Introduction
Basic transcription factor 3 (BTF3), the β subunit
of the nascent polypeptide-associated complex (NAC), is a protein
with a relative molecular mass of 27kDa (1,2). BTF3 was
originally identified to be the initiator of transcription by RNA
polymerase from proximal promoter elements (2–4).
Subsequently, it was demonstrated to be involved in cell cycle
regulation and apoptosis (5,6). Several previous studies have suggested
that BTF3 may decrease programmed death (7–9). In
addition, it was also reported to be connected with growth
development and morphogenesis (8).
An increasing volume of research has focused on the
presence of BTF3 in malignant tumors, and BTF3 has been
demonstrated to be overexpressed (9,10). By
contrast, silencing of BTF3 results in decreased expression in the
genes associated with tumor cell viability, including Eph receptors
B2 (11).
In cancer, a significant volume of research has
focused on the carcinomatous effects of BTF3, while there has been
less research on squamous cell carcinoma and cell function
(9,10). In particular, to the best of our
knowledge, there has been no research examining nasopharyngeal
carcinoma (NPC). Preliminary studies regarding biological
treatments targeting epidermal growth factor receptor (EGFR) and
angiogenesis have been performed (12). However, clinical implications of other
novel targeted therapies remain in the initial stages (12). The prognosis for advanced NPC is very
poor (12); however, early-stage NPC
is potentially curable. Therefore, early identification by
screening may lead to an improved patient outcome (13).
In the present study, the expression of BTF3 mRNA
and protein in NPC and normal samples was analyzed. siRNA-BTF3 was
then used to downregulate BTF3 expression in TCA-8113 and 5–8F
cells, and the changes in cell proliferation and colony formation
were measured to investigate the association between BTF3 and
NPC.
Materials and methods
Clinical data
Patients with NPC admitted between September 2009
and January 2013 and treated at Beijing Tongren Hospital (Beijing,
China) were included in the present study. A total of 42 male and 4
female patients with a median age of 62 years (range, 35–72), who
had not received any other treatments prior to surgical resection,
participated in the present study. All samples were confirmed
histologically and all biopsies were obtained with the written
consent of the patients. Immediately following surgical excision, a
tumor sample was obtained from the tumor area, while corresponding
peripheral normal nasopharyngeal tissue was obtained from
associated non-cancerous tissue within 1 cm of the tumor without
affecting the assessment of the tumor margins. The biopsies were
divided into two parts: One snap-frozen in liquid nitrogen
immediately following surgical resection, and stored at −80°C for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and the other fixed in 4% paraformaldehyde solution at
4°C for 12–24 h and then paraffin embedded for histological
analysis. The present study was approved by the Ethics Committee of
Beijing Tongren Hospital Capital Medical University (approval no.
TRECKY2014-027; Beijing, China).
Cell culture
The TCA-8113 and 5–8F cell lines purchased from
Shanghai GeneChem Co., Ltd., (Shanghai, China), were cultured in
complete Dulbecco's modified Eagle's medium (DMEM; Beijing Neuronbc
Laboratories Co., Ltd., Beijing, China), supplemented with 10%
fetal calf serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and incubated at 37°C in a 5% CO2
atmosphere.
Immunohistochemistry
The BTF3 antibody was purchased from Abcam
(ab66940). Tumor tissue and corresponding adjacent normal
nasopharyngeal tissue were preserved by paraffin embedding. A
positive biopsy was used for 3,3′-diaminobenzidine (DAB) staining
of the positive control, and the antibody was replaced with
phosphate-buffered saline for staining of the negative control. The
working concentration of rabbit anti-human BTF3 monoclonal antibody
was 1:100, at 4°C overnight. DAB staining was contrasted with 0.5%
hematoxylin at 37°C for 2 min. Yellow to brownish-yellow granules
in cells were considered to indicate positive cells. Positive cells
were counted in five randomly selected fields of view under a light
high-power microscope (magnification, ×400) and scored according to
the positive expression rate (0 points for <10%; 1 point for
11–20%; 3 points for 21–50%; and 4 points for >50%) and staining
intensity (0 points for no staining; 2 points for weak staining;
and 3 points for strong staining). The sum of the points for
staining intensity and positive expression rate was used as the
expression score. A score ≥3 was considered to indicate a positive
case while a score of 0–2 was classified as a negative case.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNA of clinical specimens was extracted using
the Rneasy® Micro kit (cat no. 74004; Qiagen GmbH,
Hilden, Germany) and DNA was digested from RNA specimens using the
ReverTra Ace qPCR RT Master mix with gDNA Remover (FSQ-301; Toyobo
Life Science, Osaka, Japan). Primer amplification was performed
using a SYBR Premix Ex Taq kit (Toyobo Life Science, QPS-201). The
real-time PCR included 0.5 µl upstream and downstream primers, and
1 µl template with an iCycler real time PCR instrument (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The reaction conditions
included 95°C denaturation for 15 sec, 60°C for 15 sec and 72°C for
45 sec, and were repeated for 40 cycles. The upstream and
downstream primers were: β-actin forward,
5′-GCTGCCCTGAGGCACTCTTC-3′ and reverse, 5′-ATCCTGTCGGCAATGCCAGG-3′;
and BTF3 forward, 5′-GCATCTCTGGCAGCGAACACT-3′ and reverse,
5′-GCAAGTGGTGCTTTTCCATCC-3′.
The RNA of cell specimens with BTF3 expression was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RNA was reverse transcribed using M-MLV-RTase
(Promega Corporation, Madison, WI, USA). The reaction system
included 0.5 µl upstream and downstream primers, and 1 µl template.
Primer amplification was performed using the LightCyclerFastStart
DNA SYBR-Green kit (Takara Bio, Inc., Otsu, Japan; DRR041B) with
the TP800 real time PCR instrument (Takara Bio, Inc.). The RT-PCR
conditions included 95°C denaturation for 15 sec, 95°C for 5 sec
and 60°C for 30 sec, and was repeated for 45 cycles. The upstream
and downstream primers were as follows: GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; and BTF3 forward,
5′-TCCACAGTCTACACAGTCCAG-3′ and reverse,
5′-CCAAGGTCACATAATGCCAGAG-3′.
