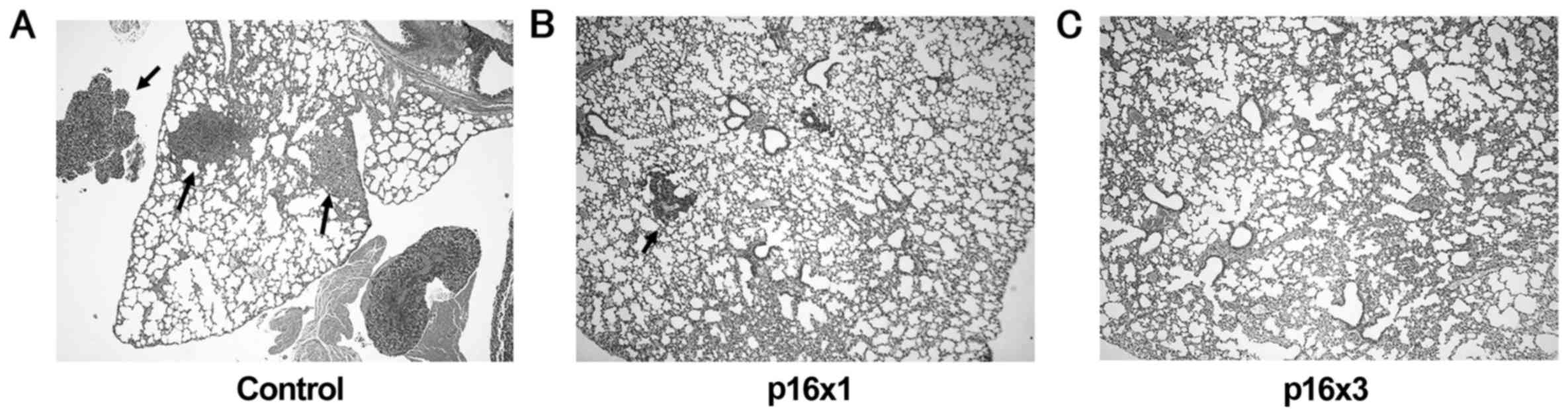
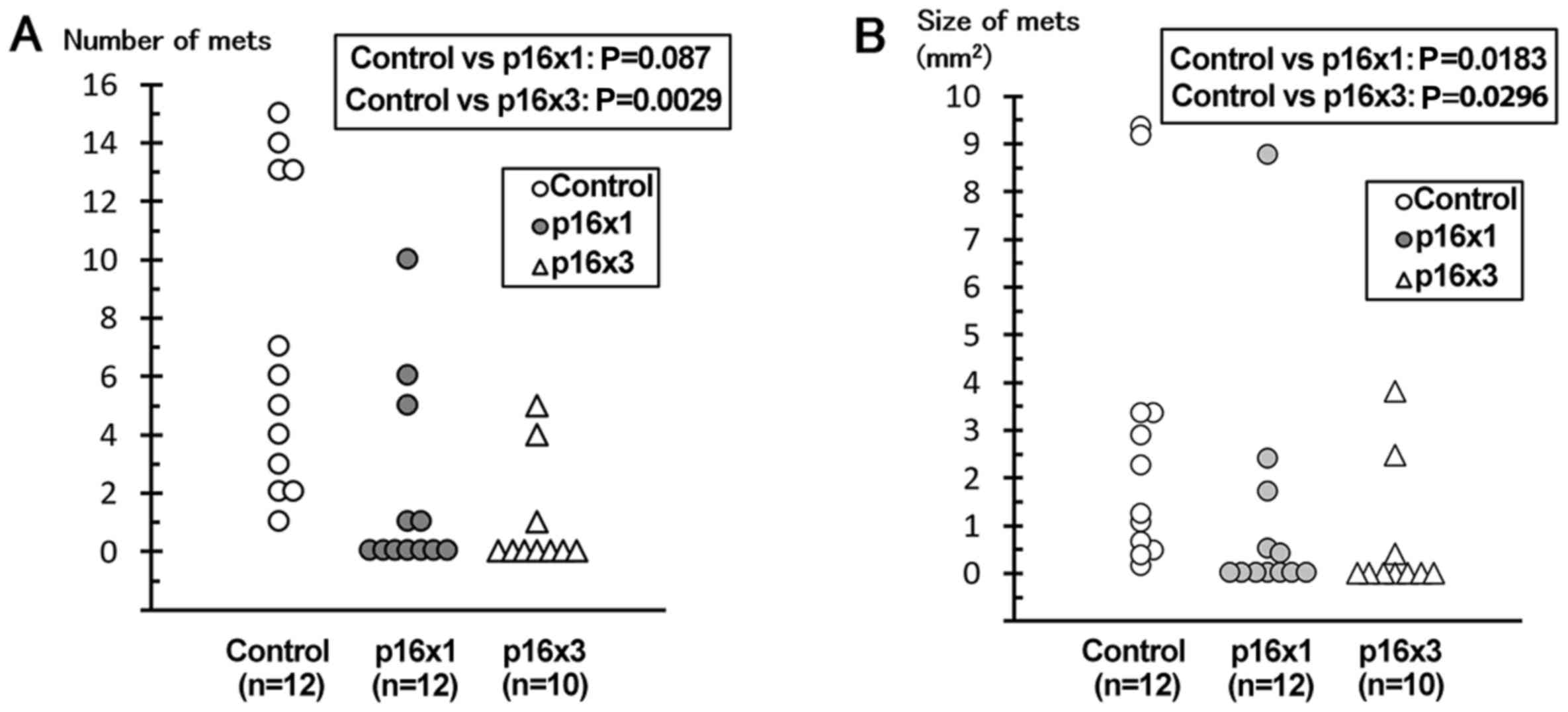
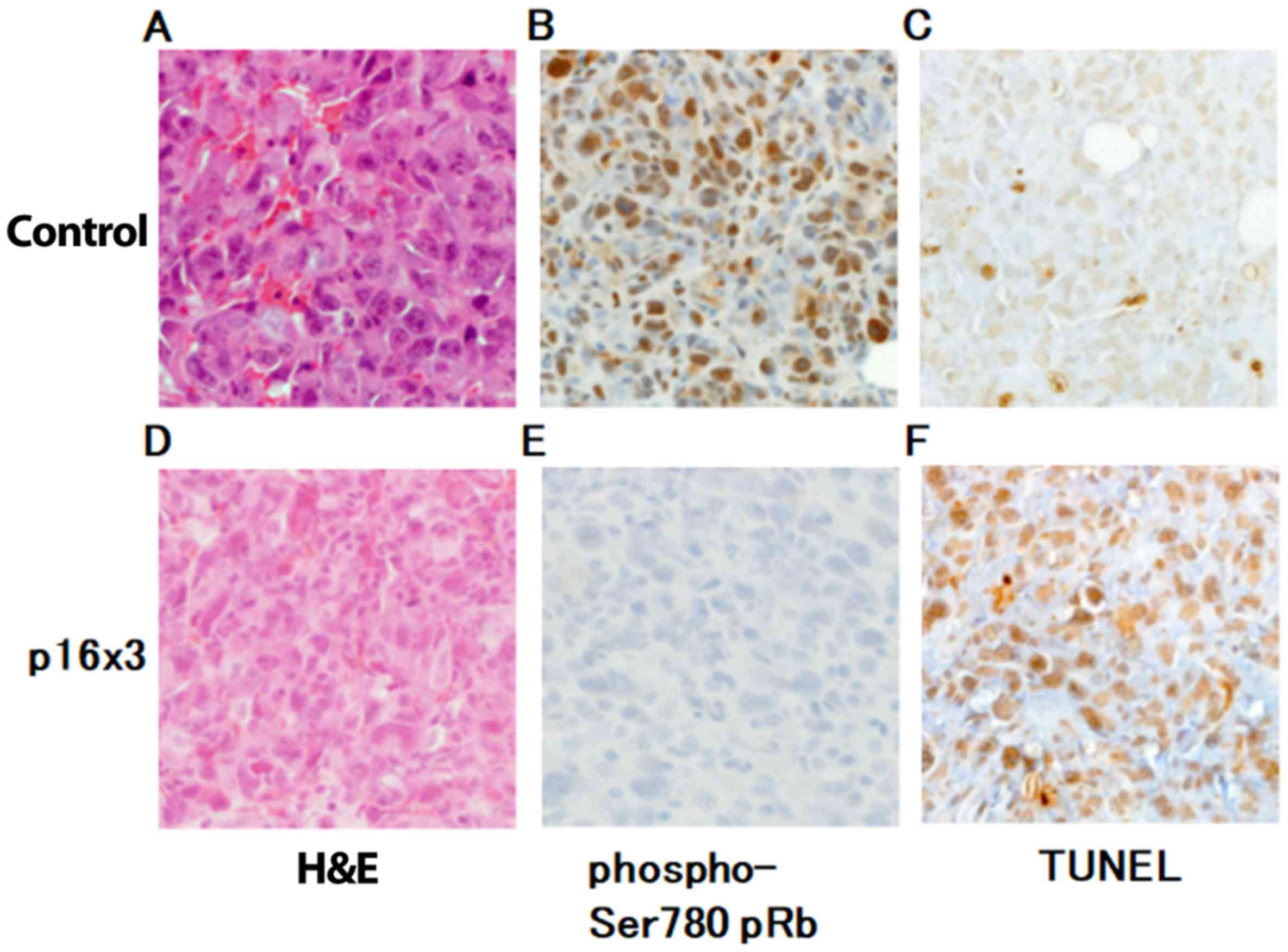
|
1
|
Shaw NJ, Georgopoulos NT, Southgate J and
Trejdosiewicz LK: Effect of loss of p53 and p16 function on life
span and survival of human urothelial cells. Int J Cancer.
116:634–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le F, rère-Belda MA, Gil Diez de Medina S,
Daher A, Martin N, Albaud B, Heudes D, Abbou CC, Thiery JP, Zafrani
ES, Radvanyi F and Chopin D: Profiles of the 2 INK4a gene products,
p16 and p14ARF, in human reference urothelium and bladder
carcinomas, according to pRb and p53 protein status. Hum Pathol.
