Introduction
Chronic lymphocytic leukemia (CLL) is the most
common type of adult leukemia in Western countries with a large
percentage of asymptomatic patients, and may be detected only
incidentally during routine examinations (1,2). CLL is a
disease that exhibits a gradual onset, and is characterized by the
accumulation of small defective CD5+ B lymphocytes in
the blood, bone marrow and lymphoid organs (3). The disease presents with subtle and
heterogeneous symptoms, including fever, night sweats, weight loss,
lymphadenopathy, anemia, and thrombocytopenia that are often missed
at early stages (1). Even following
diagnosis, due to the slow progression of CLL, patients may not
require chemotherapeutic treatment (2,3).
The heterogeneous presentation and clinical course
of CLL makes stratification of patients into appropriate risk and
treatment groups of great importance. CLL has been associated with
chromosomal abnormalities, including trisomy 12q (16%), deletion of
11q (18%) and deletion of 17p [7%, containing tumor protein p53
(TP53)], which is associated with a shorter survival time,
aggressiveness of disease and extensive lymph node involvement
(4,5).
Notably, the most common chromosomal aberration, deletion of 13q
(55%), correlates with good prognosis in 55% of patients with CLL
(4,5).
CLL heterogeneity is partially caused by microRNA (6,7),
epigenetic (8) and microenvironment
alterations (3). At present, the
strongest prognostic indicator in CLL is the mutational status of
the immunoglobulin heavy-chain variable region (IGHV), which
predicts clinical prognosis at diagnosis (9,10).
Mutation of IGHV is correlated with slower disease progression and
an increased survival time, in addition to the less advanced Binet
stage A disease (11). However,
routine determination of this biomarker is not a cost-effective
measure, and thus not widely used. Other markers, including CD38
and ZAP70, have also been investigated as surrogates for IGHV
status (12,13); however, a reliable marker has yet to
be identified. CD38 expression correlates with non-mutated IGHV,
adverse disease course and severity, whereas ZAP70 expression is
associated with mutated IGHV and an improved disease outcome
(12–18). For >40 years, Rai and Binet staging
systems have served as standardized systems to categorize patients,
provide patient care and administer adequate cancer therapy
(19,20). However, in order to continue to
improve patient outcomes, more accurate prognostic markers are
required. Additionally, important factors, such as lactate
dehydrogenase (LDH) and β2 microglobulin (B2M) indicate the
proliferation and activity of the disease (21). Lower B2M serum concentrations have
been identified as beneficial prognostic factors associated with a
higher response rate, longer disease-free time and overall survival
(OS) times (21). However, in
clinical practice, the prognostic and predictive value of B2M and
LDH remains insufficient. Therefore, it is imperative to identify
novel indicators that may aid clinicians in making an informed
decision regarding when to begin treatment.
The ‘sugar code’, or protein glycosylation pattern,
serves a role in intra- and intercellular communication, and
exhibits functions in regulatory processes, including those
dysregulated in cancer and metastasis (22–24).
Galectins are glycan-binding proteins of the lectin family, and are
central effectors of this form of communication, and their role has
previously been implicated in the development of cancer (25). All 15 of the galectins identified in
humans share a highly conserved carbohydrate recognition domain
(CRD) that recognizes and binds β-galactosides (26). This CRD-mediated pattern recognition
mechanism promotes galectin binding to specific cell surface
receptors, activating intracellular signaling pathways involved in
immune response, cell adhesion, communication, proliferation,
differentiation and migration (27,28). The
role of galectins in cancer progression is an area of interest in
current research. Different galectins are associated with tumor
progression and suppressive mechanisms that are specific to the
type of tumor (29). High plasma
levels of galectins −2, −4, −8 and −10 (Gal-2, −4, −8 and −10) have
been observed in patients with various types of malignancy,
including ovarian carcinoma, prostate cancer and metastatic
diseases (30,31). Galectins induce the secretion of
cytokines and chemokines, including granulocyte colony-stimulating
factor, interleukin-6 and chemokine (C-X-C) motif ligand 1, thereby
promoting cancer-endothelial cell adhesion and promoting metastasis
(30,32). Gal-9 also serves a central role in
tumor cell-induced T-cell exhaustion in CLL (32). Taglihoo et al (32) identified that Gal-9 levels were
increased 30-fold in the malignant cells of patients with CLL.
Levels of PL-1, a known effector of apoptosis in T cells, were also
increased. Additionally, extracellular Gal-3 has been demonstrated
to induce T-cell apoptosis in melanoma, and its suppression leads
to enhanced migration and invasion of choloangiocarcinoma cells
(33). Reduced levels of Gal-3
enhance the activation of apoptosis via cell-to-cell contact
(33). Notably, low Gal-3 levels are
associated with a progressive form of cervical neoplasia (34). In addition, intracellular Gal-3 may be
involved in mRNA splicing and correlates with poor prognosis
(35–37). Gal-9 is able to bind Tim-3, preventing
T-cell activation and triggering cell death in Tim-3+ T
cells (38–40). This dampening of the T-cell mediated
immune response is also supported by Gal-1 expression, which
induces anti-inflammatory macrophage activation and expansion of
the regulatory T-cell compartment (26,41,42). Due
to the role of galectins in cancer progression, the aim of the
current study was to evaluate serum levels of galectins as
potential prognostic factors in patients with CLL.
Materials and methods
Patients
This prospective study included 52 patients
(Tables I and II) with CLL admitted to the Department of
Internal Diseases and Oncological Chemotherapy (Mielęcki's
Independent Public Clinical Hospital of the Silesian Medical
University in Katowice) for immunochemotherapy or chemotherapy
between March 2013 and September 2017, and 30 non-CLL patients as
control subjects. Control patients were admitted to the Department
of Endocrinology (Mielęcki's Independent Public Clinical Hospital
of the Silesian Medical University in Katowice) for routine MRI
imaging of adrenal gland incidentaloma. None of the control
patients had a hormonally active tumor or CLL. Patients with CLL
were classified according to the Binet staging system (19,20). The
exclusion criteria were as follows: Other malignancies, acute
inflammatory diseases or a history of renal or liver disease or
heart failure (>New York Heart Association Functional
Classification III/IV). Based on the exclusion criteria, 4 patients
with CLL were disqualified from the study. Therefore, a total of 48
patients, 23 male and 25 female patients, with an average of 64
years of age, with CLL were included. Additionally, all patients in
the control group met the exclusion criteria. Study subjects
underwent assessment of total blood count, serum activity of LDH,
and levels of B2M, creatinine, glucose and immunoglobulins IgG, IgA
and IgM. A bone marrow biopsy was performed in each patient.
Additionally, fluorescent in situ hybridization was
performed to assess chromosomal abnormalities, including del11q,
del17p and del13q, as well as trisomy of chromosome 12. The
decision between initiation of chemotherapy or a ‘watchful waiting’
strategy was made according to the International Workshop on
Chronic Lymphocytic Leukemia (IWCLL) guidelines (43–45).
Patients with CLL who required systemic treatment received
chemotherapy comprising rituximab, fludarabine, cyclophosphamide,
vincristine, prednisone and bendamustine (R-FC, R-COP or R-B
schemes) according to schedules based on the patient's clinical
condition. The present study was performed in adherence with the
Declaration of Helsinki Guidelines and was approved by the
Bioethics Committee of Silesian Medical University in Katowice
(approval no. KNW/0022/KB1/102/13). Upon admission, all patients
provided written informed consent for their participation in this
project.
 | Table I.Clinical and laboratory patient
information in control and patients with chronic lymphocytic
leukaemia. |
Table I.
Clinical and laboratory patient
information in control and patients with chronic lymphocytic
leukaemia.
| Variable | Control | Upper-lower
quartile | CLL patients | Upper-lower
quartile | P-value |
|---|
| Age | 64 | 72–55 | 70.5 | 76–65 | 0.027 |
| Platelet
(109/l) | 238.6 | 270–205 | 160.81 | 203–111 | <0.001 |
| Haemoglobin
(g/l) | 13.93 | 15.3–12.7 | 12.23 | 14.1–10.9 | 0.001 |
| Leukocytes
(109/l) | 7.61 | 8.86–5.87 | 68.59 | 89.62–24.44 | <0.001 |
| BMI | 27.52 | 29.72–25.56 | 27.32 | 29.75–24.35 | 0.430 |
| Comorbidities |
|
|
|
|
|
|
Anaemia | 1 (33.3%) |
| 19 (36.5%) |
| <0.001 |
| Low
platelets | 3 (10.0%) |
| 23 (44.2%) |
| 0.001 |
| DM | 6 (20.0%) |
| 11 (21.2%) |
| 0.569 |
|
CHD | 13 (43.3%) |
| 21 (40.4%) |
| 0.487 |
 | Table II.Clinical patient information in
control and patients with chronic lymphocytic leukaemia. |
Table II.