Following normalization with GAPDH gene, relative
quantification of BTF3 expression was performed by the
2−ΔΔCq method (14). The
relative expression level of BTF3 gene was calculated compared with
the reference gene (GAPDH). The relative expression level of BTF3
gene was equal to 2−ΔCq, where ΔCq=Cq of the target
gene-Cq of the reference gene. The ratio of the target gene
expression level between tumor tissue and control tissue was equal
to 2−ΔΔCq, where ΔΔCq=ΔCq of tumor tissue-ΔCq of control
tissue. A value >1 represents elevated expression, while <1
represents decreased expression.
siRNA viral vector construction
The BTF3 siRNA transcript template (sense,
5′-GCCGAAGAAGCCTGGGAATCA-3′) was used to generate the viral vector,
digested with AgeI/EcoRI enzymes (cat. no.
R3552L/R3101L; New England BioLabs, Inc., Ipswich, MA, USA), and
then ligated with pGCSIL-GFP vector (New England Biolabs).
Following confirmation by agarose gel electrophoresis, the cloned
products were assessed with PCR. The positive clones were
considered to be successful siRNA viral vector constructions. PCR
primer (+), 5′-CCTATTTCCCATGATTCCTTCATA-3′ and primer (−),
5′-GTAATACGGTTATCCACGCG-3′. PCR was performed according to the
manufacturer's protocol using an Applied Biosystems 2720 thermal
cycler real time PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions included 94°C
denaturation for 30 sec, 94°C for 30 sec, 55°C for 30 sec, 72°C for
30 sec and 72°C for 6 min, and repeated for 30 cycles.
Cell interference efficiency
TCA-8113 and 5–8F cells were plated into 6-well cell
culture plate (4×105 cells) with DMEM containing 10%
FBS, with a density of ~30%. An appropriate amount of lentivirus
vector was added to the cells according to the group, the
multiplicity of infection of the two cells were 10 in the TCA-8113
group and 20 in the 5–8F group. The control group comprised normal
cells with viral infected negative control, whereas the
experimental group was normal cells with viral infected RNAi target
point. Total RNA was extracted using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and M-MLV-RTase (Promega
Corporation) was used to treat cells with green fluorescent protein
(GFP) expression. To evaluate the interference effect of the target
point, total cells were analyzed for gene mRNA expression by
RT-qPCR prior to collection at 5 days following treatment. PCR was
performed using the aforementioned primer.
Cell proliferation assay
The MTT (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) assay was used. The purple formazan crystals were
dissolved using DMSO, in order to assess proliferation. Cells were
seeded in 96-well plates at a density of 5×103
cells/well overnight, and then treated with the indicated doses of
fluorouracil (5-FU) or gemcitabine for 48 h. MTT (5 mg/ml in PBS,
pH 7.4) was added to a final concentration of 0.5 mg/ml. The
products were solubilized with acidic isopropanol and the optical
density was measured at 490 nm after 4 h of incubation at 37°C. The
growth curve was constructed and absorbance was corrected with
blank readings. The experiments were performed in triplicate and
repeated three times.
Colony forming assay
A total of 600 cells from the control and
experimental group were plated per well into 12-well plates in
triplicate. Following a 10-day culture, the colonies that had
formed were analyzed under a fluorescence microscope (XDS-100;
magnification, ×200). Images of the whole colonies were captured
with a digital camera, and then the images were used for cell
numbers counting. The data were then statistically analyzed.
Follow-up
Patients were followed up by clinical appointment
and telephone for survival status, disease progression, time of
death (if applicable) and postoperative complications. The study
cut-off was June 2015 and overall survival (OS) was defined as the
time between the first surgeon's appointment and mortality from any
cause. Participants who were alive at the end of the study period
or lost to follow-up were censored.
Statistical analysis
Survival analysis was performed using Kaplan-Meier
analysis and the multivariate Cox proportional hazard model, and
significance was analyzed using the log-rank test. Data were
analyzed using SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA). Measurement data were compared using a paired t-test.
Survival status and multivariate analyses were performed using the
Pearson's χ2 test and Cox regression analysis. The data
were presented as the mean ± standard deviation. The experiments
were performed in triplicate and repeated three times. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression levels of BTF3 are
increased in NPC tissues
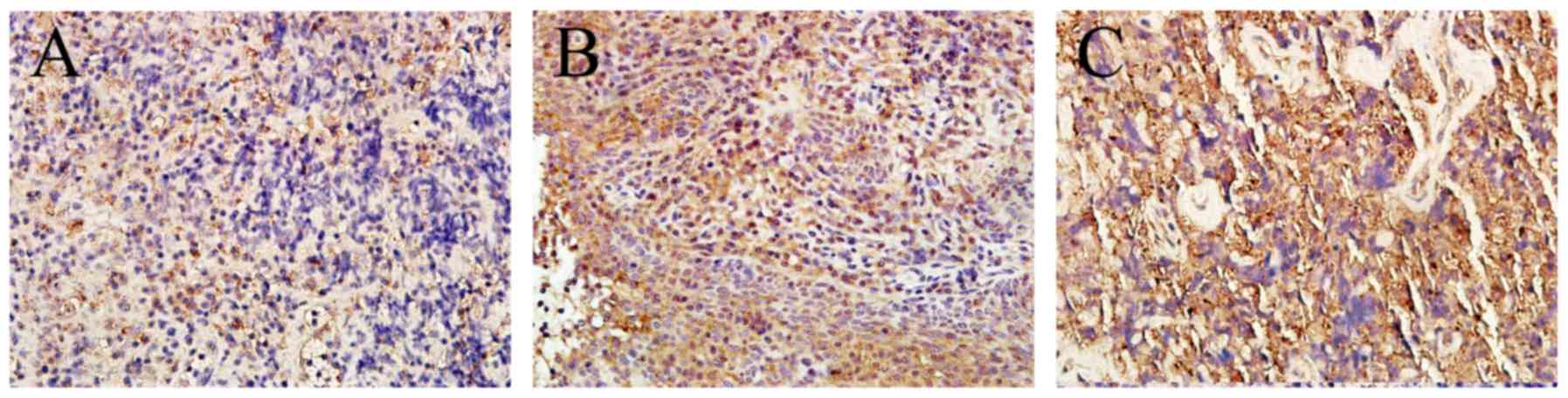
In order to identify BTF3 in nasopharyngeal tissue,
immunohistochemistry was performed. The antibody utilized
recognized the BTF3a and BTF3b isoforms. In normal tissue, weak
BTF3 staining was detected in the cytoplasm of cells and nuclei
were not stained. In tumorous tissue, BTF3 was strongly stained in
the cytoplasm and nuclei (Fig. 1).
