Introduction
Human urothelial carcinoma evolves via the
accumulation of numerous genetic alterations, with the loss of p53
and p16INK4a (p16) function representing important
stages in the development of superficial lesions and their
progression to malignant disease (1).
p16 inhibits the activities of cyclin-dependent kinases, which
maintain the retinoblastoma protein (pRb) in its active
hypophosphorylated state (2).
A p16 gene transfection study demonstrated that
growth arrest and suppression of tumorigenesis of bladder tumor
(BT) cell lines may be induced by the p16 gene (3). It was reported that the p16 antitumor
peptide notably inhibited the growth of highly aggressive
leukemia/lymphoma types through restoration of p16 function
(4). In urological cancer types,
growth of renal cell carcinoma cell line graft was also inhibited
by transduction of p16 peptide in mice (5). We previously reported the antitumor
effect of a minimal inhibitory sequence peptide of p16 (p16-MIS) on
allografts of solid tumor types, particularly urological cancer,
derived from p16-deficient BT cell lines using a Wr-T system
(6). In the present study, the
suppression of BT metastasis with a p16-Wr-T peptide transfer
system using a metastasis model of BT in mice was demonstrated,
continuing on from our previous study. In terms of animal models
for BT metastasis, a mouse lung metastasis model using the MBT-2
cell line was selected due to lung metastases occurring frequently,
and at a similar rate to liver metastases (36 and 38%,
respectively) (7). In addition, this
animal model was established previously by Horinaga et al
(8).
It has been reported that the current standard
treatment for patients with systemic disease, including distant
metastases of BT, is a combination of gemcitabine and cisplatin,
including GC therapy (9). In
addition, immune-checkpoint inhibitor, including pembrolizumab,
anti-PD-1 antibody has been reported as a second treatment option
for BT (10). Due to the fact that
the aforementioned treatments may be associated with severe adverse
events or specific immune-associated reactions, new systemic
treatments are not expected to present such adverse events.
Therefore, the present study also evaluated the toxicity in mice
associated with systemic p16 peptide transductions.
Materials and methods
Cells
The mouse BT cell line MBT-2 (Japanese Collection of
Research Bioresources Cell Bank, National Institutes of Biomedical
Innovation, Health and Nutrition, Osaka, Japan) was cultured in
RPMI-1640 containing 10% inactivated fetal bovine serum
(Immuno-Biological Laboratories, Co., Ltd., Fujioka, Japan) at 37°C
in a humidified atmosphere containing 5% CO2. The MBT-2
cell line is a p16-deficient cell line with phosphorylation of the
Rb protein (6). We previously
confirmed the lack of expression of p16 in MBT-2 and that
restoration of p16 function by peptide transduction resulted in
downregulation of phosphorylated Rb expression (6).
Mouse model for lung metastases
Six-week-old female C3H/He mice were obtained from
Charles River Laboratories Japan, Inc. (Yokohama, Japan). The mice
were kept under the following housing condition: 23.5±2.5°C
temperature; 52.5±12.5% humidity; 200Lx illumination during daytime
(5:00 to 19:00), and free access to food and water. A total of 100
µm suspension containing 1×105 MBT-2 cells in PBS was
injected into the tail vein of each mouse, and lung metastases had
developed when the mice were sacrificed by cervical dislocation on
the 14th experimental day, based on the previous study by Horinaga
et al (8). Horinaga et
al (8) observed lung metastases
between the 9th and 12th day after tail vein injection of MBT-2
cells and survival of mice decreased at the 15th day after
injection. In addition, in our pilot study, a number of mice
succumbed to severe lung metastases at the 21st day after injection
(data not shown). In total, 34 mice were classified into three
groups: A control group (n=12); a single treatment group (n=12);
and a triple treatment group (n=10). A decreased number of mice
were used in the triple treatment group due to failure (i.e.,
phlebitis and hematoma) of the tail vein injection during the
process of experiments. The initial body weight of mice in the
aforementioned three groups were 18.5±1.19, 19.2±0.970, and
18.7±0.633 g, respectively. At the end of the experiment the body
weight of mice were 19.7±1.13, 20.1±0.999, and 20.0±1.22 g,
respectively. Animal experiments performed in the present study
were approved by the Laboratory Animal Resource Center of the
University of Tsukuba (Tsukuba, Japan). All mouse procedures,
euthanasia and surgery, including injections of BT cells and
peptides, were conducted painlessly or under anesthesia using a
combination of hydrochloric acid medetonidine 0.3 mg/kg + midazolam
4 mg/kg + butorphanol tartrate 5 mg/kg within the strict guidelines
of the Laboratory Animal Resource Center of the University of
Tsukuba.
Peptide synthesis
All peptides including Wr-T and the r9-p16 MIS for
mouse were synthesized at BioGate Co. Ltd. (Yamagata, Gifu, Japan)
using standard fluorenylmethyloxycarbonyl chemistry as previously
described (6). Briefly, the 10 amino
acid residue sequence, FLDTLVVLHG, was identified as the MIS of p16
(11), and the amino acid sequence of
the Wr-T transporter peptide was identified as KET WWE TWW TEW WTE
WSQ GPG rrr rrr rrr (r, D-enantiomer arginine), as previously
described (5,6). Peptides were purified at 95% by
reverse-phase high-performance or high-pressure liquid
chromatography using Shimadzu System Control HPLC 10AVP (Shimadzu
Corporation, Kyoto, Japan), according to the manufacturer's
protocols, and a Shimadzu C18 Analytical column (Shimadzu
Corporation). Identification of all peptides was confirmed by mass
spectrometry (AXIMA CFR Kratos; Shimadzu Corporation). All peptides
precipitated from trifluoroacetic acid solutions are
trifluoroacetic acid salts. The hydrochloride form of peptides was
prepared following high-performance liquid chromatography
purification.