Clinical patient information in
control and patients with chronic lymphocytic leukaemia.
| CLL staging | Control | CLL patients | LN involvement | Spleen
involvement | LDH (SD) | B2M (SD) |
|---|
| Binet A | n/a | 14 | 10 | 1 | 157.8±23.99 | 3,444±1546 |
| Binet B | n/a | 15 | 14 | 8 | 199.9±51.53 | 4,671±2187 |
| Binet C | n/a | 19 | 20 | 15 | 231.4±99.64a | 5,169±2915 |
| CLL males | 10 | 23 |
|
|
|
|
| CLL females | 20 | 25 |
|
|
|
|
| N | 30 | 48 |
|
|
|
|
Blood samples
Blood (10 ml) was collected from each subject prior
to chemotherapy. Whole blood samples were stored in Vacutainer
tubes containing a coagulation activator, and were left to clot.
Following 20 min of incubation at room temperature, the samples
were centrifuged for 10 min at 1,000 × g at room temperature. Serum
aliquots were frozen and stored at −176°C in liquid nitrogen until
subsequent analysis. In addition, patients with CLL who underwent
treatment were re-evaluated during chemotherapy (3 months) and
following chemotherapy (6 months). Untreated patients with CLL
(with stable disease, SD) were re-evaluated for the second
measurement at 6 months. Control patients were assessed during the
same period.
Fluorescent bead-based Luminex
cytokine assay
Analysis of galectin protein concentrations was
performed in patient sera using multiplex, bead-based assays
customized for the selected galectins (cat. no., LXSAHM; R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. Galectin protein levels were analyzed
prior to chemotherapy, during chemotherapy (3 months) and following
chemotherapy (6 months). All the detection antibodies used produced
a single band in the western blot analysis, with a molecular mass
corresponding to the detected galectin (data not shown). Where
available, except standards (recombinant human galectins), external
galectin standards were used to assess potential cross-reactivity.
No significant cross-reactivity or sensitivity issues were
identified among the assayed galectins (data not shown). According
to the multiple bead-based assay manufacturer's protocol,
biotinylated anti-Gal-9 antibody has <2% cross-reactivity with
rhGalectin-1, rhGalectin-2, rhGalectin-7 and rhGalectin-8. Due to
the different dilutions required for the assay, separate assays
were performed for Gal-1 and Gal-9, and Gal-3 and Gal-3BP. Data
were acquired on a validated and calibrated Bio-Plex 200 system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and analyzed with
Bio-Plex Manager 6.0 software (Bio-Rad Laboratories, Inc.) with a
detection target of 50 beads/region, low RP1 target for CAL2
calibration, and recommended doublet discriminator gates of
8,000-16,500 for Bio-Plex. The median fluorescence intensity was
measured.
Statistical analysis
Statistical analysis was performed using Statistica
(version 10; StatSoft, Inc., Tulsa, OK, USA) and MedCalc v.14.8.1
(MedCalc Software bvba, Ostend, Belgium) software packages.
Continuous variables are expressed as the mean ± standard error of
the mean (normally distributed) or as the median and interquartile
range (non-normally distributed). The type of distribution was
established based on the Shapiro-Wilk test. Qualitative variables
were expressed as crude values and percentages. Intergroup
differences for normally distributed quantitative variables were
assessed using the paired student's t-test or one-way analysis of
variance followed by Dunnett's multiple comparison post hoc test.
The Kruskal-Wallis test was applied for parameters with a skewed
distribution. Temporal variations of different variables were
evaluated using the paired student's t-test for dependent samples
or Wilcoxon matched pairs test. Mortality or disease progression
were defined as dependent variables for the purpose of the
univariate and multivariate Cox proportional hazards model, whereas
independent variables were designated from among baseline and
laboratory parameters, including plasma galectin concentration.
Hazard ratios with 95% confidence intervals were calculated.
Kaplan-Meier estimator curves were established and compared using
the log-rank test in order to compare survival between qualitative
parameters. P<0.05 was considered to indicate a statistically
significant difference. Spearman's Rank-Order correlation was used
to perform correlation analysis between GAL-9 and LDH and GAL-9 and
B2M.
Results
Patient characteristics
Patients were stratified according to Binet staging
(Tables I and II) and subdivided according to disease
progression. Significant differences in age, platelets, hemoglobin
and leukocytes were observed between patients with CLL and the
control group (Tables I and II). No significant increases in
comorbidities were observed in the CLL group when compared with the
control group.
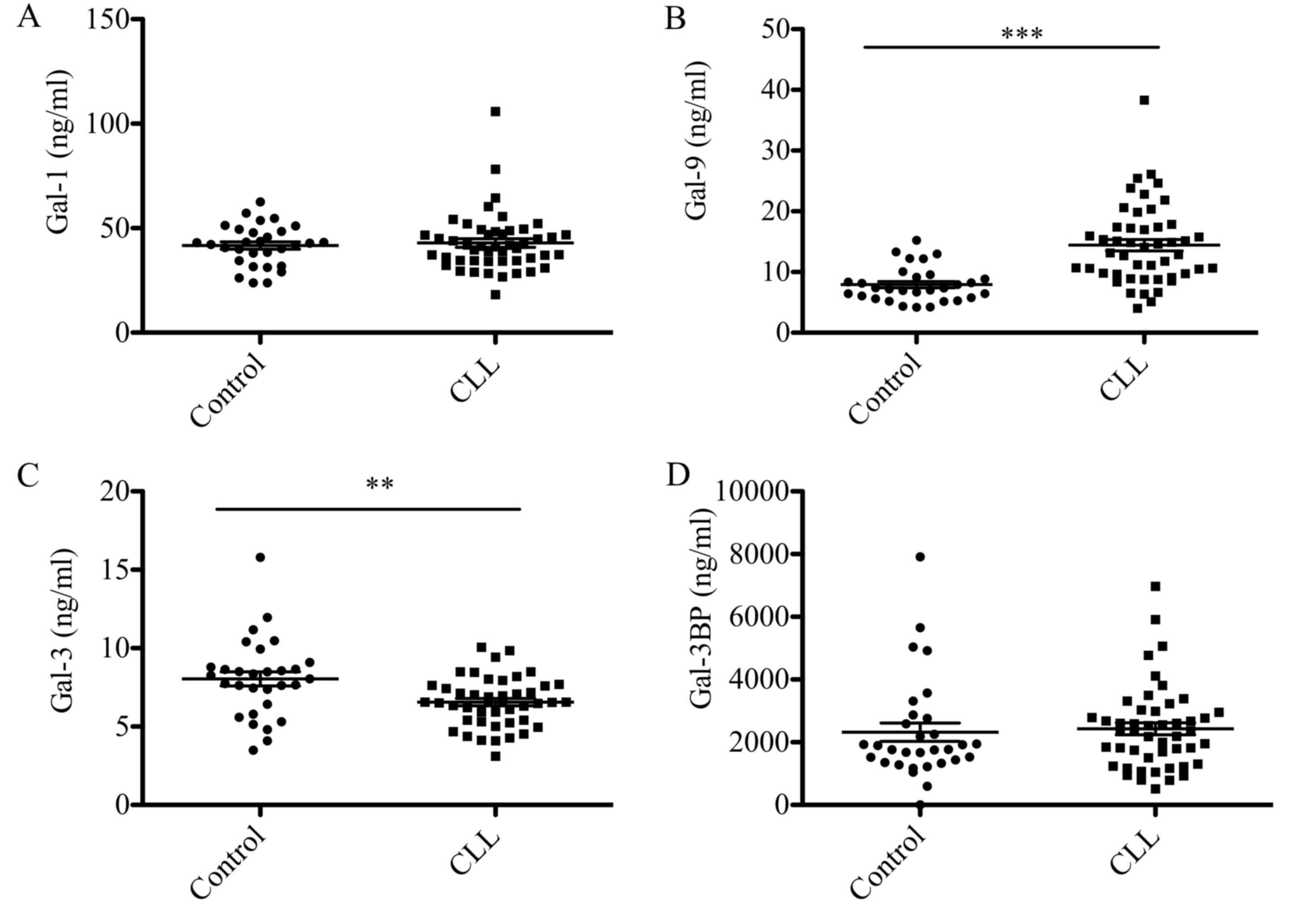
Serum concentrations of Gal-1, −3, −9
and Gal-3BP in patients with CLL
Gal-1, −3, −9 and Gal-3BP levels in the sera from 48
patients with CLL and 30 control subjects are presented in Fig. 1. Gal-9 levels were significantly
elevated in patients with CLL compared with the control group (14
vs. 8 ng/ml; P<0.0001; Fig. 1B),
whereas Gal-3 levels were significantly reduced (7 vs. 8 ng/ml;
P<0.05; Fig. 1C). No statistically
significant differences in Gal-1 and Gal-3BP protein were observed
between patients with CLL and the control group (Fig. 1A and D).