The positive staining rates of BTF3 were significantly increased in
cancerous tissues (65%; 30/46), compared with those in adjacent
tissues (24%; 11/46; P<0.01) (Table
I). These results indicated that the expression levels of BTF3
in NPC were increased. With the increase in the degree of tumor
stage, immunohistochemical staining also increased.
 | Table I.The positive staining rates of basic
transcription factor 3 in 46 cancerous tissues and adjacent
tissues. |
Table I.
The positive staining rates of basic
transcription factor 3 in 46 cancerous tissues and adjacent
tissues.
| Factor | Cancerous
tissues | Adjacent
tissues |
|---|
| Positive staining
number | 30 | 11 |
| High mRNA
expression number | 34 | 21 |
| Total number | 46 | 46 |
| Positive staining
rates (%) | 65 | 24 |
| Reverse
transcription-quantitative polymerase chain | 0.727±0.292 | 2.353±0.445 |
RT-qPCR
To analyze the potential variations in mRNA
expression levels of BTF3, RT-qPCR was used in NPC tissues and
adjacent normal tissues. The mean expression value in tumorous
tissues was 2.353±0.445 and the value in adjacent normal tissues
was 0.727±0.292, which suggested significant differences in gene
expression levels between the cancerous and adjacent tissues
(P<0.01) (Table I). In addition,
these results indicated that the expression of BTF3 in tumorous
tissue was significantly increased (P<0.05).
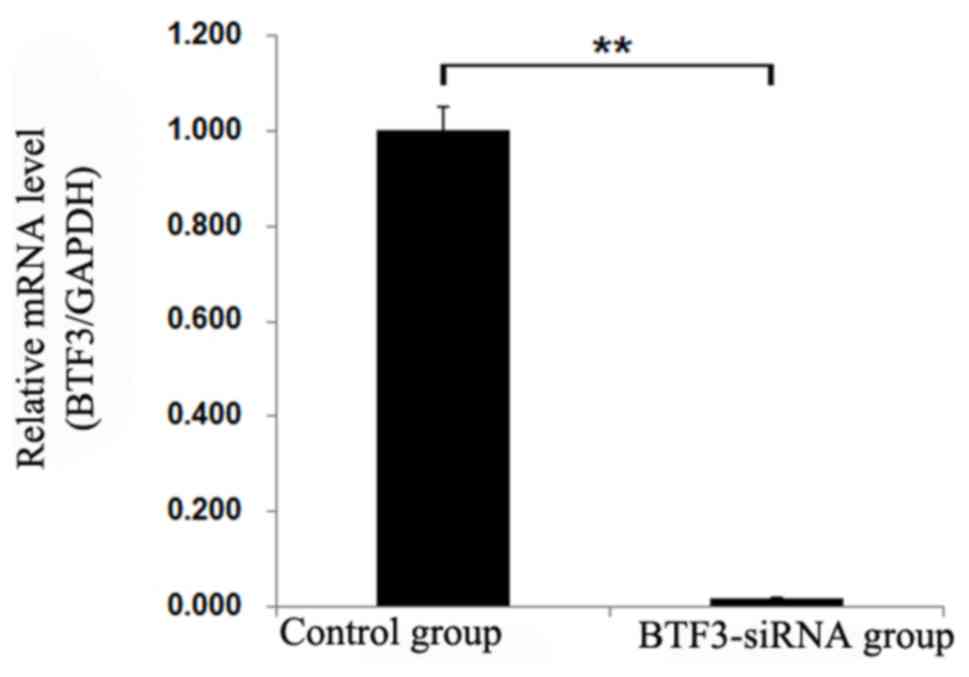
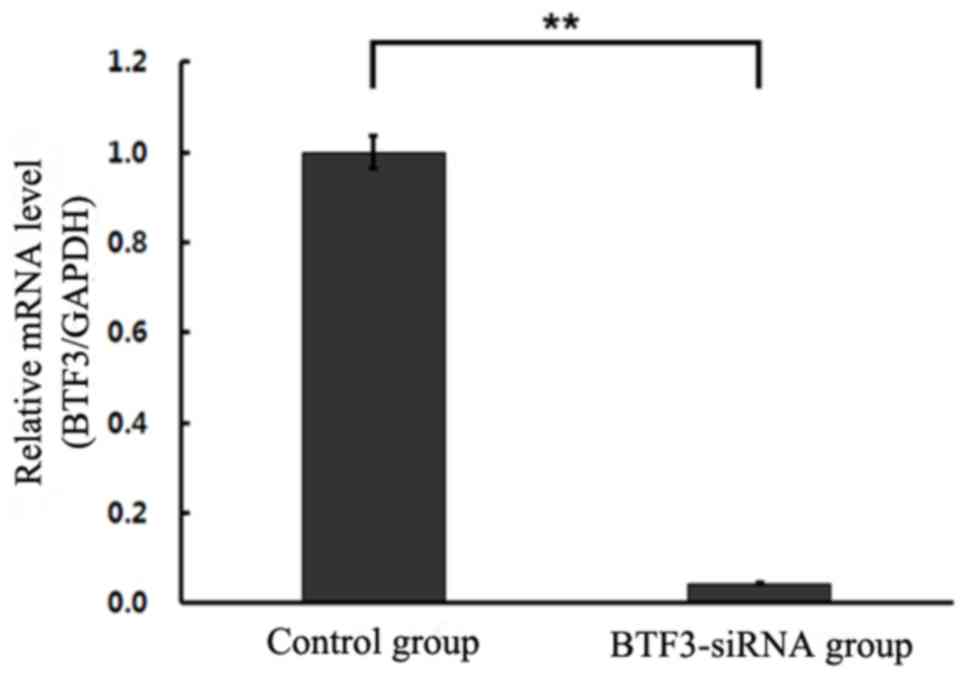
Cell interference
RT-qPCR was performed to detect potential changes in
mRNA levels of BTF3 expression in experimental and control groups.
The results illustrated significant differences in gene expression
levels (P<0.01), demonstrating that siRNA-BTF3 successfully
transfected into TCA-8113 and 5–8F cell lines led to decreased mRNA
expression (Figs. 2 and 3).
