparison between two groups. Chi-square test was used for
comparison of enumeration data. Kaplan-Meier test was used for the
survival analysis followed by a long-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
General clinical data
A total of 317 NPC patients admitted to Zhengzhou
Central Hospital Affiliated to Zhengzhou University from January
2010 to March 2015 were included. The patients were 238 males and
79 females, aging from 18 to 65 years, with a median age of 45
years. A total of 157 cases presented distant metastasis, 136 cases
had no distant metastasis, and distant metastasis was unclear in 24
cases. In addition, 172 patients had a disease-free survival (DFS)
≥3 years, and 145 patients had DFS <3 years. Tumors were
confined to the nasopharyngeal area or had infiltrated the
pharyngeal soft tissue in 56 cases. Lymph node metastasis occurred
in 308 patients (Table II). The 1-,
2-, and 3-year survival rates of the 317 patients were 82.6% (262),
64.4% (204), and 54.3% (172), respectively.
 | Table II.General clinical data of 317 NPC
patients. |
Table II.
General clinical data of 317 NPC
patients.
| Variable | n (%) |
|---|
| Age (years) |
|
| ≥45 | 156 (49.2) |
|
<45 | 161 (50.8) |
| Sex |
|
| Male | 238 (75.1) |
|
Female | 79 (24.9) |
| Distant
metastasis |
|
|
Unclear | 24 (7.6) |
|
Non-distant metastasis | 136 (42.9) |
| Distant
metastasis | 157 (49.5) |
| DFS (years) |
|
| ≥3 | 172 (54.3) |
|
<3 | 145 (45.7) |
| T period |
|
| T1 | 13 (4.1) |
| T2 | 43 (13.6) |
| T3 | 166 (52.4) |
| T4 | 95 (30.0) |
| N stages |
|
| N0 | 9 (2.8) |
| N1 | 91 (28.7) |
| N2 | 168 (53.0) |
| N3 | 49 (15.5) |
Analysis of the expression levels of
microRNA-183 and microRNA-141
The expression of microRNA-183 and microRNA-141 was
detected by RT-qPCR. The results showed that the expression levels
of microRNA-183 and microRNA-141 in lesion tissues were
significantly higher than those in adjacent tissues (p<0.05,
Table III). Patients with distant
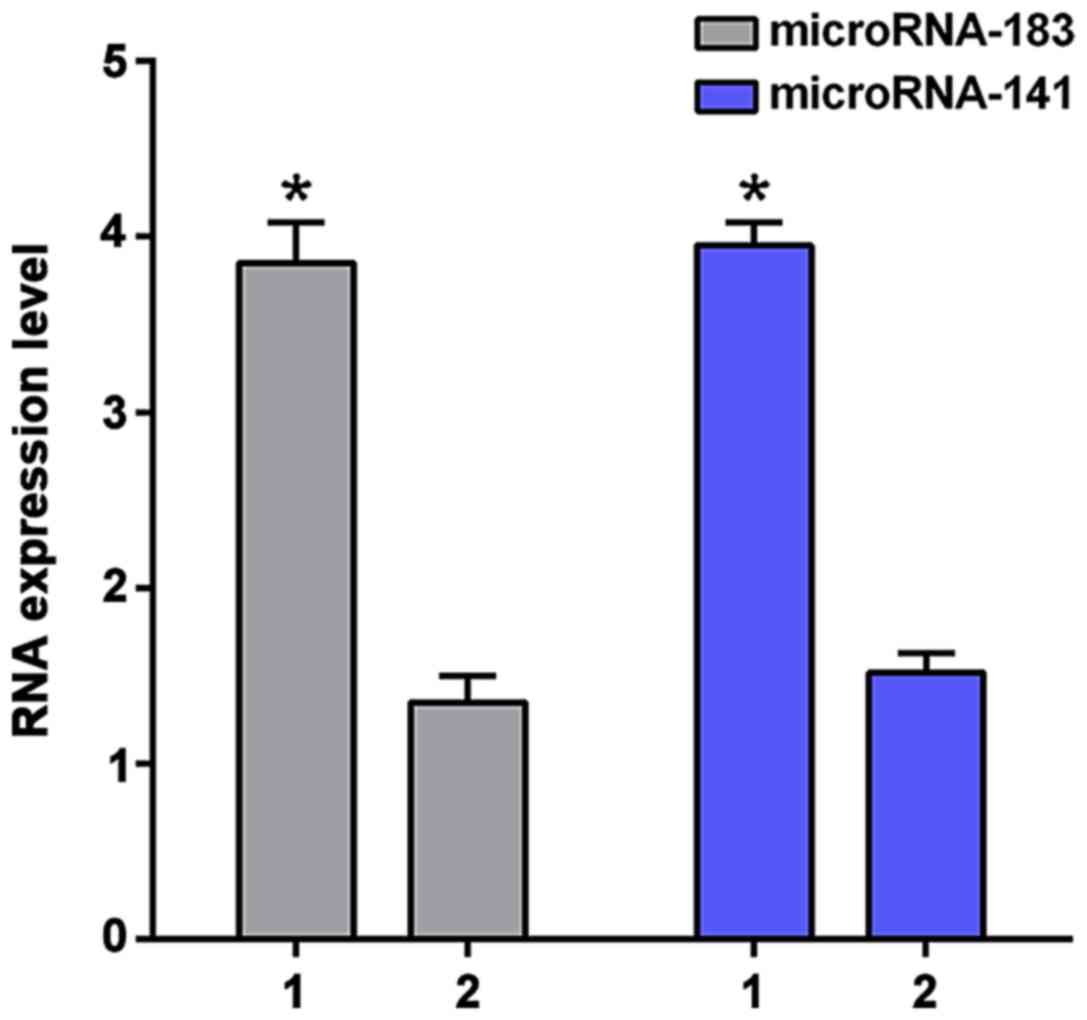
metastases had significantly higher expression levels of
microRNA-183 and microRNA-141 (3.85±0.23 and 3.95±0.13) than
patients without distant metastasis (1.35±0.15 and 1.52±0.11;
p<0.01, Fig. 1). Patients with DFS
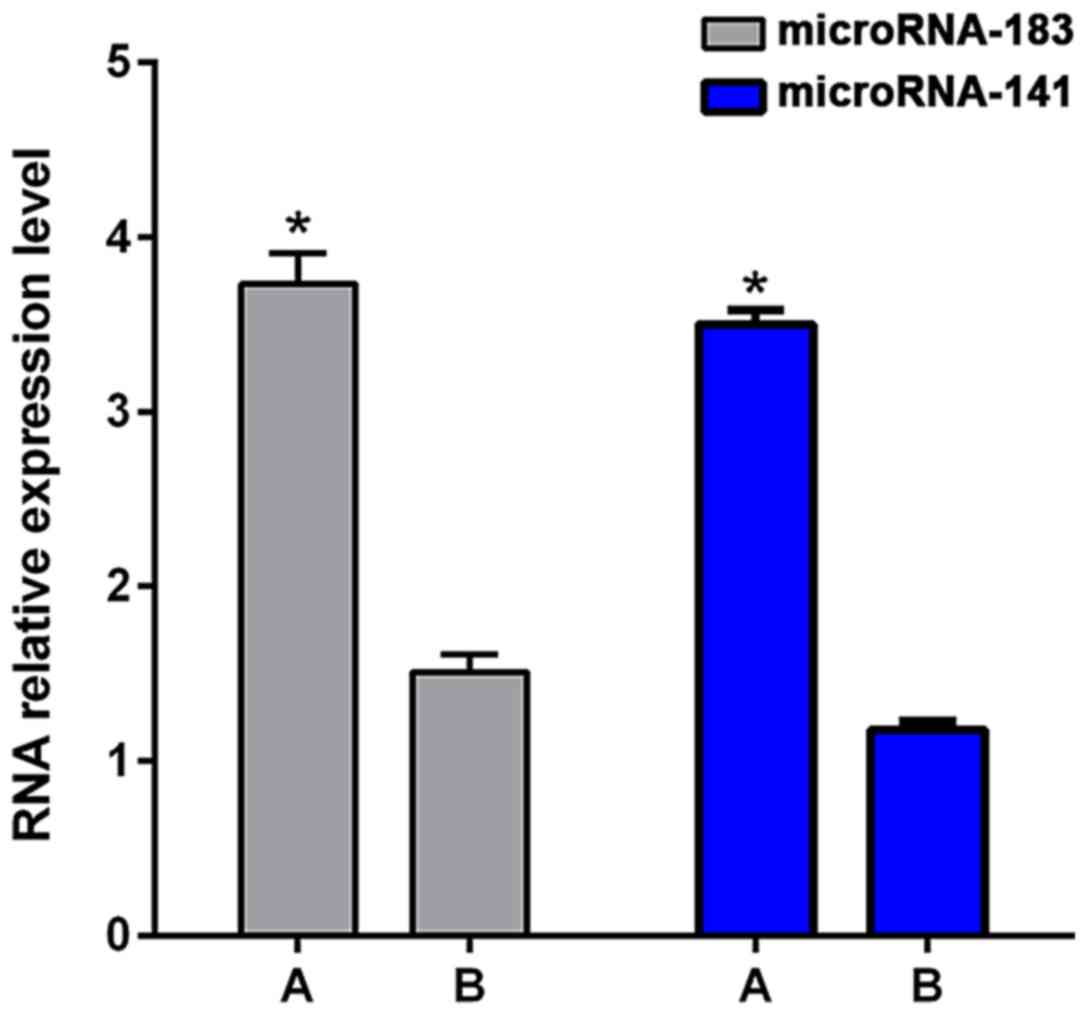
<3 years showed significantly higher levels of microRNA-183 and
microRNA-141 (3.73±0.18 and 3.50±0.08) than patients with DFS ≥3
years (1.51±0.10 and 1.18±0.05; p<0.01, Fig. 2).