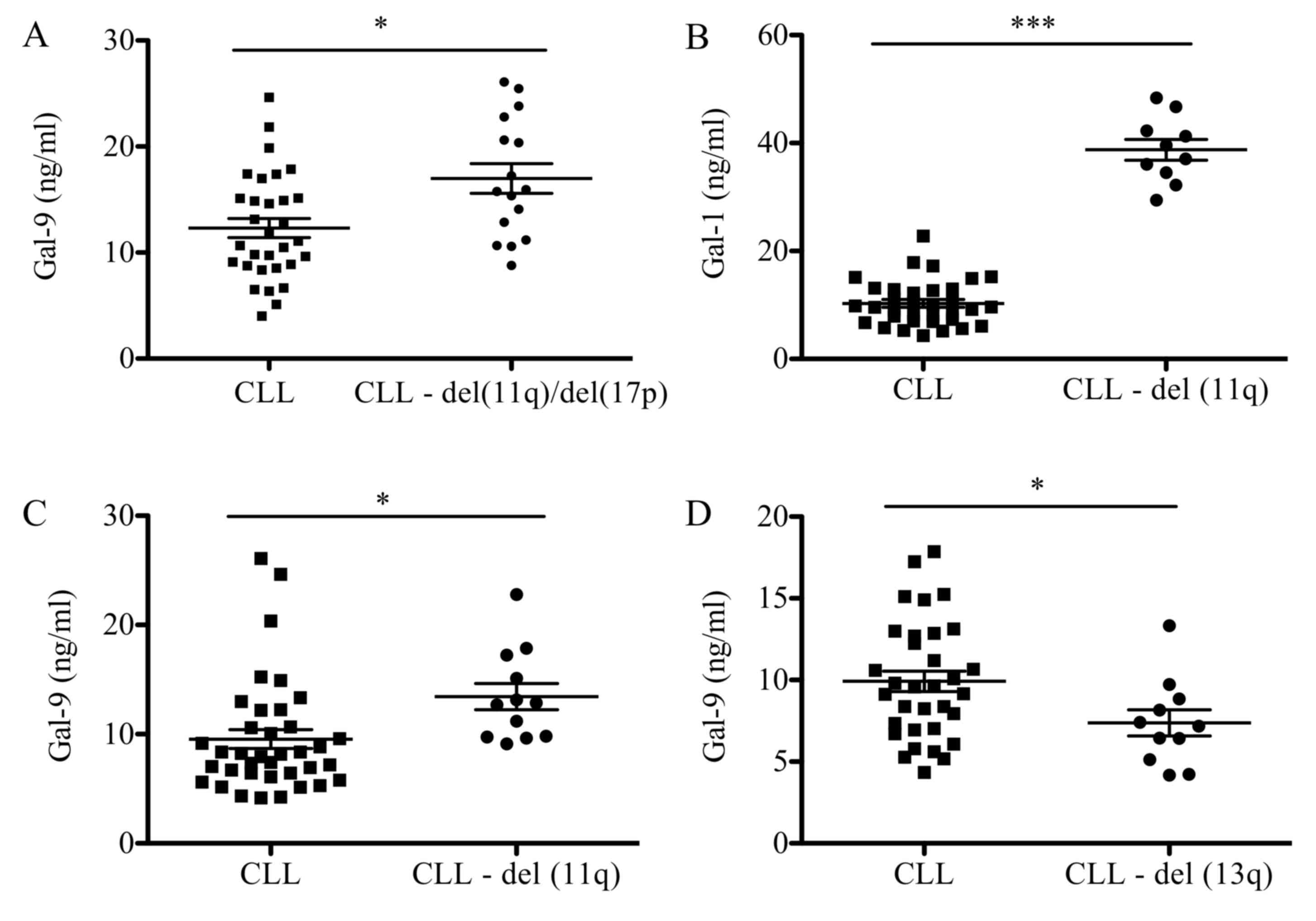
Association between galectin levels
and CLL prognostic factors
Association analysis between negative cytogenetic
factors, including deletions of 11q (del11q), 17p (del17p) and 13q
(del13q), were analyzed (Fig. 2).
Patients with del11q and del17p presented with higher levels of
Gal-9 when compared with patients with CLL and no chromosomal
abnormalities (17 vs. 12 ng/ml; P<0.05; Fig. 2A). When analyzed individually,
patients with del11q exhibited a statistically significant increase
in levels of Gal-1 (39 vs. 10 ng/ml; P<0.001) and Gal-9 (13 vs.
9 ng/ml; P<0.05) compared with patients with CLL and no
chromosomal abnormalities (Fig. 2B and
C). Conversely, patients with deletions in del13q presented a
significant decrease in Gal-9 levels (13 vs. 18 ng/ml; P<0.05)
when compared with the CLL group with no chromosomal abnormalities
(Fig. 2D). No significant differences
were observed in galectin levels when patients presented with
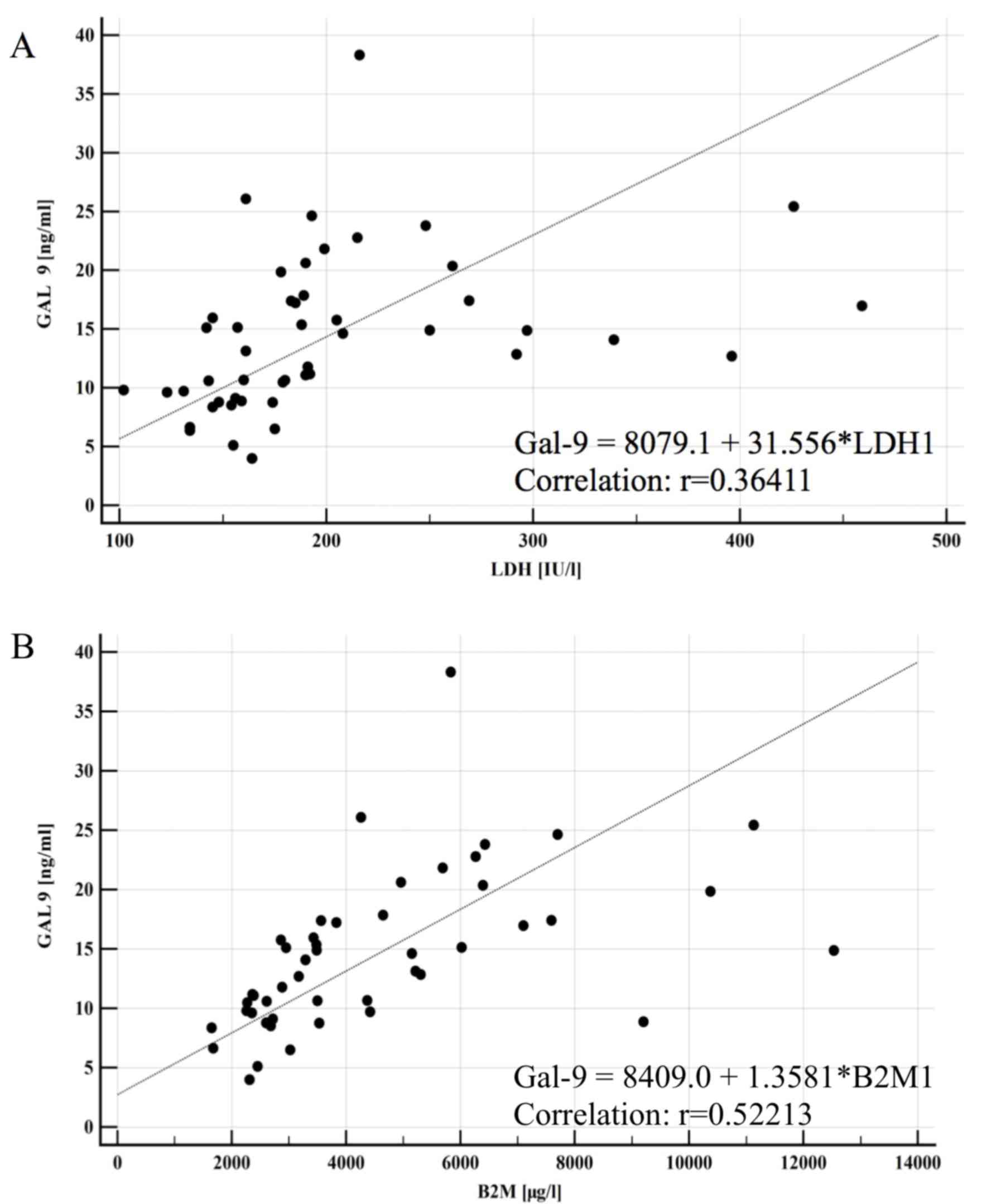
del17p (data not shown). Furthermore, a significant correlation was
observed between Gal-9 serum concentration and CLL prognostic
factors, including LDH activity (P<0.0001) and B2M level
(P<0.0001; Fig. 3).