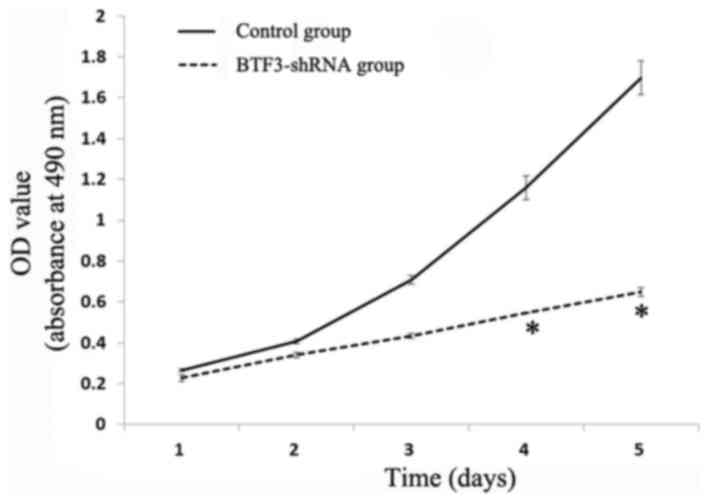
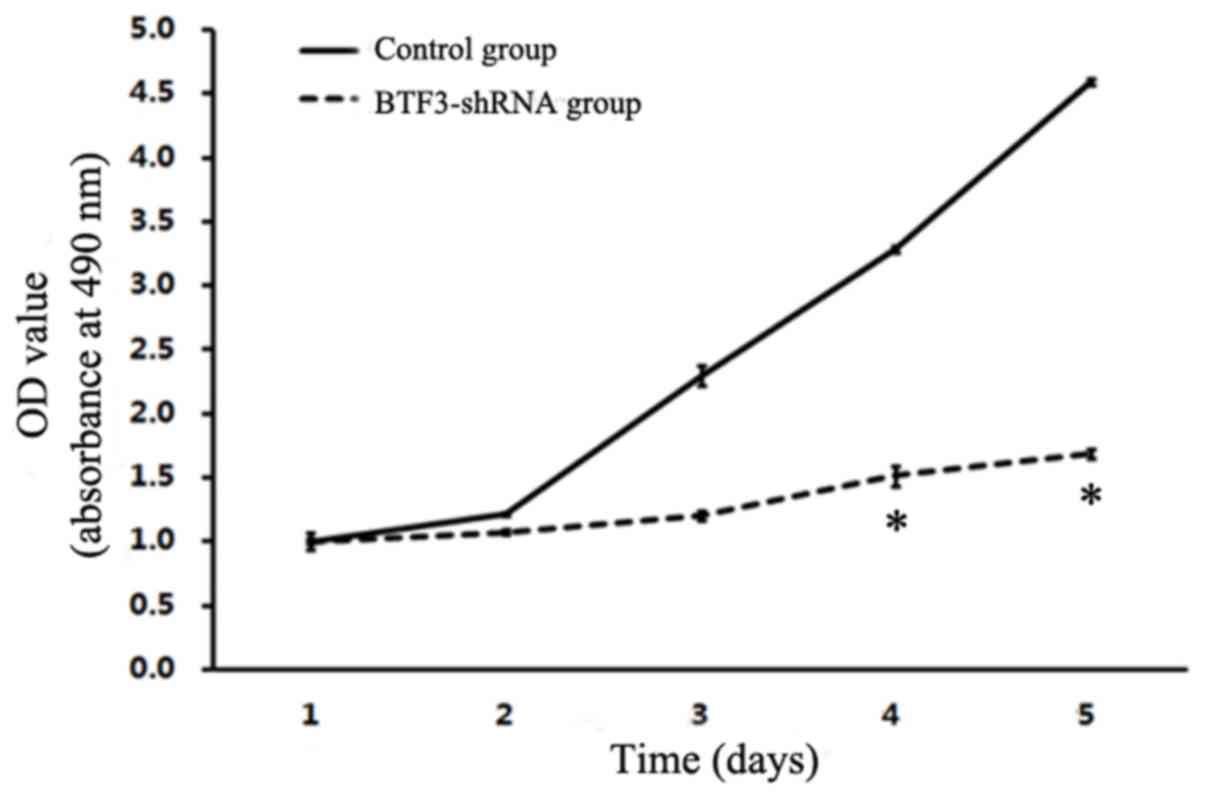
Cell proliferation assay
Results are presented as growth curves from day 1 to
day 5, and suggest that MTT value ratios (multiplication rate) were
significantly reduced in 5–8F and TCA-8113 experimental groups
(Figs. 4 and 5). The results indicated that there was a
significant inhibition of 5–8F and TCA-8113 proliferation following
siRNA lentiviral transfection (P<0.05).