 | Table III.Expression of microRNA-183 and
microRNA-141 in lesions and adjacent tissues (mean ± SD). |
Table III.
Expression of microRNA-183 and
microRNA-141 in lesions and adjacent tissues (mean ± SD).
| Groups | Lesions | Adjacent tissues | t | P-value |
|---|
| microRNA-183 | 3.11±0.09 | 1.21±0.10 | 4.771 | 0.041 |
| microRNA-141 | 2.98±0.12 | 1.13±0.08 | 8.215 | 0.014 |
Relationship between microRNA-183 and
microRNA-141 and prognosis
The relative expression levels of microRNA-183 and
microRNA-141 in the lesion tissue of NPC patients were 2.78±0.35
and 2.52±0.43, respectively. With the average value as threshold,
patients were divided into high and low expression groups. There
were 163 cases with high expression of microRNA-183, 154 cases with
low expression of microRNA-183, 161 cases with high expression of
microRNA-141, and 156 cases with low expression of microRNA-141.
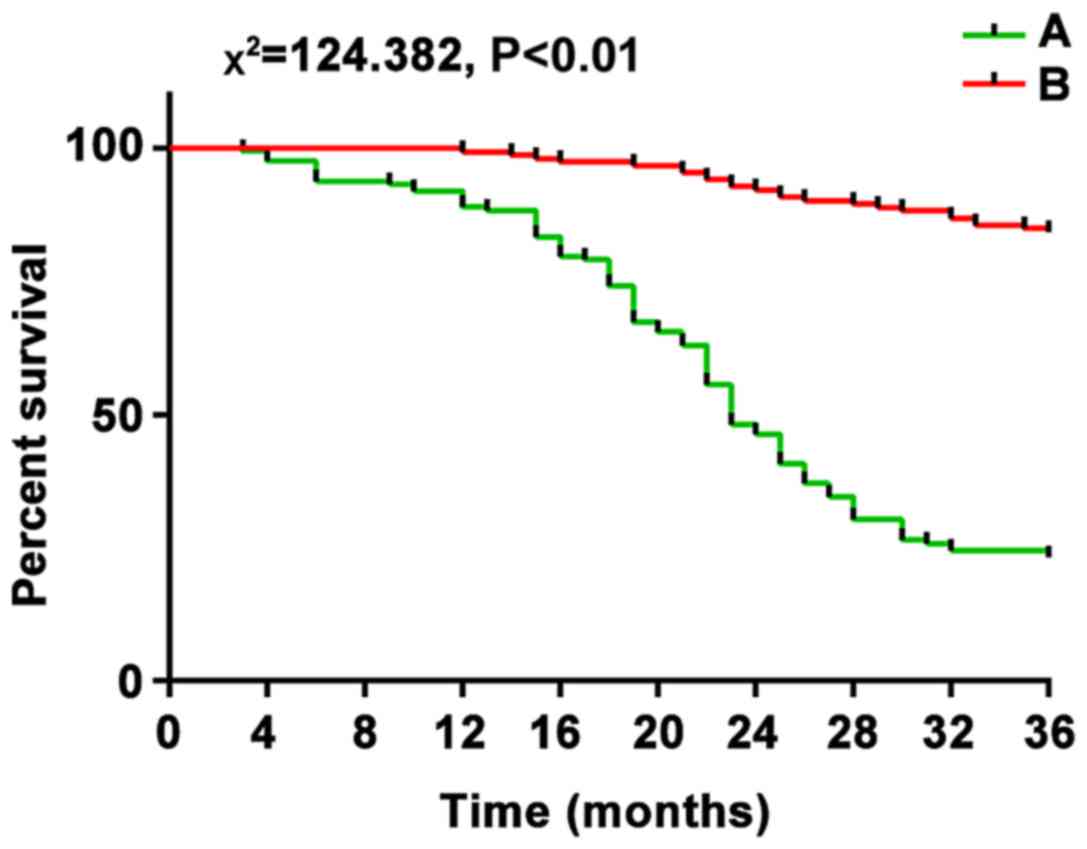
After 3 years of follow-up for these patients, 163 patients with
high microRNA-183 expression had recurrence and the total number of
deaths was 124 (76.1%), and 154 patients with low microRNA-183
expression had recurrence and the total number of deaths was 21
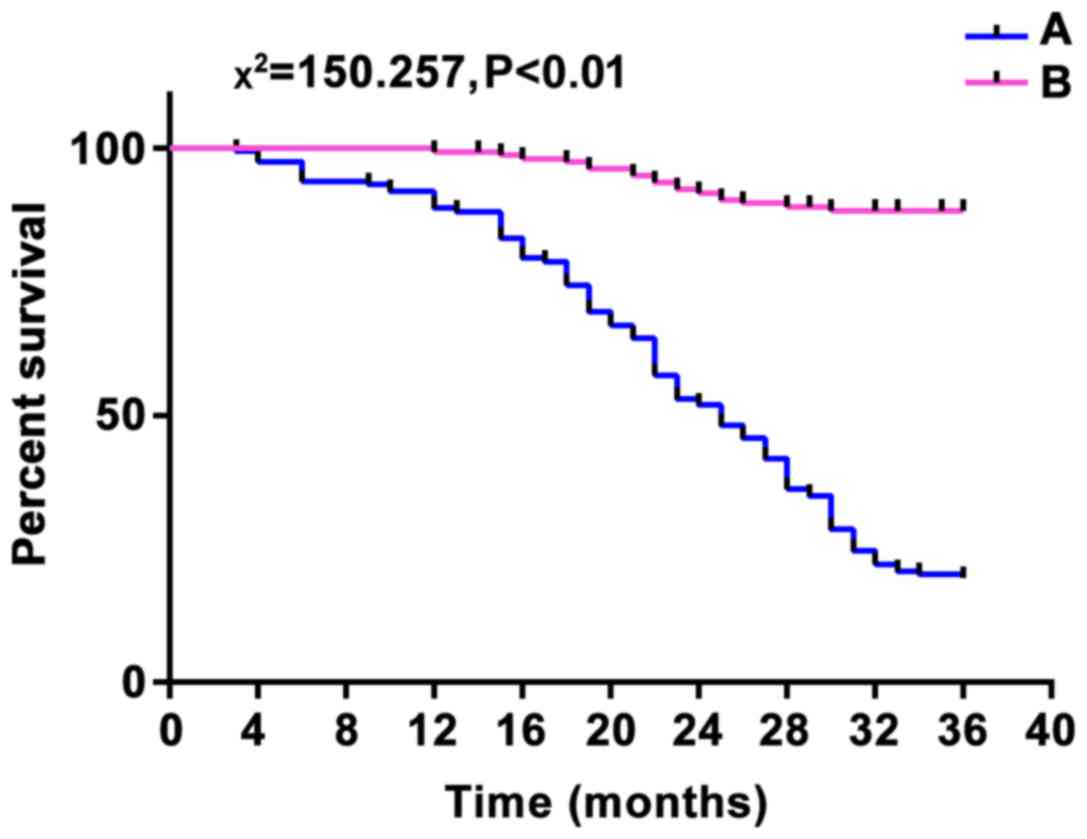
(13.6%). A total of 161 patients with high expression of
microRNA-141 had recurrence and the total number of deaths was 128
(79.5%), and 156 patients with low expression of microRNA-141 had
recurrence and the total number of deaths was 17 (10.9%). The
recurrence and mortality rates of high and low expression groups of
microRNA-183 and microRNA-141 were significantly different from
each other (χ2 was 124.382 and 150.257, respectively;
p<0.01), suggesting that the high expression of microRNA-183 and
microRNA-141 was associated with poor prognosis (Figs. 3 and 4).