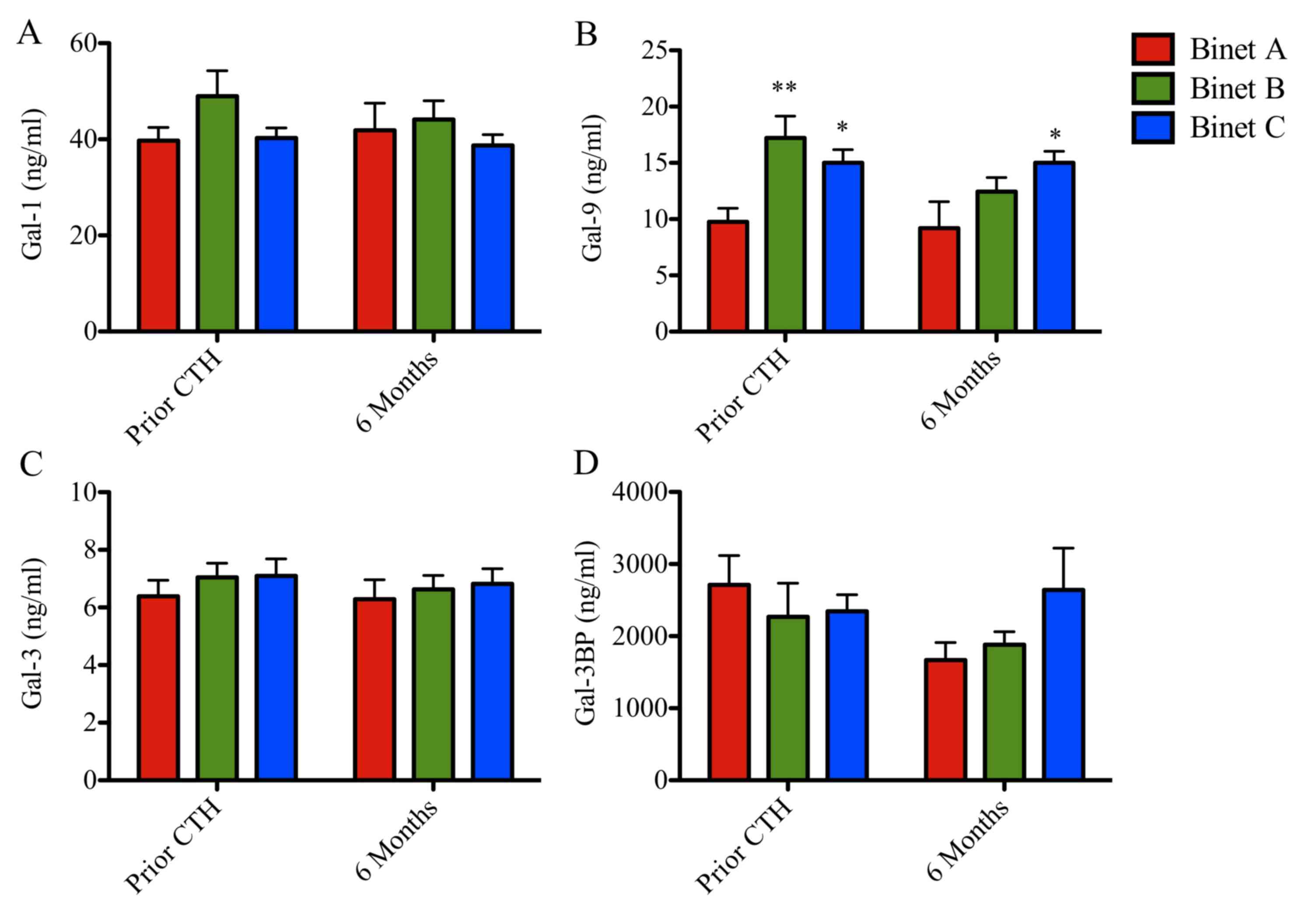
Serum concentration of Gal-9
correlates with clinical stage of CLL
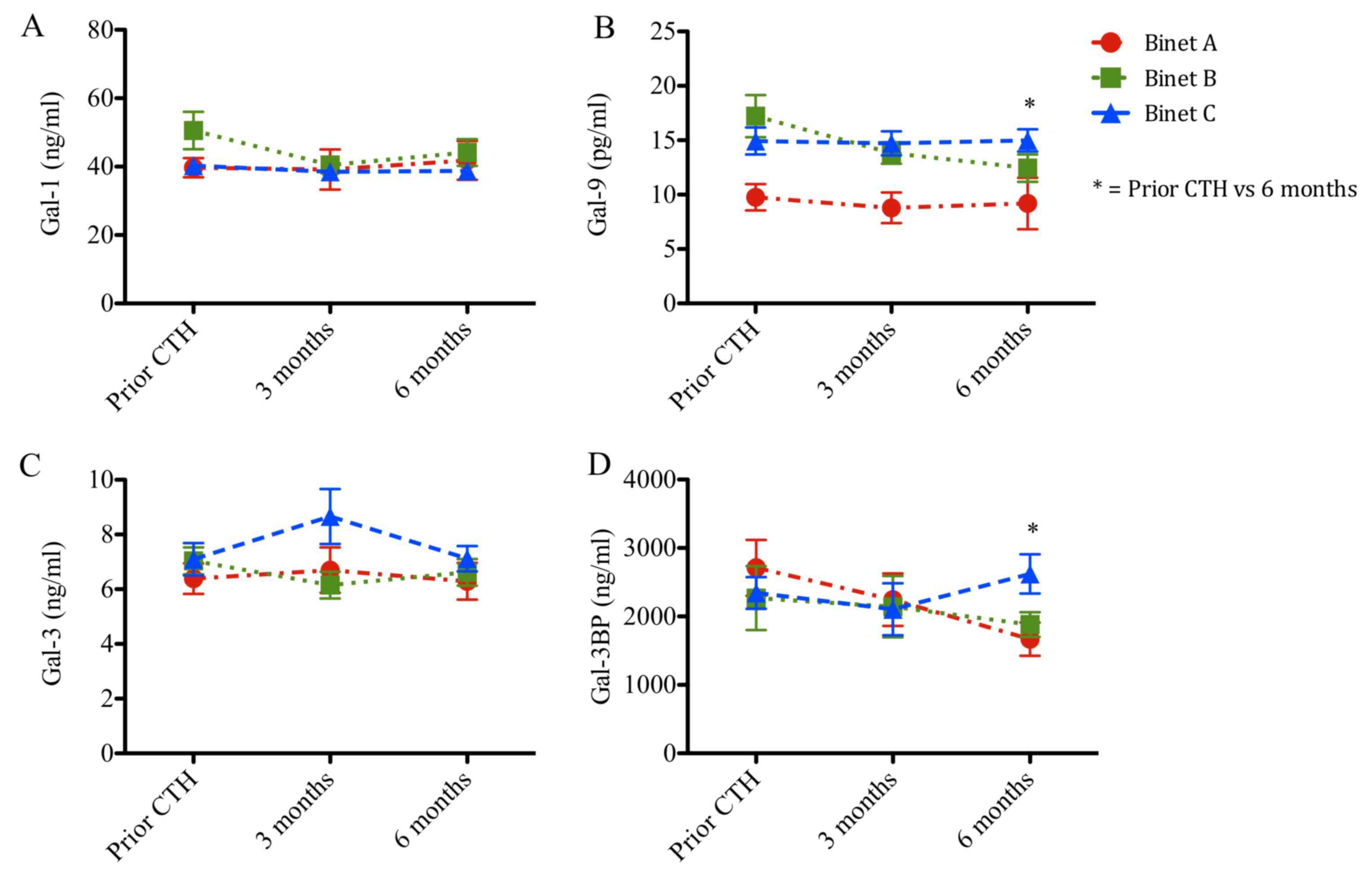
Serum concentrations of Gal-1, −9 and −3 and Gal-3BP
stratified according to patient stage are presented in Figs. 4 and 5
and Tables I and II. Prior to chemotherapy, patients with CLL
at Binet stages B and C exhibited increased levels of Gal-9 (17 and
15 ng/ml; P<0.001 and P<0.05, respectively), when compared
with patients with CLL at Binet stage A (10 ng/ml; Fig. 4). Following chemotherapy, Gal-9 levels
remained elevated in patients with Binet C CLL when compared with
patients with Binet A CLL (Fig. 4B).