Peptide transduction
In vivo peptide delivery to the lung
metastatic tumor model of mice was performed by injecting the
Wr-T/r9-p16 MIS peptide mix (Wr-T, 50 nmol; and r9-p16 MIS, 80
nmol) into the tail vein. Following the MBT-2 cell injection, the
peptide mix was delivered by tail vein injection on the 3rd day or
the 3rd, 5th, and 7th days for the single and triple treatment
group, respectively. The control group received no tail vein
injection.
Evaluation of lung metastases
following peptide transduction
Lungs were fixed in 10% neutral buffered formalin
(Fujifilm Wako Pure Chemical Corp., Tokyo, Japan) overnight at room
temperature and subsequently embedded into paraffin. Serial
sections (4 µm) were used for hematoxylin and eosin (H&E) and
immunohistochemical staining. The sections were stained with
hematoxylin and eosin for 1.5 and 4 min, respectively, at room
temperature. Lung metastases following the peptide transfer were
evaluated histologically by H&E staining under a light
microscope (magnification ×40-400; OLYMPUS BX41; Olympus Corp.,
Tokyo, Japan). To evaluate lung metastases, the number and size of
metastatic nodules in each group was quantified using ImageJ 1.48v
(National Institutes of Health, Bethesda, MD, USA). The number of
metastases was counted on random H&E-stained sections of lungs
from each mouse. Additionally, the size of the tumor was
represented by the area of the tumor, calculated by tracing its
contour line, on the H&E-stained sections, using ImageJ 1.48
software. For the number and area of the tumor samples, the
differences between groups were analyzed.
Immunohistochemical staining of
p16-associated molecules and terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining
transduction
Lungs were fixed in 10% neutral buffered formalin
overnight at room temperature and subsequently embedded into
paraffin. According to previous studies (6,12), 4-µm
serial paraffin sections were stained using a rabbit polyclonal
anti-phospho-Ser780 pRB antibody (dilution, 1:100; cat. no. 9307S;
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C, followed by the Universal Immuno-enzyme Polymer method using
the N-Histofine Simple Stain Mouse MAX PO reagent (cat. no. 714342;
Nichirei Biosciences, Inc., Tokyo, Japan), according to the
manufacturer's protocol. The sections were developed with
3,3′-diaminobenzidine tetrahydrochloride containing 0.03% hydrogen
peroxide for 5 min at room temperature and counterstained with
hematoxylin for 90 sec at room temperature. Apoptotic cells were
detected in tumors harvested from mice 48 h after the peptide
administration. Apoptosis in tumor sections was determined by the
TUNEL assay method with the use of ApopTag Peroxidase In Situ
Apoptosis Detection kit (cat. no. S7100; Nippon Chemi-Con Co.,
Tokyo, Japan), according to the manufacturer's protocol.
Histological findings were observed in five fields under a light
microscope (magnification, ×400; OLYMPUS BX41; Olympus Corp.,
Tokyo, Japan).
Evaluation of toxicity of systemic p16
MIS peptide transfer
To evaluate the toxicity of systemic p16 MIS peptide
transduction, a single low or high dose of the p16 MIS peptide was
injected into the hearts of mice as follows: A single low dose
peptide transfer was defined as 5 nmol Wr-T and 20 nmol p16 MIS;
and a single high dose was defined as 25 nmol Wr-T and 100 nmol p16
MIS. In contrast, late phase multiple high dose peptide
transduction was evaluated at 12 weeks after the initial peptide
administration, followed by four weekly peptide transfers of 25
nmol Wr-T and 100 nmol p16 MIS, respectively. There was no group
with multiple low dose peptide transduction in late phase. Each
group including the control group contained three male and three
female mice, and toxicities were evaluated by body weight and blood
analyses, which included a whole blood count and biochemistry, and
by histological examination, including aforementioned H&E
staining of bone marrow, thymus, lung, bronchus, pericardium,
stomach, intestine, liver, spleen, kidney, testis, ovary and brain
of mice. The mice used for toxicity evaluation were obtained from
Japan SLC, Inc. (Hamamatsu, Japan) and were kept under the same
housing condition previously described.