Discussion
The occurrence and development process of NPC is
complex, and distant metastasis is an important cause of death in
NPC patients (13). Therefore, it is
particularly important to explore the relevant molecular mechanisms
of NPC distant metastasis.
MicroRNA plays an important role in the invasion of
tumor cells. Recent studies have found that microRNA exerts the
dual role of oncogenes and tumor suppressor gene in the development
of tumors (14).
MicroRNA-183 can inhibit metastasis of cancer cells
by inhibiting ezrin protein in lung (15) and breast cancer (16). In addition, it can also inhibit
migration of lung cancer cells by downregulating WISP2 protein or
upregulating the expression of S100P protein (17). MicroRNA-183 inhibits the expression of
cell death factor 4 (PDCD4) in liver cancer and inhibits cell
apoptosis (18). MicroRNA-183
inhibits the metastasis of colon cancer cells by inhibiting the
expression of EGR1 and PTEN (19). In
addition, the role of microRNA-183 in tumors is tissue-specific.
MicroRNA-183 can play a tumor suppressive role and can also promote
tumor invasion and migration in different cancer cells (20). In this study it was found that the
expression level of microRNA-183 in patients with distant
metastasis is significantly higher than that in patients without
distant metastasis (p<0.01), suggesting that the expression
level of microRNA-183 can be used to predict distant metastasis in
NPC patients. When distant metastasis occurs, the expression of
microRNA-183 increases, and microRNA-183 plays an important role in
promoting the metastasis of tumor cells in NPC. Sarver et al
(21) have found that the
upregulation of microRNA-183 in colon cancer plays an important
role in promoting the development of cancer. In the present study,
it was found that the expression level of microRNA-183 in patients
with DFS <3 years is significantly higher than that in patients
with DFS ≥3 years (p<0.01), suggesting that microRNA-183
expression level is related to postoperative survival. At the end
of the follow-up, recurrence and mortality were significantly
higher in the microRNA-183 high expression group than in the low
expression group, suggesting that high microRNA-183 expression is
closely related to poor prognosis.
MicroRNA-141 is a member of microRNA-200 family. Xia
et al (22) have found that
the expression level of microRNA-141 in NPC cell lines with low
differentiation is higher than that in highly differentiated NPC
cell lines. It was considered that the increase of microRNA-141
expression attributes to NPC occurrence at early stage. In
addition, microRNA-141 expression pattern is extremely different in
different tumor cells, such as high expression in ovarian cancer
(23), and low expression in hepatoma
cells (24). In this study, the
expression level of microRNA-141 was significantly higher in
patients with distant metastasis than in patients without distant
metastasis (p<0.01). Ahmed et al (25) have found that the appearance of lymph
node and distant metastasis is accompanied by the increased
expression level of microRNA-141, which is consistent with the
findings in this study. The expression level of microRNA-141 in
patients with DFS <3 years was significantly higher than that in
patients with DFS ≥3 years (p<0.01). Furthermore, recurrence and
mortality of patients with high expression of microRNA-141 were
also significantly higher than those of patients with low
expression, suggesting that increased expression of microRNA-141
predicts distant metastasis and shortened DFS, and leads to poor
prognosis.