Gal-1, Gal-3 and Gal-3BP levels remained unchanged (Fig. 4A, C and D). Patients in Binet A stage
exhibited a significant reduction in Gal-9 protein concentration
compared with patients in Binet C stage at 6 months following
chemotherapy (9 vs. 15 ng/ml; P<0.05; Fig. 4B).
Furthermore, galectin protein levels were analyzed
prior to chemotherapy, during chemotherapy (3 months) and following
chemotherapy (6 months; Fig. 5). The
follow-up Gal-1 and Gal-3 levels remained unchanged (Fig. 5A and C). Notably, patients in Binet B
stage exhibited a progressive reduction in Gal-9 levels during the
follow-up time (17, 14 and 12 ng/ml, respectively; P<0.05;
Fig. 5B). Additionally, Gal-3BP
levels increased in patients in Binet C stage compared with
patients in Binet A stage, following chemotherapy (23 vs. 17 ng/ml;
P<0.05; Fig. 5D).
Galectins as prognostic markers for
response to treatment and survival
Galectin levels in patients with CLL were evaluated
according to the type of response to chemotherapy (Fig. 6). The response to chemotherapy was
evaluated according to IWCLL criteria (43–45).
Patients with SD exhibited a significant reduction in Gal-9
concentration compared with patients who achieved partial response
(PR; 9 vs. 18 ng/ml; P<0.05). No significant associations
between Gal-1 or Gal-3BP (Fig. 6A and
D) and response to treatment were identified. Notably,
untreated patients with SD exhibited lower Gal-9 levels compared
with patients who achieved complete remission (CR) following
chemotherapy, as well as with patients who exhibited disease
progression (PD) and patients who succumbed to the disease during
the treatment (Fig. 6B). Notably,
Gal-3 levels were significantly elevated in patients who succumbed
to the disease compared with patients with CR, PR, SD and PD, with
the PD group exhibiting the lowest Gal-3 concentration (Fig. 6C). The established negative prognostic
factor for CLL, LDH, did not exhibit a statistically significant
difference.
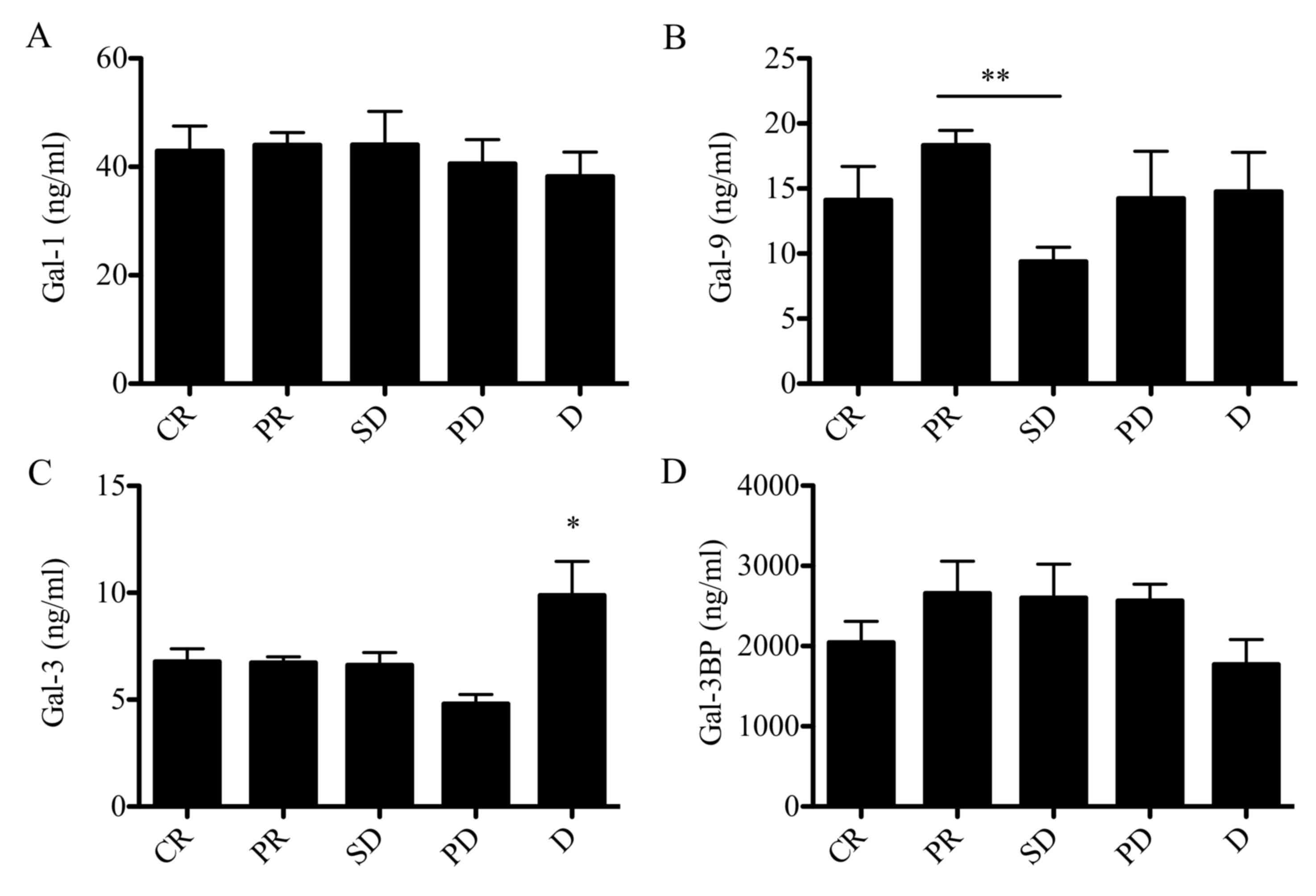 | Figure 6.Galectin protein levels in patients
with CLL stratified according to IWCLL criteria. Protein
concentration of (A) galectin-1, (B) galectin-9, (C) galectin-3 and
(D) galectin 3-binding protein in patients with CLL at CR, PR, SD,
PD or D. Data are presented as the mean ± standard error of the
mean. Data were analyzed using one-way analysis of variance
followed by Dunnett's multiple comparison post-hoc test. *P<0.05
and **P<0.001. CLL, chronic lymphocytic leukemia; CR, complete
remission; PR, partial remission; SD, stable disease; PD,
progressive disease; D, death. |
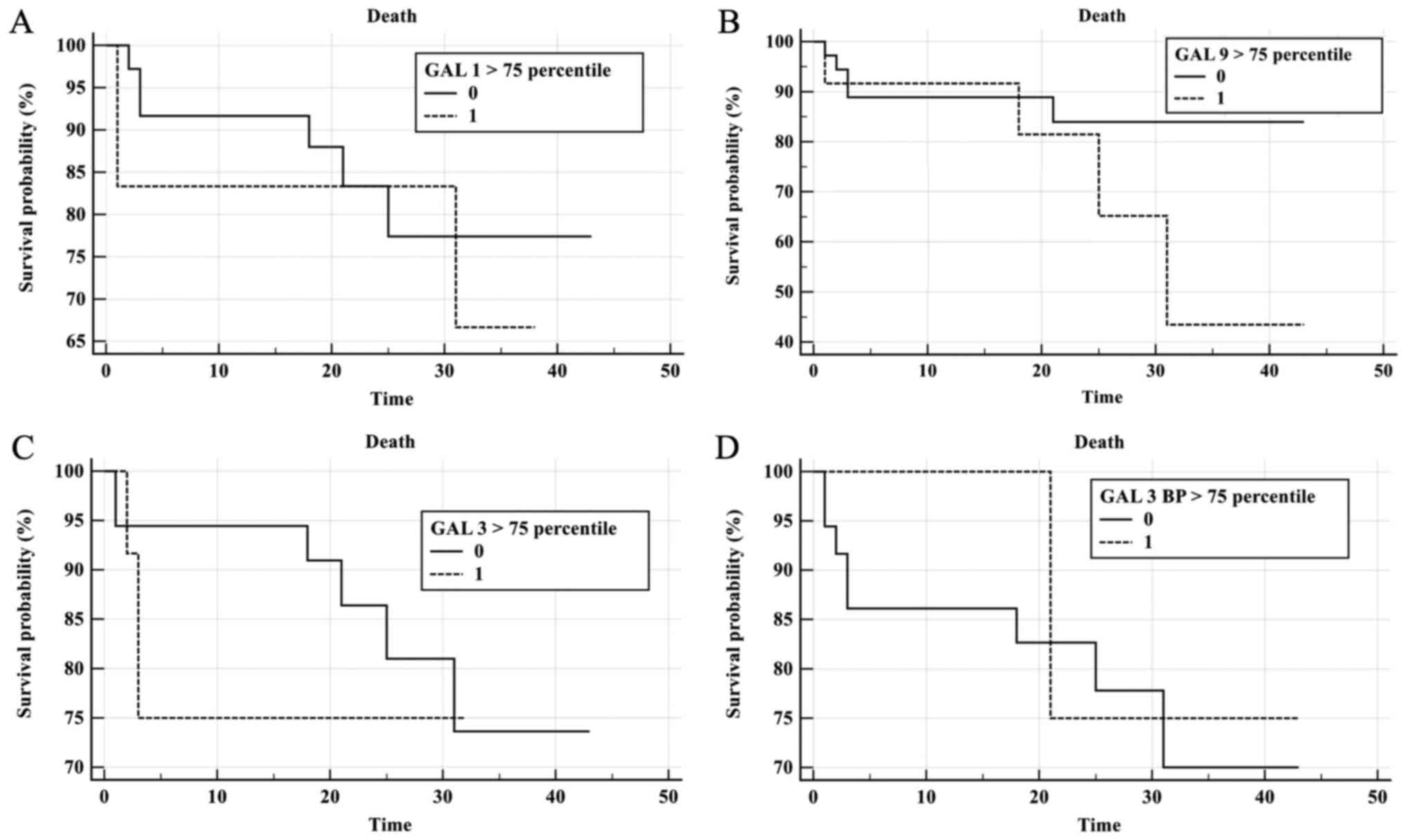
To evaluate the prognostic value of galectin levels
in CLL patient sera, Kaplan-Meier survival curves were plotted for
Gal-1, Gal-3, Gal-9 and Gal-3BP according to galectin concentration
>75th percentile (Fig. 7). During
follow-up, 9 patients succumbed and 10 exhibited PD. The median
time of progression free survival (PFS) for the CLL group was 19.5
months and an elevated risk of mortality was observed when Gal-9
levels were elevated; however, this was not statistically
significant (P=0.19; Fig. 7B). No
significant results were observed for elevated Gal-1, Gal-3 and
Gal-3BP in the prediction of shorter OS or PFS times (Fig. 7A, C and D). COX analysis indicated no
significant results for galectins in the prediction of mortality or
progression. A trend was observed in Gal-3 concentration measured
prior to treatment (P=0.056; Fig.
7C).
Discussion
Galectins are members of the lectin family, which