Statistical analyses
Each value in blood analyses and body weight was
presented as the mean ± standard deviation. Statistical analysis
was performed with JMP® 9.0.0 supported by SAS (SAS
Institute Inc., Cary, NC, USA). One way analysis of variance
(ANOVA) and the post-hoc Steel-Dwass test (non-parametric test)
were used to compare the number and area of lung metastases, the
blood analyses and animal body weight between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibition of lung metastasis of BT
cells following tail vein injection of p16 MIS peptide in a mouse
lung metastasis model
Due to the practical potential of the Wr-T and p16
MIS peptide delivery system, the efficacy and toxicity of this
system for the treatment of lung metastasis of MBT-2 mouse BT cells
in C3H mice was investigated. At the 14th day after BT cell
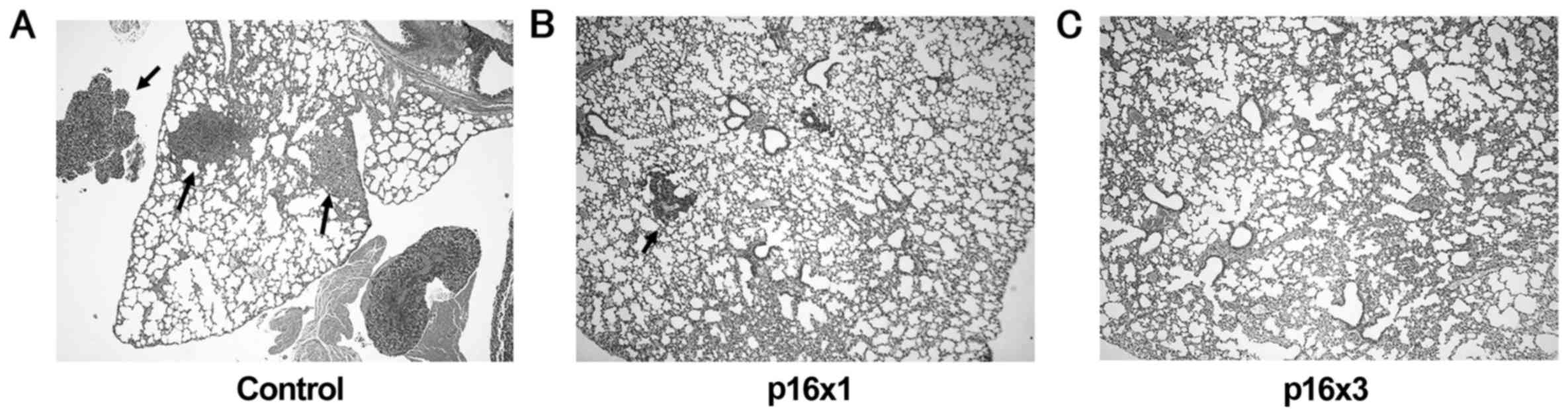
injection, lung metastases were observed in 100% (12/12), 41.7%
(5/12) and 30% (3/10) of control, single and triple doses of p16
groups, respectively. Lung tumors were notably decreased in number
and size in the p16 MIS peptide transfer groups, compared with the
control group (Fig. 1).
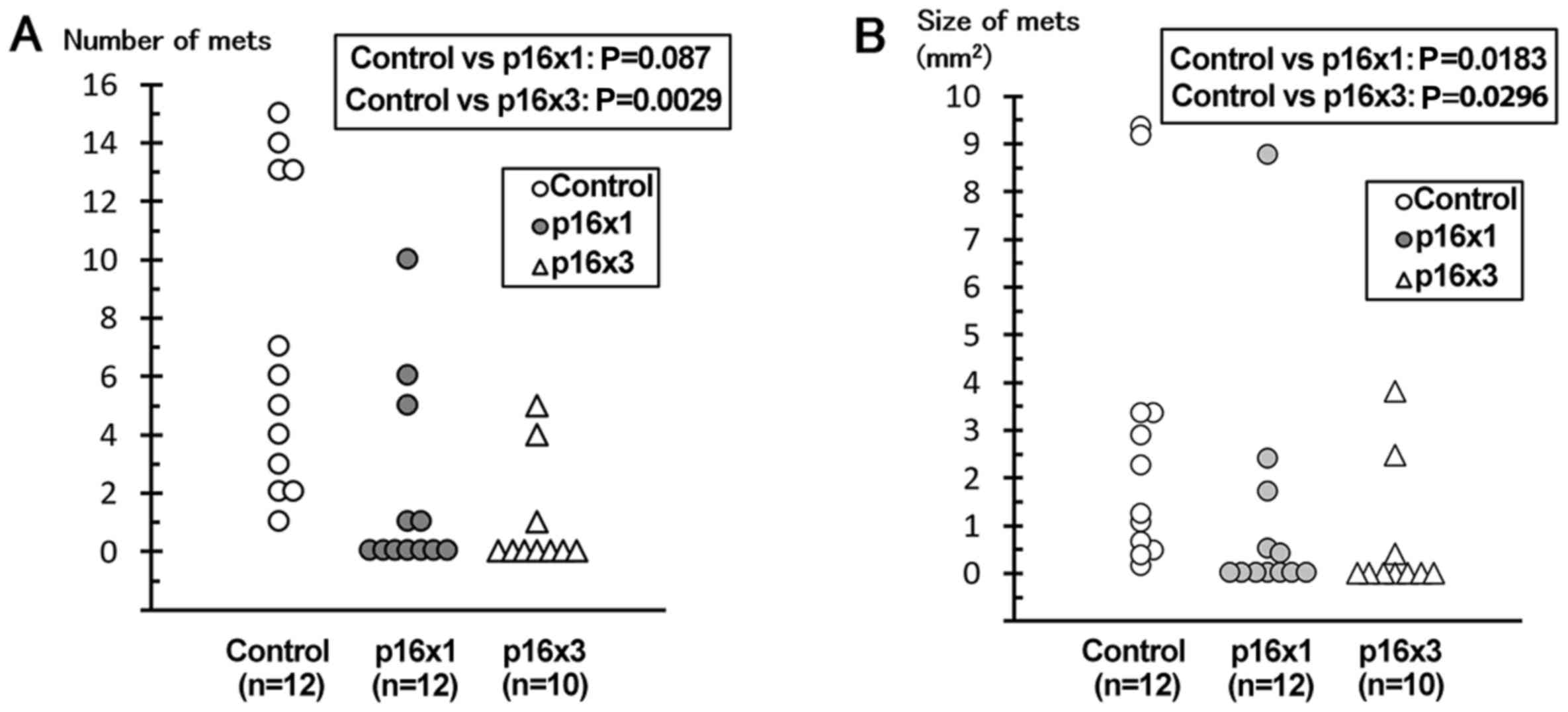
A statistically significant decrease in tumor number
and size was observed in the p16 peptide groups, compared with the
control group (control vs. single or triple doses of p16 MIS
peptide for the number, P=0.0087 and 0.0029, and in area, P=0.0183
and 0.0296, of tumors, respectively; Fig.