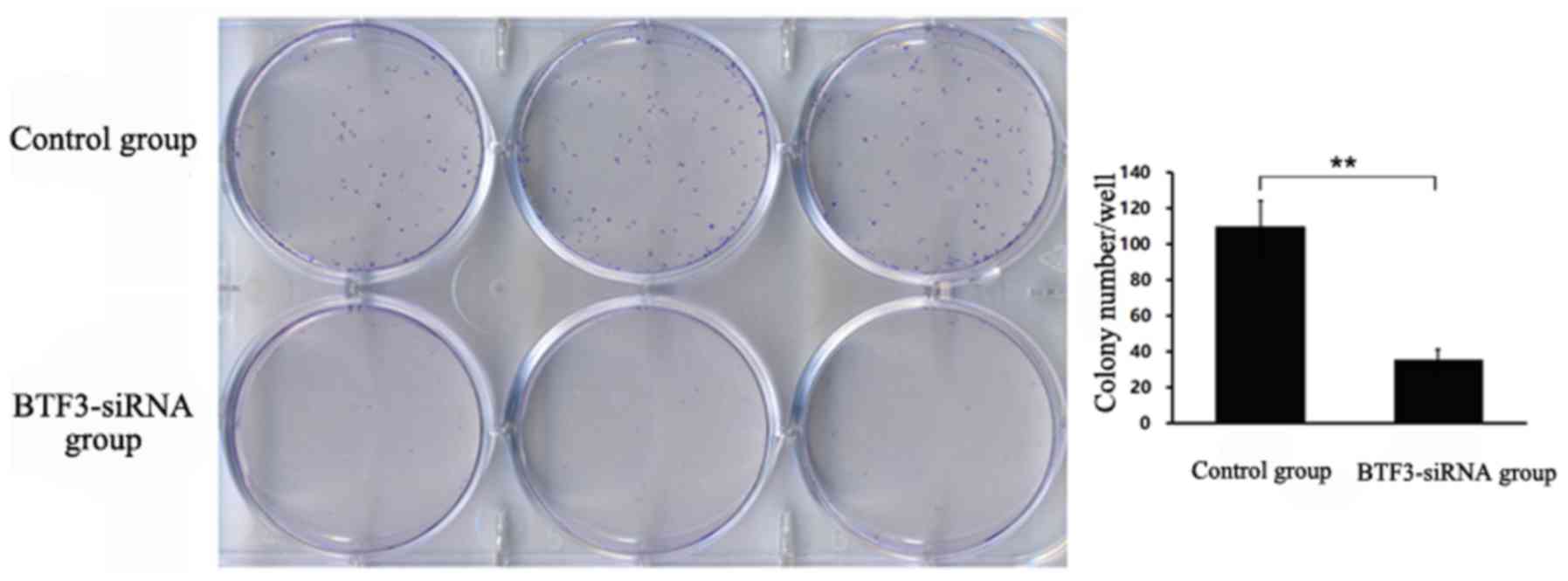
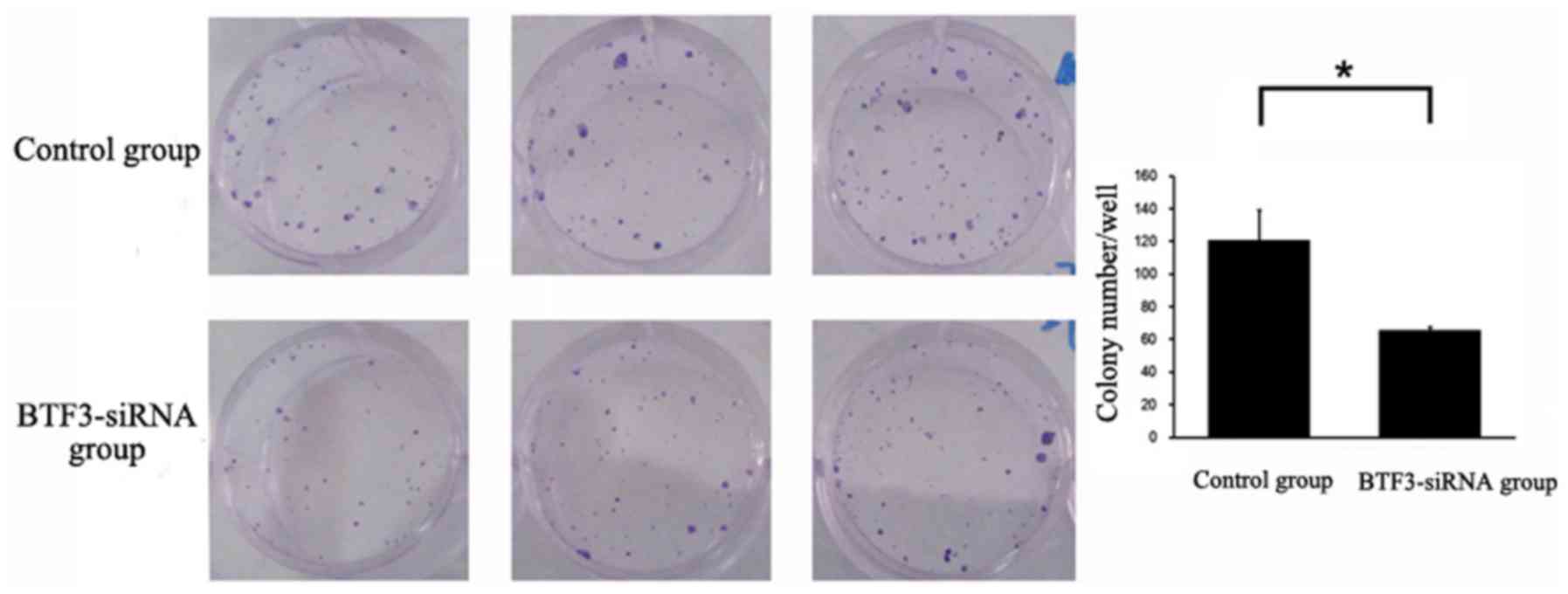
Cell colony formation assay
In 5–8F cells, a mean of 36 colonies was detected in
the siRNA BTF3 group, whereas a significantly higher mean of 110
colonies was detected in the control group (P<0.01; Fig. 6). Similarly, in TCA-8113 cells, a mean
of 65 colonies was detected in the siRNA BTF3 group, whereas a
significantly higher mean of 121 colonies was detected in the
control group (P<0.05; Fig. 7).
Compared with the control group, the examined cell lines exhibited
a significantly lower colony formation ability following
BTF3-silencing.
Follow-up
In the 46 cases of nasopharyngeal tissue, the BTF3
gene was associated with tumor stage, lymph node metastasis and
distant metastasis (Table II). The
patients with later tumor stage, lymph node metastasis or distant
metastasis exhibited a stronger BTF3 expression compared with those
with early tumor stage, negative lymph node metastasis or distant
metastasis (P<0.05).
 | Table II.Association between protein and mRNA
expression of BTF3 and clinicopathological characteristics in 46
nasopharyngeal carcinoma cases. |
Table II.
Association between protein and mRNA
expression of BTF3 and clinicopathological characteristics in 46
nasopharyngeal carcinoma cases.