exhibit a high affinity for β-galactosides. The galectin-glycan
conjugate serves a fundamental role in metastasis, angiogenesis,
tumor immunity, proliferation and apoptosis (25,26).
Unfortunately, the mechanism of action and potential therapeutic
implications of galectins in cancer are not yet fully understood
(29,32,46). In a
study by Asgarian-Omran et al (29), low mRNA expression of the Gal-3 gene
was observed in the leukemic cells of patients with CLL, and no
significant difference was observed in Gal-1 mRNA between CLL
patients and control subjects. In addition, expression of Gal-3
mRNA was lower in patients with progressive disease. In the current
study, a reduction in Gal-3 plasma levels was observed in patients
with CLL compared with controls. In a study by Taghiloo et
al (32), galectin levels were
compared in 25 patients with CLL and 15 healthy subjects, and high
mRNA expression levels of Gal-9 were observed in mononuclear cells
of patients with CLL. In a previous study, plasma levels of Gal-1
were measured in 49 patients with CLL and 40 healthy subjects, the
study revealed an increase in Gal-1 in patients with CLL compared
with healthy subjects (32). In
patients at Binet C stage, plasma Gal-1 levels were identified to
be increased >2-fold when compared with patients at stage Binet
A (517 vs. 208 ng/ml) (46). However,
in the current study, Gal-1 levels were similar between CLL and
controls. There are limitations to the present study, for example,
at the design stage, the association between IGHV, and Gal-1 and
Gal-3 levels were not investigated as according to the literature,
the mutational status of the IGHV has no effect on the expression
of Gal-1 and Gal-3 (29).
Additionally, due to the limited number of patients enrolled in the
present study, statistical analyses to assess the association
between galectin level and mutational status of IGHV were not
performed. The beta value of statistical analysis would not be
strong enough to support a possible negative result indicating that
there is no correlation between galectin levels and mutational
status.
To the best of our knowledge, the current study is
the first to describe that patients with CLL exhibit higher serum
concentrations of Gal-9, which is associated with the stage of
disease. Consistent with these results, Kikushige et al
(47) observed higher concentrations
of Gal-9 in sera of patients and mice with acute myeloid leukemia
(AML) compared with healthy subjects. AML cells secrete Gal-9 via
an autocrine loop, which leads to activation of T-cell
immunoglobulin and mucin domain-containing protein 3 receptor
(Tim-3). Gal-9-dependent T-cell activation promotes leukemic stem
cell self-renewal (47). Furthermore,
Gal-9 and Tim-3 proteins inhibit the activation of natural killer
(NK) cells, blocking the action of NKs on AML cells (48). Subsequently, the Gal-9/Tim-3 signaling
pathway is associated with inhibition of the response from Th1
cells in patients with osteosarcoma (49). The Tim-3 receptor, as well as
receptors including cytotoxic T-lymphocyte antigen-4 (CTLA-4),
programmed cell death-1 and −2 (PD-1 and PD-2) and lymphocyte
activation gene 3 (LAG3), are associated with the transmission of
inhibitory signals to lymphocytes (49). These inhibitory signals impair
anti-tumor immunity (50). At
present, anti-CTLA-4 antibody (ipilimumab in melanoma), anti-PD-1
(nivolumab and pembrolizumab in melanoma and lung cancer) and
anti-programmed death ligand 1 (atezolizumab in lung cancer) have
been used in cancer immunotherapy (51–55).