2).
Change in expression of p16-associated
molecules and apoptosis after p16 peptide treatment
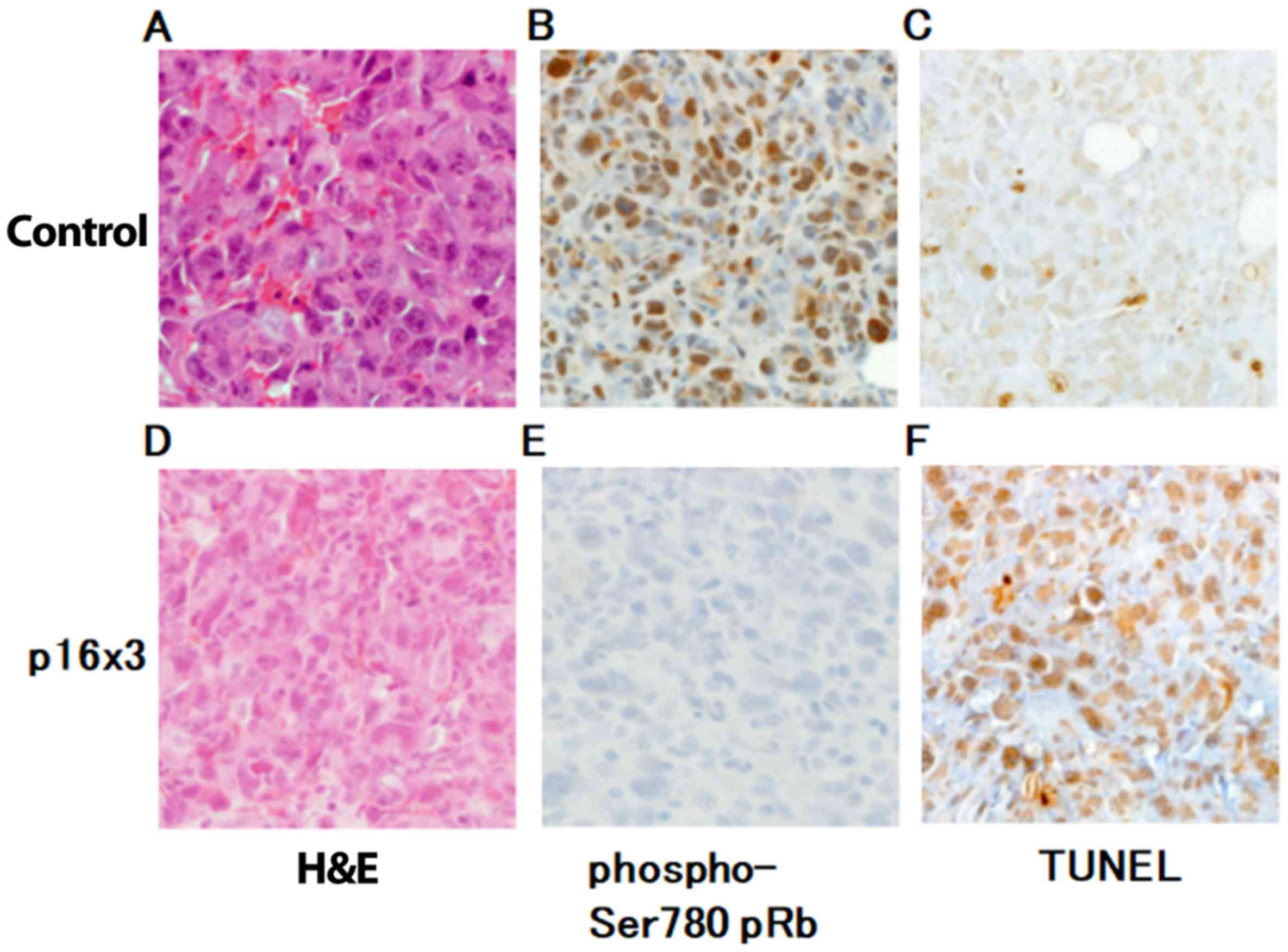
Fig. 3A, B and C
demonstrate H&E staining, immunohistochemistry using an
antibody against phosphorylated Rb, and TUNEL staining in control
group, respectively. In H&E staining, no microscopic change in
lung metastasis was observed between control and triple p16 MIS
peptide treatment group (Fig. 3D).