In conclusion, microRNA-183 and microRNA-141 can be
used as markers for the prediction of distant metastasis of NPC,
and have a reference value for the prognosis of NPC. The specific
molecular mechanism of microRNA-183 and microRNA-141 in the
occurrence and development of NPC remains to be further
studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL wrote the manuscript. JL and MY were responsible
for cDNA synthesis. JL and YL performed PCR. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhengzhou Central Hospital Affiliated to Zhengzhou University
(Zhengzhou, China). Signed informed consents were obtained from the
patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chan KC, Hung EC, Woo JK, Chan PK, Leung
SF, Lai FP, Cheng AS, Yeung SW, Chan YW, Tsui TK, et al: Early
detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus
DNA analysis in a surveillance program. Cancer. 119:1838–1844.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marsh GM, Youk AO and Morfeld P:
Mis-specified and non-robust mortality risk models for
nasopharyngeal cancer in the National Cancer Institute formaldehyde
worker cohort study. Regul Toxicol Pharmacol. 47:59–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang B and Tang PZ: Introduction to ‘NCCN
clinical practice guidelines in head and neck cancer (2009 Chinese
version)’. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
44:707–709. 2009.(In Chinese). PubMed/NCBI
|
|
4
|
Lee N, Harris J, Garden AS, Straube W,
Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, et
al: Intensity-modulated radiation therapy with or without
chemotherapy for nasopharyngeal carcinoma: Radiation therapy
oncology group phase II trial 0225. J Clin Oncol. 27:3684–3690.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Zong J, Wu J and Pan J: Prognostic
analysis of nasopharyngeal carcinoma patients with distant
metastasis after curative radiotherapy. Zhonghua Zhong Liu Za Zhi.
37:216–221. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Lin Y, Ukaji T, Koide N and Umezawa K:
Inhibition of late and early phases of cancer metastasis by the
NF-κB inhibitor DHMEQ derived from microbial bioactive metabolite
epoxyquinomicin: A review. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
7
|
Engels BM and Hutvagner G: Principles and
effects of microRNA-mediated post-transcriptional gene regulation.
Oncogene. 25:6163–6169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma MiR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie YJ, Long ZF and He XS: Involvement of
EBV-encoded BART-miRNAs and dysregulated cellular miRNAs in
nasopharyngeal carcinoma genesis. Asian Pac J Cancer Prev.
14:5637–5644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao Z, Ching Chow S, Han Li C, Chun Tang
S, Tsui SK, Lin Z and Chen Y: Role of microRNA-95 in the anticancer
activity of Brucein D in hepatocellular carcinoma. Eur J Pharmacol.
728:141–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, Mao W and Zheng S: MicroRNA-183
regulates Ezrin expression in lung cancer cells. FEBS Lett.
582:3663–3668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang R, Li FX, Shao WF, Wen QS, Yu XR and
Xiong JB: Protein-protein interaction between ezrin and p65 in
human breast cancer cells. Genet Mol Res. 15:152016. View Article : Google Scholar
|
|
17
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Targeting of integrin beta1 and kinesin 2alpha by
microRNA 183. J Biol Chem. 285:5461–5471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J,
Qin Y, Sun Z and Zheng X: miR-183 inhibits TGF-beta1-induced
apoptosis by downregulation of PDCD4 expression in human
hepatocellular carcinoma cells. BMC Cancer. 10:3542010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim J, Kang HS, Lee YJ, Lee HJ, Yun J,
Shin JH, Lee CW, Kwon BM and Hong SH: EGR1-dependent PTEN
upregulation by 2-benzoyloxycinnamaldehyde attenuates cell invasion
and EMT in colon cancer. Cancer Lett. 349:35–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng F, Li Z, Yan J, Manjanatha M, Shelton
S, Yarborough S and Chen T: Tissue-specific microRNA responses in
rats treated with mutagenic and carcinogenic doses of aristolochic
acid. Mutagenesis. 29:357–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarver AL, Li L and Subramanian S:
MicroRNA miR-183 functions as an oncogene by targeting the
transcription factor EGR1 and promoting tumor cell migration.
Cancer Res. 70:9570–9580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia H, Ng SS, Jiang S, Cheung WK, Sze J,
Bian XW, Kung HF and Lin MC: miR-200a-mediated downregulation of
ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma
cell growth, migration and invasion. Biochem Biophys Res Commun.
391:535–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Jaarsveld MT, Helleman J, Boersma AW,
van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH,
Berns EM, Verweij J, et al: miR-141 regulates KEAP1 and modulates
cisplatin sensitivity in ovarian cancer cells. Oncogene.
32:4284–4293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue J, Niu YF, Huang J, Peng G, Wang LX,
Yang YH and Li YQ: miR-141 suppresses the growth and metastasis of
HCC cells by targeting E2F3. Tumour Biol. 35:12103–12107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmed FE, Amed NC, Vos PW, Bonnerup C,
Atkins JN, Casey M, Nuovo GJ, Naziri W, Wiley JE and Allison RR:
Diagnostic microRNA markers to screen for sporadic human colon
cancer in blood. Cancer Genomics Proteomics. 9:179–192.
2012.PubMed/NCBI
|