|
|
| BTF3 |
|---|
|
|
|
|
|---|
| Factor | Cases | Protein (+) No,
% | χ2 test
P-value | High mRNA No,
% | χ2 test
P-value |
|---|
| Age, years |
|
|
|
| 0.75 |
|
≥60 | 20 | 12 | 0.18 | 15 |
|
|
<60 | 26 | 18 |
| 19 |
|
| Sex |
|
|
|
| 0.24 |
|
Male | 41 | 27 | 0.38 | 30 |
|
|
Female | 5 | 3 |
| 4 |
|
| Tumor
stagea |
|
|
|
| <0.01 |
|
I–II | 14 | 5 | 0.01 | 6 |
|
|
III–IV | 32 | 25 |
| 28 |
|
| LNM |
|
|
|
| 0.01 |
|
None | 18 | 10 | 0.03 | 9 |
|
|
Yes | 28 | 20 |
| 25 |
|
| Distant
metastasis |
|
|
|
| 0.01 |
|
None | 37 | 23 | 0.02 | 26 |
|
|
Yes | 9 | 7 |
| 8 |
|
| Smoking |
|
|
|
| 0.20 |
|
Frequently | 30 | 19 | 0.37 | 23 |
|
|
None | 16 | 11 |
| 11 |
|
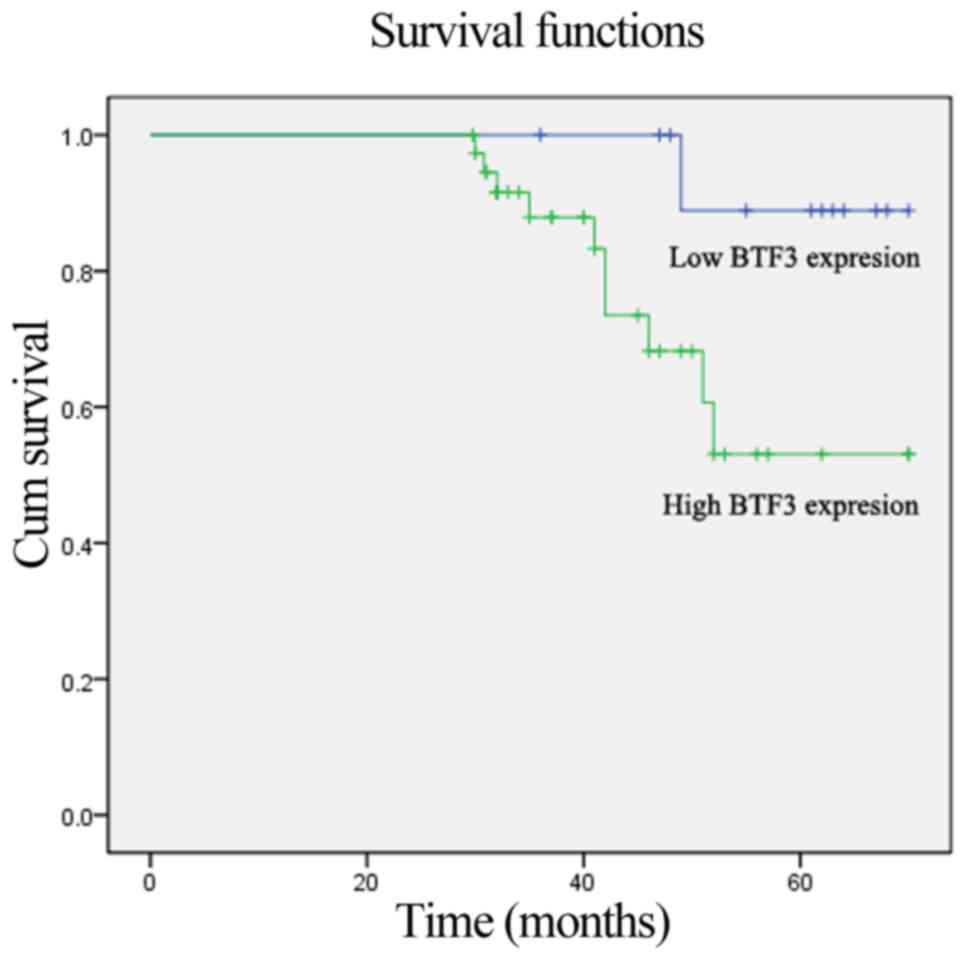
Survival analysis for BTF3
expression
The Kaplan-Meier analysis of survival with respect
to mortality from all causes is illustrated in Fig. 8. A total of 10 mortalities occurred
from any cause. Compared with patients with low expression of the
BTF3 gene, the patients with high expression had a significantly
increased risk of mortality (Log-rank, 7.55; P=0.01). Next,
survival status was determined using the multivariate Cox
proportional hazard model. Metastasis was a significant risk factor
in patients with NPC (HR, 0.09; 95% CI, 0.16–0.47; P=0.01).
Furthermore, the positivity for BTF3 (HR, 47.48; 95% CI,
1.35–1809.34; P=0.03) was a significant risk factor for the shorter
OS periods observed in patients with NPC (Table III).
 | Table III.Survival status and multivariate
analyses in 46 nasopharyngeal carcinoma tissues. |
Table III.
Survival status and multivariate
analyses in 46 nasopharyngeal carcinoma tissues.
|
|
| Survival
status | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | Total cases | Alive | Dead | χ2 test
P-value | P-value | Hazard risk (95%
CI) |
|---|
| BTF3 |
|
|
|
|
| 47.48
(1.35–1809.34) |
| Low
expression | 16 | 14 | 1 | <0.01 | 0.03 |
|
| High
expression | 30 | 21 | 9 |
|
|
|
| Tumor
stagea |
|
|
|
|
| 1.98
(0.21–18.46) |
|
I–II | 14 | 12 | 2 | 0.05 | 0.55 |
|
|
III–IV | 32 | 24 | 8 |
|
|
|
| LNM |
|
|
|
|
| 4.95
(0.67–36.63) |
|
None | 18 | 15 | 2 | <0.01 | 0.12 |
|
|
Yes | 28 | 21 | 8 |
|
|
|
| Distant
metastasis |
|
|
|
|
| 0.09
(0.16–0.47) |
|
None | 37 | 33 | 4 | <0.01 | 0.01 |
|
|
Yes | 9 | 3 | 6 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
≥60 | 20 | 15 | 5 | 0.31 |
|
|
|
<60 | 26 | 21 | 5 |
|
|
|
| Sex |
|
|
|
|
|
|
|
Male | 41 | 33 | 9 | 0.73 |
|
|
|
Female | 5 | 3 | 1 |
|
|
|
| Smoking |
|
|
|
|
|
|
|
Frequently | 30 | 25 | 6 | 0.40 |
|
|
|
None | 16 | 11 | 4 |
|
|
|
Discussion
Nasopharyngeal carcinoma (NPC) is highly prevalent
in Southeast Asia, Southern China, Hong Kong and Taiwan (15). In 2010, data demonstrated that the
incidence rate of NPC was 19.5/100,000, while mortality rates was
7.7/100,000 in Southern China (16).
The biological behavior and prognosis may be significantly
different in patients with NPC with the same stage, histological
type or differentiation grade (17).
Recently, BTF3 has been revealed to be overexpressed in numerous
types of malignant tumor, and has a great influence on gene
expression, which is associated with tumor formation and
development (9,10).