The expression of Rb phosphorylation was notably decreased in the
triple p16 MIS peptide treatment group (Fig. 3E), compared with the control group
(Fig. 3B). The TUNEL staining
demonstrated that apoptosis was increased in the triple p16 MIS
peptide treatment group (Fig. 3F)
compared with the control group (Fig.
3B).
Toxicity of systemic transduction of
the p16 MIS peptide assessed by body weight, blood analyses and
histological data
Changes in blood count and biochemistry following a
single treatment in the early phase of the 2nd experimental week
are demonstrated in Table I and
indicated no significant statistical difference between control and
p16 MIS peptide treatment groups in both males and females. Changes
in mouse body weights during administration of multiple p16 MIS
doses are displayed in Table II. No
significant statistical difference was observed in body weight
between groups. Additionally, Table
III indicates that no statistical difference in blood tests
between the control and multiple p16 peptide treatment groups was
observed in the late phase of the 12th experimental week. The white
blood cell count was reduced in the group with multiple treatments,
but there was no statistical difference.
 | Table I.Change in blood analyses of single
treatment. |
Table I.
Change in blood analyses of single
treatment.
| A, Male |
|---|
|
|---|
| Group | Alb (g/dl) | ALT (U/l) | AST (U/l) | Creat (mg/dl) | WBC (/µl) | RBC
(104/µl) | Hb (g/dl) | Plt
(104/µl) |
|---|
| Controla | 3.3 | 86 | 30 | 0.01 | 5,430 | 748 | 12.4 | 37.8 |
|
| (0.06) | (23) | (11.5) | (0.02) | (1,036) | (34.8) | (0.62) | (7.0) |
| 20 nmola | 3.5 | 74 | 19 | 0.02 | 5,207 | 741 | 11.7 | 35.5 |
|
| (0.27) | (17) | (3.1) | (0.02) | (1,952) | (127.7) | (2.1) | (16.5) |
| 100 nmola | 3.2 | 60 | 16 | 0.05 | 6,160 | 805 | 13.0 | 60.2 |
|
| (0.15) | (8.7) | (1.7) | (0.01) | (1,384) | (30.6) | (0.1) | (15.0) |
| P-value | 0.779 | 0.189 | 0.179 | 0.176 | 0.900 | 0.390 | 0.784 | 0.188 |
|
| B, Female |
|
| Group | Alb
(g/dl) | ALT
(U/l) | AST
(U/l) | Creat
(mg/dl) | WBC
(/µl) | RBC
(104/µl) | Hb
(g/dl) | Plt
(104/µl) |
|
|
Controla | 3.5 | 81 | 22 | 0.07 | 6,360 | 783 | 13.0 | 54.0 |
|
| (0.28) | (5.0) | (5.6) | (0.01) | (2,786) | (7.07) | (0.0) | (1.78) |
| 20
nmola | 3.7 | 91 | 19 | 0.1 | 3,430 | 873 | 14.4 | 52.2 |
|
| (0.36) | (3.0) | (2.3) | (0.03) | (1,488) | (55.9) | (1.3) | (15.0) |
| 100
nmola | 3.6 | 82 | 26 | 0.08 | 5,763 | 816 | 13.9 | 50.3 |
|
| (0.17) | (1.0) | (5.6) | (0.02) | (1,762) | (27.7) | (0.06) | (8.5) |
| P-value | 0.944 | 0.999 | 0.955 | 0.824 | 0.999 | 0.309 | 0.281 | 0.955 |
 | Table II.Change in body weight. |
Table II.
Change in body weight.
| A, Male |
|---|
|
|---|
| Group | Day 0 (g) | Day 7 (g) | Day 14 (g) | Day 21 (g) |
|---|
|
Controla | 20.5 | 38.5 | 42.6 | 44.7 |
|
| (0.305) | (3.10) | (3.30) | (5.00) |
| 100
nmola | 21.2 | 38.2 | 44.9 | 47.5 |
|
| (0.173) | (0.451) | (0.721) | (2.78) |
| P-value | 0.077 | 0.999 | 0.663 | 0.663 |
|
| B,
Female |
|
| Group | Day 0
(g) | Day 7
(g) | Day 14
(g) | Day 21
(g) |
|
|
Controla | 20.5 | 33.3 | 35.6 | 38.0 |
|
| (0.737) | (2.41) | (1.77) | (1.45) |
| 100
nmola | 20.8 | 31.3 | 34.1 | 36.9 |
|
| (0.814) | (1.34) | (4.16) | (4.17) |
| P-value | 0.507 | 0.383 | 0.663 | 0.663 |
 | Table III.Change in blood analyses of multiple
treatments. |
Table III.