35:817–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Q, Possati L, Montesi M, Gualandi F,
Rimessi P, Morelli C, Trabanelli C and Barbanti-Brodano G: Growth
arrest and suppression of tumorigenicity of bladder-carcinoma cell
lines induced by the P16/CDKN2 (p16INK4A, MTS1) gene and other loci
on human chromosome 9. Int J Cancer. 65:840–846. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo E, Seto M, Yoshikawa K and Yoshino
T: Highly efficient delivery of p16 antitumor peptide into
aggressive leukemia/lymphoma cells using a novel transporter
system. Mol Cancer Ther. 3:1623–1630. 2004.PubMed/NCBI
|
|
5
|
Zennami K, Yoshikawa K, Kondo E, Nakamura
K, Upsilonamada Y, De Velasco MA, Tanaka M, Uemura H, Shimazui T,
Akaza H, et al: A new molecular targeted therapeutic approach for
renal cell carcinoma with a p16 functional peptide using a novel
transporter system. Oncol Rep. 26:327–333. 2011.PubMed/NCBI
|
|
6
|
Shimazui T, Yoshikawa K, Miyazaki J,
Kojima T, Inai H, Ando S, Uemura H, Uchida K and Nishiyama H:
Systemic transduction of p16INK4A antitumor peptide inhibits the
growth of MBT-2 mouse bladder tumor cell line grafts. Int J Oncol.
42:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babaian RJ, Johnson DE, Llamas L and Ayala
AG: Metastases from transitional cell carcinoma of urinary bladder.