Restoring the function of T lymphocytes by blocking a signaling
pathway associated with Gal-9/Tim-3 may be a potential therapeutic
option for patients with cancer, including those with CLL.
In the current study, higher levels of Gal-9 in
patients with CLL were correlated with disease progression,
negative prognostic factors and response to treatment. However, no
significant effects of galectins on OS and PFS were observed, this
is potentially due to the small number of patients in the patient
cohort. Furthermore, patients in Binet B stage exhibited higher
concentrations of Gal-9 compared with patients in Binet C stage.
Patients whose disease was advanced (Binet C) exhibited more severe
symptoms compared with those in the intermediate stage of the
disease (Binet B), including lymph node involvement, splenomegaly
and white blood cell count. An altered lymph node microenvironment
retains Gal-9, reducing circulating levels of Gal-9. Furthermore,
galectin production by tumor cells during disease progression may
be negatively regulated by accumulation of Gal-9 in the
extracellular matrix, acting as an autocrine regulatory signal.
Finally, elevated Gal-9 levels may also result from clonal
expansion and dynamic development of CLL. A large body of evidence
suggests that CLL is a clonal disease, consisting of cells of
different phenotypes (56). Patients
with a lower disease stage may develop a clonal expansion of tumor
cells with a different growth rate and needs, compared with clone
cells in patients with more advanced disease. Additionally,
different Gal-9 isoforms are expressed in tumor clonal cells, which
may stimulate the production of other isoforms by such clones
(57).
In summary, the potential prognostic factor of Gal-9
in patients with CLL requires further investigation. In the present
study, a difference in Gal-9 concentration between the study and
control groups was demonstrated, and a correlation was identified
between Gal-9 levels and negative prognostic factors, including
del11q, del17p, LDH, B2M and response to treatment. An association
was observed between Gal-9 levels and progression of CLL or patient
mortality. However, these findings are limited by the number of
patients investigated in the present study. Therefore, further
studies with larger groups of patients should be performed. Future
therapeutic strategies for the treatment of CLL may involve the use
of galectin inhibitors, including competitive carbohydrates, small
non-carbohydrate binding molecules and antibodies.
Acknowledgements
Not applicable.
Funding
The present study was supported by internal grant
from the Medical University of Silesia (grant no.
KNW-1-025/K/7/K).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF, PF and KW made substantial contributions to the
conception and the design of the present study and were involved in
the acquisition analysis and interpretation of data. EC was
involved in analysis and interpretation of data, and drafting the
manuscript. NA, MW, MK and IG performed the statistical analyses
and were involved in data management and critical appraise of the
manuscript. TF, JC and JW contributed to the study design and the
critical revision and final approval of the present manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in adherence with the
Declaration of Helsinki Guidelines and was approved by the
Bioethics Committee of Silesian Medical University in Katowice
(approval no. KNW/0022/KB1/102/13). Upon admission, all patients
provided written informed consent for their participation in this
project.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shanafelt TD, Byrd JC, Call TG, Zent CS
and Kay NE: Narrative review: Initial management of newly
diagnosed, early-stage chronic lymphocytic leukemia. Ann Intern
Med. 145:435–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zenz T, Mertens D, Küppers R, Döhner H and
Stilgenbauer S: From pathogenesis to treatment of chronic
lymphocytic leukaemia. Nat Rev Cancer. 10:37–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S and Kipps T: The pathogenesis of
chronic lymphocytic leukemia. Annu Rev Pathol. 9:103–118. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Döhner H, Stilgenbauer S, Benner A,
Leupolt E, Kröber A, Bullinger L, Döhner K, Bentz M and Lichter P:
Genomic aberrations and survival in chronic lymphocytic leukemia. N
Engl J Med. 343:1910–1916. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kipps TJ, Stevenson FK, Wu CJ, Croce CM,
Packham G, Wierda WG, O'Brien S, Gribben J and Rai K: Chronic
lymphocytic leukaemia. Nat Rev Dis Primers. 3:160962017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balatti V, Pekarky Y and Croce CM: Role of
microRNA in chronic lymphocytic leukemia onset and progression. J
Hematol Oncol. 8:122015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabbri M, Bottoni A, Shimizu M, Spizzo R,
Nicoloso MS, Rossi S, Barbarotto E, Cimmino A, Adair B, Wojcik SE,
et al: Association of a microRNA/TP53 feedback circuitry with
pathogenesis and outcome of B-cell chronic lymphocytic leukemia.
JAMA. 305:59–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martín-Subero JI, López-Otín C and Campo
E: Genetic and epigenetic basis of chronic lymphocytic leukemia.
Curr Opin Hematol. 20:362–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kröber A, Seiler T, Benner A, Bullinger L,
Brückle E, Lichter P, Döhner H and Stilgenbauer S: V(H) mutation
status, CD38 expression level, genomic aberrations, and survival in
chronic lymphocytic leukemia. Blood. 100:1410–1416. 2002.PubMed/NCBI
|
|
10
|
Ghia P, Stamatopoulos K, Belessi C, Moreno
C, Stilgenbauer S, Stevenson F, Davi F and Rosenquist R: European
Research Initiative on CLL: ERIC recommendations on IGHV gene
mutational status analysis in chronic lymphocytic leukemia.
Leukemia. 21:1–3. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amaya-Chanaga CI and Rassenti LZ:
Biomarkers in chronic lymphocytic leukemia: Clinical applications
and prognostic markers. Best Pract Res Clin Haematol. 29:79–89.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Arena G, Musto P, Cascavilla N,
Dell'Olio M, Di Renzo N, Perla G, Savino L and Carotenuto M: CD38
expression correlates with adverse biological features and predicts
poor clinical outcome in B-cell chronic lymphocytic leukemia. Leuk
Lymphoma. 42:109–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayat A, O'Brien D, O'Rourke P, McGuckin
S, Fitzgerald T, Conneally E, Browne PV, McCann SR, Lawler MP and
Vandenberghe E: CD38 expression level and pattern of expression
remains a reliable and robust marker of progressive disease in
chronic lymphocytic leukemia. Leuk Lymphoma. 47:2371–2379. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oscier DG, Rose-Zerilli M, Winkelmann N,
Gonzalez de Castro D, Gomez B, Forster J, Parker H, Parker A,
Gardiner A, Collins A, et al: The clinical significance of NOTCH1
and SF3B1 mutations in the UK LRF CLL4 trial. Blood. 121:468–475.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dürig J, Naschar M, Schmücker U,
Renzing-Köhler K, Hölter T, Hüttmann A and Dührsen U: CD38
expression is an important prognostic marker in chronic lymphocytic
leukaemia. Leukemia. 16:30–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Del Poeta G, Maurillo L, Venditti A,
Buccisano F, Epiceno AM, Capelli G, Tamburini A, Suppo G, Battaglia
A, Del Principe MI, et al: Clinical significance of CD38 expression
in chronic lymphocytic leukemia. Blood. 98:2633–2639. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rassenti LZ, Jain S, Keating ML, Wierda
WG, Grever MR, Byrd JC, Kay NE, Brown JR, Gribben JG, Neuberg DS,
et al: Relative value of ZAP-70, CD38, and immunoglobulin mutation
status in predicting aggressive disease in chronic lymphocytic
leukemia. Blood. 112:1923–1930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghia P, Guida G, Stella S, Gottardi D,
Geuna M, Strola G, Scielzo C and Caligaris-Cappio F: The pattern of
CD38 expression defines a distinct subset of chronic lymphocytic
leukemia (CLL) patients at risk of disease progression. Blood.
101:1262–1269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rai KR, Sawitsky A, Cronkite EP, Chanana
AD, Levy RN and Pasternack BS: Clinical staging of chronic
lymphocytic leukemia. Blood. 46:219–234. 1975.PubMed/NCBI
|
|
20
|
Binet JL, Auquier A, Dighiero G, Chastang
C, Piguet H, Goasguen J, Vaugier G, Potron G, Colona P, Oberling F,
et al: A new prognostic classification of chronic lymphocytic
leukemia derived from a multivariate survival analysis. Cancer.
48:198–206. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wierda WG, O'Brien S, Wang X, Faderl S,
Ferrajoli A, Do KA, Cortes J, Thomas D, Garcia-Manero G, Koller C,
et al: Prognostic nomogram and index for overall survival in
previously untreated patients with chronic lymphocytic leukemia.