BTF3 was initially purified from HeLa whole cell
extracts (3,4), and is present in two isoforms, BTF3α and
BTF3β, which are spliced by the same mRNA (18–20). BTF3α
has all the characteristics of purified BTF3, whereas BTF3β is
transcriptionally inactive and lacks the first 44 amino acids of
the BTF3α N-terminus, despite its ability to bind RNA pol II
(1,5,21,22). At an early stage of transcription
initiation with RNA polymerase II, transcription factor class II D
(TFIID) is required to be stably bound to the proximal promoter
region, such as the TATA box (2). The
BTF3 protein, an additional TF II-related protein, is not directly
associated with the proximal promoter, but forms a stable complex
with RNA polymerase II and is part of the gene transcription
initiation complex (23–26).
In addition, BTF3 was demonstrated to be involved in
cell cycle regulation and apoptosis (27–29). Using
a subclone of the human Burkitt lymphoma BL60 cell line, Brockstedt
et al (5) initially discovered
that the BTF3 gene was associated with anti-IgM antibody-mediated
apoptosis. Downregulation of BTF3 is involved in the inhibition of
transcription and protein synthesis in the apoptotic K562 cells
induced by harringtonine (7). Later,
BTF3 was identified as ICD-1 (Inhibitor of Cell Death-1) in
Caenorhabditis elegans, a previously uncharacterized
suppressor of apoptosis (6). Bloss
et al (6) reported that that
loss of ICD-1 leads to inappropriate apoptosis in developing and
differentiated cells in various tissues, while overexpression of
ICD-1 with CED-4 (caspase-4) participation inhibits the apoptosis
of cells that are normally programmed to die (6).
In addition, BTF3 is important in growth development
and morphogenesis (30). Deng and
Behringer (8) transmitted a BTF3
mutation through the germline of chimeric mice, and identified that
mice homozygous for the mutant allele died soon after implantation
(8).
In the present study, the BTF3 gene was demonstrated
to be overexpressed in NPC tissues compared with adjacent normal
tissues, which is consistent with results in glioblastoma multiform
(31–33), hepatic and gastric (34–36),
pancreatic (9) and prostatic cancer
(10). In addition,
immunohistochemical staining is also increased with an increasing
degree of tumor differentiation, which is consistent with
pancreatic (36), prostatic (10) and colorectal cancer (27–29). The
expression of BTF3 is much higher in advanced tumor stages and
lymph node metastasis. In addition, at follow-up, BTF3 was
identified to have a significant association with tumor stage,
lymph node metastasis and distant metastasis. Furthermore, the data
demonstrated that patients with high BTF3 expression had a
significantly lower survival rate than those with low levels of
BTF3. This may be due to the BTF3 gene participating in cell
apoptosis, decreasing programmed cell death and promoting the
growth of tumor cells (6,30).
In the present study, siRNA-BTF3 was used to
downregulate BTF3 expression in 5–8F and TCA-8113 cells.
BTF3-silencing was demonstrated to decrease cell proliferation,
particularly in TCA-8113 cells. Furthermore, compared with the
control group, following BTF3-silencing, cells exhibited a
significantly lower colony formation ability. This may be
associated with cell cycle arrest following BTF3-silencing.
BTF3-silencing may induce G1 to G2/M or S phase failure, which
leads to the inhibition of cell cycle completion and postponement
of cell multiplication. By contrast, the BTF3 gene participates in
the process of apoptosis (9),
including anti-IgM antibody-mediated apoptosis (30) or apoptosis with caspase-4 (CED-4)
participation (6). In addition, BTF3
may inhibit the apoptosis path by affecting several tumor
correlation factors.
A number of limitations are indicated in the present
study. The possibility of selection biases exists, which may have
affected either the patients referred to the institution or the
patients recruited in the present study. In addition, the
performance of apoptosis experiments and cell cycle experiments are
required in future examination of BTF3 gene function.
In summary, the results of the present study
demonstrated that BTF3 mRNA and protein were overexpressed in
patients with NPC, which was associated with tumor stage, lymph
node metastasis and distant metastasis. In addition, patients with
a high expression of BTF3 had a significantly increased risk of
overall death, which indicated that the BTF3 gene was associated
with the progression and prognosis of NPC. Furthermore, silencing
of BTF3 may inhibit tumor growth and cloning ability, due of its
association with cell proliferation and the process of apoptosis.
Therefore, the results of the present study indicated that BTF3 may
contribute toward the development, progression and prognosis of
NPC, and may provide evidence at the molecular level for the
targeted therapy of NPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing
Health System High-level Health Technology Talents Training Project
Funding (grant no. 2015-3-022); the National Natural Science
Foundation of China, (grant no. 81670946); the Beijing Municipal
Administration of Hospitals Clinical Medicine Development of
Special Funding Support (grant no. XMLX201507); Capital's Funds for
Health Improvement and Research (grant no. CFH2018-1-2052) and the
Beijing Municipal Administration of Hospitals Incubating Program
(grant no. PX2017032).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC performed the histological examination and was a
major contributor in writing the manuscript. QZ made contributions
on conception and design and analyzed the patient data. ZL
interpreted the patient data and cell experiment data. YZ and ZH
made substantial contributions to conception and interpreted the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Tongren Hospital Capital Medical University
(approval no. TRECKY2014-027; Beijing China) and informed consent
in written form was obtained from all participants.
Patient consent for publication
The patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanno M, Chalut C and Egly JM: Genomc
structure of the putative BTF3 transcription factor. Gene.
117:219–228. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng XM, Black D, Chambon P and Egly JM:
Sequencing and expression of complementary DNA for the general
transcription factor BTF3. Nature. 344:556–559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cavallini B, Huet J, Plassat JL, Sentenac
A, Egly JM and Chambon P: A yeast activity can substitute for the
HeLa cell TATA box factor. Nature. 334:77–80. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng XM, Moncollin V, Egly JM and Chambon
P: A general transcription factor forms a stable complex with RNA
polymerase B (II). Cell. 50:361–368. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brockstedt E, Otto A, Rickers A, Bommert K
and Wittmann-Liebold B: Preparative high-resolution two-dimensional
electrophoresis enables the identification of RNA polymerase B
transcription factor 3 as an apoptosis-associated protein in the
human BL60-2 Burkitt lymphoma cell line. J Protein Chem.