Change in blood analyses of multiple
treatments.
| A, Male |
|---|
|
|---|
| Group | Alb (g/dl) | ALT (U/l) | AST (U/l) | Creat (mg/dl) | WBC (/µl) | RBC
(1×104/µl) | Hb (g/dl) | Plt
(1×104/µl) |
|---|
|
Controla | 3.4 | 97 | 23 | 0.09 | 3,843 | 882 | 13.6 | 51.9 |
|
| (0.15) | (23) | (1.0) | (0.03) | (1,846) | (51.4) | (0.78) | (19.7) |
| 100
nmola | 3.5 | 69 | 18 | 0.08 | 5,953 | 863 | 13.3 | 46.6 |
|
| (0.10) | (19.7) | (2.8) | (0.03) | (1,638) | (30.4) | (0.43) | (13.9) |
| P-value | 0.369 | 0.190 | 0.077 | 0.653 | 0.383 | 0.663 | 0.663 | 0.663 |
|
| B,
Female |
|
| Group | Alb
(g/dl) | ALT
(U/l) | AST
(U/l) | Creat
(mg/dl) | WBC
(/µl) | RBC
(1×104/µl) | Hb
(g/dl) | Plt
(1×104/µl) |
|
|
Controla | 3.5 | 77 | 18 | 0.10 | 3,173 | 845 | 13.4 | 40.4 |
|
| (0.15) | (18.2) | (4.5) | (0.02) | (867) | (32.2) | (0.06) | (2.2) |
| 100
nmola | 3.4 | 72 | 20 | 0.10 | 4,230 | 861 | 13.3 | 42.7 |
|
| (0.26) | (35.7) | (4.0) | (0.04) | (1,142) | (8.96) | (0.35) | (8.2) |
| P-value | 0.507 | 0.999 | 0.663 | 0.999 | 0.383 | 0.663 | 0.822 | 0.663 |
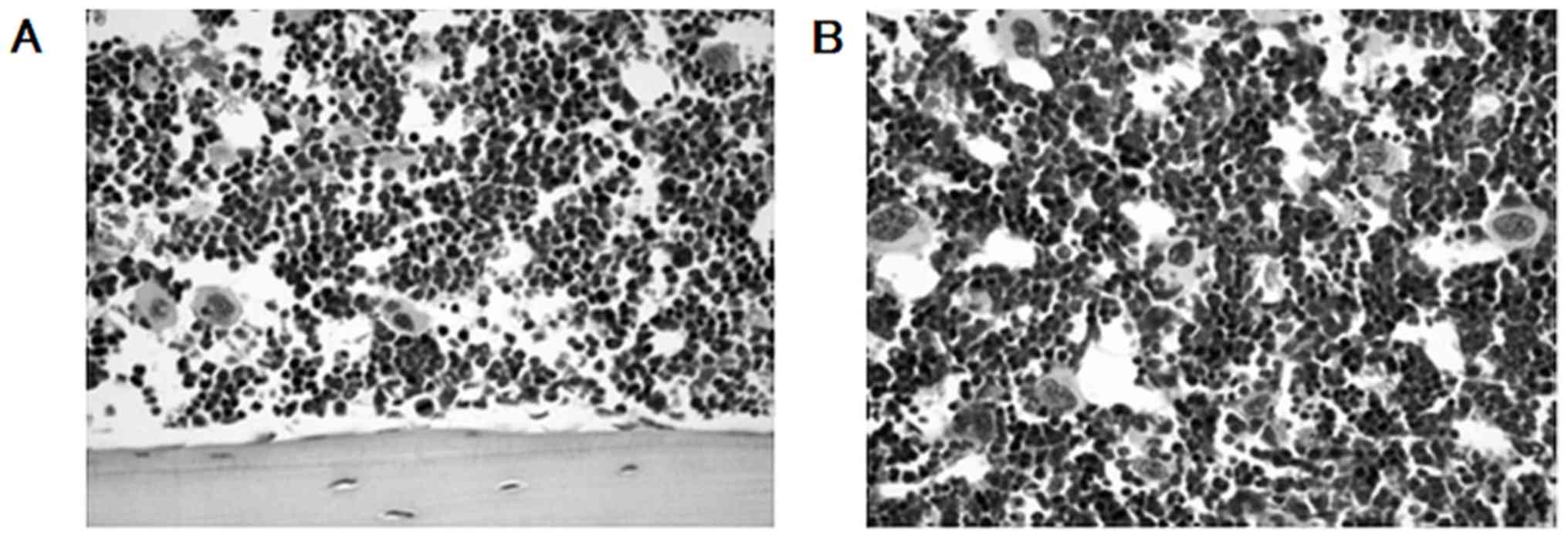
There was no histological change observed in the
thymus, lung, bronchus, pericardium, stomach, intestine, liver,
spleen, kidney, testis, ovary, brain or bone marrow. No severe
myelosuppression was histologically observed in the bone marrow
subsequent to systemic p16 MIS peptide transduction (Fig. 4).
Discussion
In the present study, systemic transduction of the
p16 MIS antitumor peptide successfully inhibited lung metastasis
induced by venous injection in a dose-dependent manner. The
decrease in the number and size of metastatic nodules, as
demonstrated by histological data, indicated that the
anti-metastatic effect may be based on inhibition of implantation
and growth of tumor cells by cell cycle arrest (6) and that it induced apoptosis signaling.
Immunohistochemistry also indicated that a candidate indicator to
predict the anti-metastatic activity of the p16 MIS peptide could
be the elevated expression of phosphorylated Rb in BT cells, due to
p16 expression not always being absent or decreased but also it is
overexpressed in a number of BT types, as previously described
(6,13,14).