Blood. 109:4679–4685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Varki A, Kannagi R and Toole BP:
Glycosylation changes in cancer. In Essentials of Glycobiology.
2nd. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi
CR, Hart GW and Etzler ME: Cold spring harbor laboratory press; NY:
2017
|
|
24
|
Oliveira-Ferrer L, Legler K and
Milde-Langosch K: Role of protein glycosylation in cancer
metastasis. Semin Cancer Biol. 44:141–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ebrahim AH, Alalawi Z, Mirandola L,
Rakhshanda R, Dahlbeck S, Nguyen D, Jenkins M, Grizzi F, Cobos E,
Figueroa JA and Chiriva-Internati M: Galectins in cancer :
Carcinogenesis, diagnosis and therapy. Ann Transl Med.
2:882014.PubMed/NCBI
|
|
26
|
Giordano M, Croci DO and Rabinovich GA:
Galectins in hematological malignancies. Curr Opin Hematol.
20:327–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pena C, Mirandola L, Figueroa JA,
Hosiriluck N, Suvorava N, Trotter K, Reidy A, Rakhshanda R, Payne
D, Jenkins M, et al: Galectins as therapeutic targets for
hematological malignancies: A hopeful sweetness. Ann Transl Med.
2:872014.PubMed/NCBI
|
|
28
|
Cousin JM and Cloninger MJ: The role of
galectin-1 in cancer progression, and synthetic multivalent systems
for the study of Galectin-1. Int J Mol Sci. 17(pii): E15662016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asgarian-Omran H, Forghani P,
Hojjat-Farsangi M, Roohi A, Sharifian RA, Razavi SM, Jeddi-Tehrani
M, Rabbani H and Shokri F: Expression profile of galectin-1 and
galectin-3 molecules in different subtypes of chronic lymphocytic
leukemia. Cancer Invest. 28:717–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen C, Duckworth CA, Zhao Q, Pritchard
DM, Rhodes JM and Yu LG: Increased circulation of galectin-3 in
cancer induces secretion of metastasis-promoting cytokines from
blood vascular endothelium. Clin Cancer Res. 19:1693–1704. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Colomb F, Wang W, Simpson D, Zafar M,
Beynon R, Rhodes JM and Yu LG: Galectin-3 interacts with the
cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion
of metastasis-promoting cytokines from vascular endothelial cells.
J Biol Chem. 292:8381–8389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taghiloo S, Allahmoradi E, Ebadi R,
Tehrani M, Hosseini-Khah Z, Janbabaei G, Shekarriz R and
Asgarian-Omran H: Upregulation of Galectin-9 and PD-L1 immune
checkpoints molecules in patients with chronic lymphocytic
leukemia. Asian Pac J Cancer Prev. 18:2269–2274. 2017.PubMed/NCBI
|
|
33
|
Junking M and Wongkham C: Decreased
expression of galectin-3 is associated with metastatic potential of
liver fluke-associated cholangiocarcinoma. Eur J Cancer.
44:6192008-626. View Article : Google Scholar
|
|
34
|
Lee JW, Song SY, Choi JJ, Choi CH, Kim TJ,
Kim J, Lee JH, Kim BG and Bae DS: Decreased galectin-3 expression
during the progression of cervical neoplasia. J Cancer Res Clin
Oncol. 132:241–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsuda Y, Yamagiwa Y, Fukushima K, Ueno Y
and Shimosegawa T: Expression of galectin-3 involved in prognosis
of patients with hepatocellular carcinoma. Hepatol Res.
38:1098–1111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prieto VG, Mourad-Zeidan AA, Melnikova V,
Johnson MM, Lopez A, Diwan AH, Lazar AJ, Shen SS, Zhang PS, Reed
JA, et al: Galectin-3 expression is associated with tumor
progression and pattern of sun exposure in melanoma. Clin Cancer
Res. 12:6709–6715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakahara S, Oka N and Raz A: On the role
of galectin-3 in cancer apoptosis. Apoptosis. 10:267–275. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koyama S, Akbay E, Li YY, Herter-Sprie GS,
Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina
H, et al: Adaptive resistance to therapeutic PD-1 blockade is
associated with upregulation of alternative immune checkpoints. Nat
Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Q, Munger M, Veenstra RG, Weigel BJ,
Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK
and NBlazar BR: Coexpression of Tim-3 and PD-1 identifies a CD8+
T-cell exhaustion phenotype in mice with disseminated acute
myelogenous leukemia. Blood,. 117:4501–4510. 2011. View Article : Google Scholar
|
|
41
|
Dalotto-Moreno T, Croci D, Cerliani JP,
Martinez-Allo VC, Dergan-Dylon S, Méndez-Huergo SP, Stupirski JC,
Mazal D, Osinaga E, Toscano MA, et al: Targeting galectin-1
overcomes breast cancer-associated immunosuppression and prevents
metastatic disease. Cancer Res. 73:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cedeno-Laurent F, Watanabe R, Teague JE,
Kupper TS, Clark RA and Dimitroff CJ: Galectin-1 inhibits the
viability, proliferation, and Th1 cytokine production of
nonmalignant T cells in patients with leukemic cutaneous T-cell
lymphoma. Blood. 119:3534–3538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hallek M, Cheson BD, Catovsky D,
Caligaris-cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ,
Montserrat E, Rai KR, et al: Guidelines for the diagnosis and
treatment of chronic lymphocytic leukemia: A report from the
international workshop on chronic lymphocytic leukemia updating the
national cancer institute-Working Group 1996 guidelines. Blood.
111:5446–5456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hallek M: Chronic lymphocytic leukemia:
2015 update on diagnosis, risk stratification, and treatment. Am J
Hematol. 90:446–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eichhorst B, Robak T, Montserrat E, Ghia
P, Hillmen P, Hallek M and Buske C: ESMO Guidelines Committee:
Chronic lymphocytic leukaemia: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 26 (Suppl
5):v78–v84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Croci DO, Morande PE, Dergan-Dylon S,
Borge M, Toscano MA, Stupirski JC, Bezares RF, Avalos JS, Narbaitz
M, Gamberale R, et al: Nurse-like cells control the activity of
chronic lymphocytic leukemia B cells via galectin-1. Leukemia.
27:1413–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kikushige Y, Miyamoto T, Yuda J,
Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, Niiro H, Yurino A,
Miyawaki K, Takenaka K, et al: A TIM-3/Gal-9 autocrine stimulatory
loop drives self-renewal of human myeloid leukemia stem cells and
leukemic progression. Cell Stem Cell. 17:341–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gonçalves Silva I, Yasinska IM, Sakhnevych
SS, Fiedler W, Wellbrock J, Bardelli M, Varani L, Hussain R,
Siligardi G, Ceccone G, et al: The Tim-3-galectin-9 secretory
pathway is involved in the immune escape of human acute myeloid
leukemia cells. EBioMedicine. 22:44–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li X, Chen Y, Liu X, Zhang J, He X, Teng G
and Yu D: Tim3/Gal9 interactions between T cells and monocytes
result in an immunosuppressive feedback loop that inhibits Th1
responses in osteosarcoma patients. Int Immunopharmacol.
44:153–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ozkazanc D, Yoyen-Ermis D, Tavukcuoglu E,
Buyukasik Y and Esendagli G: Functional exhaustion of
CD4+ T cells induced by co-stimulatory signals from
myeloid leukaemia cells. Immunology. 149:460–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hurtado AM, Chen-Liang TH, Przychodzen B,
Hamedi C, Muñoz-Ballester J, Dienes B, García-Malo MD, Antón AI, de
Arriba F, Teruel-Montoya R, et al: Prognostic signature and
clonality pattern of recurrently mutated genes in inactive chronic
lymphocytic leukemia. Blood Cancer J. 5:e3422015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sato M, Nishi N, Shoji H, Seki M,
Hashidate T, Hirabayashi J, Kasai Ki K, Hata Y, Suzuki S, Hirashima
M and Nakamura T: Functional analysis of the carbohydrate
recognition domains and a linker peptide of galectin-9 as to
eosinophil chemoattractant activity. Glycobiology. 12:191–197.
2002. View Article : Google Scholar : PubMed/NCBI
|