Urology. 16:142–144. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horinaga M, Fukuyama R, Nishiyama T,
Harsch KM, Cicek M, Heston W, Sizemore N, Casey G and Larchian W:
Novel enhanced lung-colonizing variant of murine MBT-2 bladder
cancer cells. Urology. 66:676–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fåhraeus R, Laín S, Ball KL and Lane DP:
Characterization of the cyclin-dependent kinase inhibitory domain
of the INK4 family as a model for a synthetic tumour suppressor
molecule. Oncogene. 16:587–596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park GC, Lee M, Roh JL, Choi SH, Nam SY,
Kim SY and Cho KJ: Phospho-Rb (Ser780) as a biomarker in patients
with cervical lymph node metastases from an unknown primary tumour:
A retrospective cohort study. Clin Otolaryngol. 38:313–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakazawa K, Murata S, Yuminamochi T, Ishii
Y, Ohno S, Nakazawa T, Kondo T and Katoh R: p16(INK4a) expression
analysis as an ancillary tool for cytologic diagnosis of urothelial
carcinoma. Am J Clin Pathol. 132:776–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asamoto M, Hori T, Baba-Toriyama H, Sano
M, Takahashi S, Tsuda H and Shirai T: p16 gene overexpression in
mouse bladder carcinomas. Cancer Lett. 127:9–13. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo E, Tanaka T, Miyake T, Ichikawa T,
Hirai M, Adachi M, Yoshikawa K, Ichimura K, Ohara N, Moriwaki A, et
al: Potent synergy of dual antitumor peptides for growth
suppression of human glioblastoma cell lines. Mol Cancer Ther.
7:1461–1471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higa M, Katagiri C, Shimizu-Okabe C,
Tsumuraya T, Sunagawa M, Nakamura M, Ishiuchi S, Takayama C, Kondo
E and Matsushita M: Identification of a novel cell-penetrating
peptide targeting human glioblastoma cell lines as a cancer-homing
transporter. Biochem Biophys Res Commun. 457:206–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kondo E, Saito K, Tashiro Y, Kamide K, Uno
S, Furuya T, Mashita M, Nakajima K, Tsumuraya T, Kobayashi N, et
al: Tumour lineage-homing cell-penetrating peptides as anticancer
molecular delivery systems. Nat Commun. 3:9512012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim KJ, Sung BH, Shin JR, Lee YW, Kim DJ,
Yang KS and Kim SC: A cancer specific cell-penetrating peptide,
BR2, for the efficient delivery of an scFv into cancer cells. PLoS
One. 8:e660842013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi W, Porten S, Kim S, Willis D, Plimack
ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al:
Identification of distinct basal and luminal subtypes of
muscle-invasive bladder cancer with different sensitivities to
frontline chemotherapy. Cancer Cell. 25:152–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rebouissou S, Hérault A, Letouzé E,
Neuzillet Y, Laplanche A, Ofualuka K, Maillé P, Leroy K, Riou A,
Lepage ML, et al: CDKN2A homozygous deletion is associated with
muscle invasion in FRFR3-mutated urothelial bladder carcinoma. J
Pathol. 227:315–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, He F, Mendelsohn CL, Tang MS,
Huang C and Wu XR: FGFR3b extracellular loop mutation lacks
tumorigenicity in vivo but collaborates with p53/pRB deficiency to
induce high-grade papillary urothelial carcinoma. Sci Rep.
6:255962016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nogova L, Sequist LV, Perez Garcia JM,
Andre F, Delord JP, Hidalgo M, Schellens JH, Cassier PA, Camidge
DR, Schuler M, et al: Evaluation of BGJ398, a fibroblast growth
factor receptor 1-3 kinase inhibitor, in patients with advanced
solid tumors harboring genetic alterations in fibroblast growth
factor receptors: Results of a global phase I, dose-escalation and
dose-expansion study. J Clin Oncol. 35:157–165. 2017. View Article : Google Scholar : PubMed/NCBI
|