In previous studies regarding in vivo
transduction of the antitumor peptide, adverse effects were not
investigated (6,15); therefore, in the present study,
whether or not systemic transduction of the p16 MIS peptide could
cause toxicities of normal organs was analyzed using blood tests
and histological data. Furthermore, systemic p16 peptide
transduction did not inhibit the function of vital organs and bone
marrow at the early and late phases following peptide
administration. Although a number of statistical differences were
observed in serum transaminase, no association with hepatic
dysfunction was apparent. In H&E-stained sections, no notable
histological difference was observed between control and p16 MIS
peptide transfer groups at any experimental stage. This may
indicate that overexpression of p16 would not suppress non-tumorous
cells in mice, regardless of the activation state of
proliferation.
Although the p16 MIS peptide used in the present
study would be transferred into various cells in a non-selective
manner, metastatic tumor in the lung could be a good candidate for
non-specific transduction, for example the peptide from the present
study following injection into the systemic circulation. Higa et
al (16) developed a novel
cancer-homing peptide, which has a high affinity to the
cell-penetrating peptide with p16 MIS peptide, to glioblastoma
cells. They screened cell-penetrating peptides as a novel
biomedical delivery system and fused it with a functional p16 MIS
peptide to improve therapeutic efficacy and decrease side effects
on normal tissues (17,18). This system may become an ideal
therapeutic system using functional peptides to improve antitumor
activity against lung metastases of the present model in the
future.
It may indicate that lung metastases can be
controlled by the inactivation of a p16-associated cell cycle
activator, including phosphorylated Rb, as demonstrated in the
immunohistochemistry of the present study. Considering that p53 may
be a key molecule in BT, restoration of p14 or p21, as an activator
of impaired p53 function, could inhibit lung metastases. Kondo
et al (15) previously
reported that simultaneous induction of two tumor suppressor
peptides (p14 and p16 or p16 and p21) suppressed the growth of a
glioblastoma cell line that contained a missense mutation in
p53.
Another possible strategy could be combination with
a novel molecular target detected with whole genome mRNA expression
profiling (19). For instance,
although one of the key genes, fibroblast growth factor receptor 3
(FGFR3), has detected muscle invasive bladder cancer by the MD
Anderson Cancer Center molecular subtyping (19,20), it
was recently reported that FGFR3 mutations have a limited role in
urothelial carcinogenesis and must collaborate with other genetic
events, including inactivation of pRb/p53 (21). Therefore, a combination therapy of
functional peptide transduction with an FGFR3 inhibitor, such as
BGJ398 (22), may demonstrate an
enhanced inhibition of advanced bladder cancer cells in the
future.
A limitation of the present study was the lack of
information regarding in vivo kinetics in mice following
administration of the p16 MIS peptide. If the accumulation of p16
MIS peptide in lung and metastatic tumor is detectable using
whole-body fluorescent imaging, for example the IVIS Imaging
System, it could assist with analyzing the biological modification
of p16-associated molecules following peptide transduction in mice.
Additionally, immunogenicity of the p16 MIS peptide should be
analyzed to predict the production of antibodies against this
peptide, which may be a limitation of future clinical
application.
To conclude, systemic administration of the p16 MIS
peptide through the tail vein significantly inhibited the number
and size of lung metastases of BT in mice models. The toxicity of
the p16 MIS peptide had no notable effect on the mice, according to
blood and histological analyses, following short and long exposures
to even a high dose of the peptide.
Acknowledgements
The authors would like to thank Mrs Noriko Kunita
and Mrs Taeko Asano, Department of Urology, University of Tsukuba
for their technical support.
Funding
The present study was supported by Grants-in-Aid
from the Japan Society for the Promotion of Science, Tokyo, Japan
(grant no. 24592375).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS performed and analyzed the animal experiments
regarding the inhibition of lung metastasis. KY analyzed animal
data regarding the toxicity of the peptides. RI, TK, SK and TY
assisted with the animal experiments and its data analyses. JM
analyzed histological investigation regarding the
immunohistochemistry. KU supervised and analyzed molecular analysis
regarding inhibition of lung metastasis. HN analyzed the molecular
and histological analyses regarding inhibition of lung metastasis
and revised the manuscript.
Ethics approval and consent to
participate
Animal experiments performed in the present study
were approved by the Laboratory Animal Resource Center, University
of Tsukuba (approval no. 12-373).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaw NJ, Georgopoulos NT, Southgate J and
Trejdosiewicz LK: Effect of loss of p53 and p16 function on life
span and survival of human urothelial cells. Int J Cancer.
116:634–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le F, rère-Belda MA, Gil Diez de Medina S,
Daher A, Martin N, Albaud B, Heudes D, Abbou CC, Thiery JP, Zafrani
ES, Radvanyi F and Chopin D: Profiles of the 2 INK4a gene products,
p16 and p14ARF, in human reference urothelium and bladder
carcinomas, according to pRb and p53 protein status. Hum Pathol.