18:225–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bloss TA, Witze ES and Rothman JH:
Suppression of CED-3-independent apoptosis by mitochondrial betaNAC
in Caenorhabditis elegans. Nature. 424:1066–1071. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li R, Liu XL, Du QF, Zhang S, Luo RC and
Zhou SY: Proteome analysis of apoptotic K562 cells induced by
harringtonine. Zhonghua Xue Ye Xue Za Zhi. 25:323–327. 2004.(In
Chinese). PubMed/NCBI
|
|
8
|
Deng JM and Behringer RR: An insertional
mutation in the BTF3 transcription factor gene leads to an early
postimplantation lethality in mice. Transgenic Res. 4:264–269.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kusumawidjaja G, Kayed H, Giese N, Bauer
A, Erkan M, Giese T, Hoheise JD, Friess H and Kleeff J: Basic
transcription factor 3 (BTF3) regulates transcription of
tumor-associated genes in pancreatic cancer cells. Cancer Biol
Ther. 6:367–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Symes AJ, Eilertsen M, Millar M, Nariculam
J, Freeman A, Notara M, Feneley MR, Patel HR, Masters JR and Ahmed
A: Quantitative analysis of BTF3, HINT1, NDRG1 and ODC1 protein
over-expression in human prostate cancer tissue. PLoS One.
8:e842952013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010.Surawska H, Ma PC and Salgia R: The role of
ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev 15:
419–433, 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiang AK, Mak NK and Ng WT: Translational
research in nasopharyngeal carcinoma. Oral Oncol. 50:345–352. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Wu S, Zhou J and Chen XY:
Screening for nasopharyngeal cancer. Cochrane Database Syst Rev.
11:CD0084232015.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chia WK, Teo M, Wang WW, Lee B, Ang SF,
Tai WM, Chee CL, Ng J, Kan R, Lim WT, et al: Adoptive T-cell
transfer and chemotherapy in the first-line treatment of metastatic
and/or locally recurrent nasopharyngeal carcinoma. Mol Ther.
22:132–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong Kong Cancer Registry. http://www3.ha.org.hk/cancereg/Statistics.html20–August.
2013
|
|
17
|
Chen ZT, Liang ZG and Zhu XD: A review:
Proteomics in nasopharyngeal carcinoma. Int J Mol Sci.
16:15497–15530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi S, Andoh T and Tani T: EGD1
(β-NAC) mRNA is localized in a novel cytoplasmic structure in
Saccharomyces cerevisiae. Genes Cells. 16:316–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirstein-Miles J, Scior A, Deuerling E and
Morimoto RI: The nascent polypeptide-associated complex is a key
regulator of proteostasis. EMBO J. 32:1451–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Berndt U, Gölz H, Tais A,
Oellerer S, Wölfle T, Fitzke E and Rospert S: NAC functions as a
modulator of SRP during the early steps of protein targeting to the
endoplasmic reticulum. Mol Biol Cell. 23:3027–3040. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beatrix B, Sakai H and Wiedmann M: The
alpha and beta subunit of the nascent polypeptide-associated
complex have distinct functions. J Biol Chem. 275:37838–37845.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franke J, Reimann B, Hartmann E, Köhlerl M
and Wiedmann B: Evidence for a nuclear passage of nascent
polypeptide-associated complex subunits in yeast. J Cell Sci.
114:2641–2648. 2001.PubMed/NCBI
|
|
23
|
Bukau B, Deuerling E, Pfund C and Craig
EA: Getting newly synthesized proteins into shape. Cell.
101:119–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartl FU and Hayer-Hartl M: Molecular
chaperones in the cytosol: From nascent chain to folded protein.
Science. 295:1852–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wegrzyn RD and Deuerling E: Molecular
guardians for newborn proteins: ribosome-associated chaperones and
their role in protein folding. Cell Mol Life Sci. 62:2727–2738.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powers T and Walter P: The nascent
polypeptide-associated complex modulates interactions between the
signal recognition particle and the ribosome. Curr Biol. 6:331–338.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thiede B, Dimmler C, Siejak F and Rudel T:
Predominant identification of RNA-binding proteins in Fas-induced
apoptosis by proteome analysis. J Biol Chem. 276:26044–26050. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thakur D, Saxena R, Singh V, Haq W, Katti
SB, Singh BN and Tripathi RK: Human beta casein fragment (54–59)
modulates M. bovis BCG survival and basic transcription
factor 3 (BTF3) expression in THP-1 cell line. PLoS One.
7:e459052012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pham VC, Pitti R, Anania VG, Bakalarski
CE, Bustos D, Jhunjhunwala S, Phung QT, Yu K, Forrest WF,
Kirkpatrick DS, et al: Complementary proteomic tools for the
dissection of apoptotic proteolysis events. J Proteome Res.
11:2947–2954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang KS, Kim HS, Jin UH, Lee SS, Park JA,
Lim YP and Pai HS: Silencing of NbBTF3 results in developmental
defects and disturbed gene expression in chloroplasts and
mitochondria of higher plants. Planta. 225:1459–1469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Odreman F, Vindigni M, Gonzales ML,
Niccolini B, Candiano G, Zanotti B, Skrap M, Pizzolitto S, Stanta G
and Vindigni A: Proteomic studies on low- and high-grade human
brain astrocytomas. J Proteome Res. 4:698–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kunkle BW, Yoo C and Roy D: Reverse
engineering of modified genes by Bayesian network analysis defines
molecular determinants critical to the development of glioblastoma.
PLoS One. 8:e641402013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jain R, Kulkarni P, Dhali S, Rapole S and
Srivastava S: Quantitative proteomic analysis of global effect of
LLL12 on U87 cell's proteome: An insight into the molecular
mechanism of LLL12. J Proteomics. 113:127–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uhlen M, Oksvold P, Fagerberg L, Lundberg
E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S,
et al: Towards a knowledge-based human protein atlas. Nat
Biotechnol. 28:1248–1250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Q, Zhou JP, Li B, Huang ZC, Dong HY,
Li GY, Zhou K and Nie SL: Basic transcription factor 3 is involved
in gastric cancer development and progression. World J
Gastroenterol. 19:4495–4503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roy L, Laboissière S, Abdou E, Thibault G,
Hamel N, Taheri M, Boismenu D, Lanoix J, Kearney RE and Paiement J:
Proteomic analysis of the transitional endoplasmic reticulum in
hepatocellular carcinoma: An organelle perspective on cancer.
Biochim Biophys Acta. 1804:1869–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amin MB, Edge SB, Greene FL, et al: AJCC
cancer staging manual. 8th edition. New York: Springer; 2017,
View Article : Google Scholar
|