35:817–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Q, Possati L, Montesi M, Gualandi F,
Rimessi P, Morelli C, Trabanelli C and Barbanti-Brodano G: Growth
arrest and suppression of tumorigenicity of bladder-carcinoma cell
lines induced by the P16/CDKN2 (p16INK4A, MTS1) gene and other loci
on human chromosome 9. Int J Cancer. 65:840–846. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo E, Seto M, Yoshikawa K and Yoshino
T: Highly efficient delivery of p16 antitumor peptide into
aggressive leukemia/lymphoma cells using a novel transporter
system. Mol Cancer Ther. 3:1623–1630. 2004.PubMed/NCBI
|
|
5
|
Zennami K, Yoshikawa K, Kondo E, Nakamura
K, Upsilonamada Y, De Velasco MA, Tanaka M, Uemura H, Shimazui T,
Akaza H, et al: A new molecular targeted therapeutic approach for
renal cell carcinoma with a p16 functional peptide using a novel
transporter system. Oncol Rep. 26:327–333. 2011.PubMed/NCBI
|
|
6
|
Shimazui T, Yoshikawa K, Miyazaki J,
Kojima T, Inai H, Ando S, Uemura H, Uchida K and Nishiyama H:
Systemic transduction of p16INK4A antitumor peptide inhibits the
growth of MBT-2 mouse bladder tumor cell line grafts. Int J Oncol.
42:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Babaian RJ, Johnson DE, Llamas L and Ayala
AG: Metastases from transitional cell carcinoma of urinary bladder.
Urology. 16:142–144. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horinaga M, Fukuyama R, Nishiyama T,
Harsch KM, Cicek M, Heston W, Sizemore N, Casey G and Larchian W:
Novel enhanced lung-colonizing variant of murine MBT-2 bladder
cancer cells. Urology. 66:676–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fåhraeus R, Laín S, Ball KL and Lane DP:
Characterization of the cyclin-dependent kinase inhibitory domain
of the INK4 family as a model for a synthetic tumour suppressor
molecule. Oncogene. 16:587–596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park GC, Lee M, Roh JL, Choi SH, Nam SY,
Kim SY and Cho KJ: Phospho-Rb (Ser780) as a biomarker in patients
with cervical lymph node metastases from an unknown primary tumour:
A retrospective cohort study. Clin Otolaryngol. 38:313–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakazawa K, Murata S, Yuminamochi T, Ishii
Y, Ohno S, Nakazawa T, Kondo T and Katoh R: p16(INK4a) expression
analysis as an ancillary tool for cytologic diagnosis of urothelial
carcinoma. Am J Clin Pathol. 132:776–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asamoto M, Hori T, Baba-Toriyama H, Sano
M, Takahashi S, Tsuda H and Shirai T: p16 gene overexpression in
mouse bladder carcinomas. Cancer Lett. 127:9–13. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo E, Tanaka T, Miyake T, Ichikawa T,
Hirai M, Adachi M, Yoshikawa K, Ichimura K, Ohara N, Moriwaki A, et
al: Potent synergy of dual antitumor peptides for growth
suppression of human glioblastoma cell lines. Mol Cancer Ther.
7:1461–1471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higa M, Katagiri C, Shimizu-Okabe C,
Tsumuraya T, Sunagawa M, Nakamura M, Ishiuchi S, Takayama C, Kondo
E and Matsushita M: Identification of a novel cell-penetrating
peptide targeting human glioblastoma cell lines as a cancer-homing
transporter. Biochem Biophys Res Commun. 457:206–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kondo E, Saito K, Tashiro Y, Kamide K, Uno
S, Furuya T, Mashita M, Nakajima K, Tsumuraya T, Kobayashi N, et
al: Tumour lineage-homing cell-penetrating peptides as anticancer
molecular delivery systems. Nat Commun. 3:9512012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim KJ, Sung BH, Shin JR, Lee YW, Kim DJ,
Yang KS and Kim SC: A cancer specific cell-penetrating peptide,
BR2, for the efficient delivery of an scFv into cancer cells. PLoS
One. 8:e660842013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi W, Porten S, Kim S, Willis D, Plimack
ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al:
Identification of distinct basal and luminal subtypes of
muscle-invasive bladder cancer with different sensitivities to
frontline chemotherapy. Cancer Cell. 25:152–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rebouissou S, Hérault A, Letouzé E,
Neuzillet Y, Laplanche A, Ofualuka K, Maillé P, Leroy K, Riou A,
Lepage ML, et al: CDKN2A homozygous deletion is associated with
muscle invasion in FRFR3-mutated urothelial bladder carcinoma. J
Pathol. 227:315–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, He F, Mendelsohn CL, Tang MS,
Huang C and Wu XR: FGFR3b extracellular loop mutation lacks
tumorigenicity in vivo but collaborates with p53/pRB deficiency to
induce high-grade papillary urothelial carcinoma. Sci Rep.
6:255962016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nogova L, Sequist LV, Perez Garcia JM,
Andre F, Delord JP, Hidalgo M, Schellens JH, Cassier PA, Camidge
DR, Schuler M, et al: Evaluation of BGJ398, a fibroblast growth
factor receptor 1-3 kinase inhibitor, in patients with advanced
solid tumors harboring genetic alterations in fibroblast growth
factor receptors: Results of a global phase I, dose-escalation and
dose-expansion study. J Clin Oncol. 35:157–165. 2017. View Article : Google Scholar : PubMed/NCBI
|