Introduction
Lung cancer is a common malignancy, with the highest
incidence and mortality rates of all malignant tumors worldwide
(1). Clinical therapy for lung cancer
typically includes surgery, chemotherapy, radiotherapy and targeted
therapy (2). However, the 5-year
survival rate is <15% (3),
therefore, developing novel, effective methods to treat lung cancer
is of great importance. Gene-targeted therapy is one of the major
methods used as a therapy for lung cancers (4). It is important to clarify the molecular
mechanism of lung cancer tumorigenesis. Many tumor suppressor genes
and oncogenes are altered in lung cancer; these contribute to
tumorigenesis, development, migration and metastasis (5,6).
The phosphatase and tensin homolog (PTEN) is
a tumor suppressor gene that was first identified in 1997 (7). It has been reported that PTEN is
frequently deleted or mutated in human cancer types, including
breast cancer (8,9), pancreatic cancer (10), colorectal cancer (11,12), liver
cancer (13), prostate cancer
(14), gastric cancer (15) and non-small cell lung cancer (NSCLC)
(16). PTEN serves an important role
in the nucleus by maintaining genomic stability via the regulation
of RAD51 (17). Loss or gene
disruption of PTEN is associated with poor prognosis in
human cancer types (18). Under
normal circumstances, RAD51 is phosphorylated by the
phosphoinositide 3-kinase (PI3K) family and dephosphorylated by the
phosphatase PTEN to generate phosphatidylinositol (PI)-(4,5)-P2.
However, loss of PTEN increases the levels of PI-(3,4,5)-triphosphate, which in turn activates the
PI3K-protein kinase B (Akt) signaling pathway to promote cell
proliferation and survival (19,20).
Elucidating the molecular mechanism of NSCLC would
provide a basis for clinical therapies to treat patients with lung
cancer. Lu et al (21) have
previously reported that PTEN inhibits cell proliferation, promotes
cell apoptosis and induces cell cycle arrest via downregulation of
the PI3K/AKT/human telomerase reverse transcriptase (hTERT) pathway
in lung adenocarcinoma A549 cells. In the current study, additional
lung cancer cell lines, including H460, SK-MES-1, H1299 and A549,
were used in order to investigate the regulatory mechanisms of
NSCLC cells. The current study demonstrated that PTEN regulates
cell phase progression and cell apoptosis, possibly by regulating
the levels of S-phase kinase-associated protein 2 (Skp2). Future
studies providing further clarification regarding the role of PTEN
in human NSCLC cell proliferation may provide a basis for the
development of novel gene therapies.
Materials and methods
Cell lines, shRNA, plasmid and
reagents
The immortalized human bronchial epithelial cell
line BEAS-2B was obtained from Shanghai Bioleaf Biotech Co., Ltd.
(Shanghai, China). Human MRC-5 cells, (cat. no. AA-CELL-79) were
purchased from Zhixing Biological Technology Corporation
(Guangzhou, China; http://action.binzhuang.com/). A549 (cat. no.
zs100735), H1299 (cat. no. as100207) and H460 (cat. no. zs101010)
cells were purchased from Zishi Biotechnology Corporation
(Shanghai, China; http://pozuchou1004.cn.globalimporter.net/). SK-MES-1
(cat. no. XB-0170) was purchased from Aolu Biological Corporation
(Shanghai, China; http://www.chem17.com/st310034/Intro.html). The cell
lines were cultured in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (cat. no. SH30071.03; HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere
containing 5% CO2. MTT was obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). The recombinant plasmid of PTEN
was constructed and provided by Cyagen Biology Technology
Corporation (Suzhou, China; http://www.cyagen.com/cn/zh-cn/), with open reading
frames of PTEN digested with HindIII and BamHI and
subcloned into a pcDNA3.1 (+) plasmid. Here, the empty pcDNA3.1 (+)
plasmid was used as a negative control. PTEN short hairpin (sh)RNA
(h2; cat. no. sc-44272-SH) and a scrambled control shRNA (cat. no.
sc-108060) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The A549 and SK-MES-1 cells, BEAS-2B and H460
cells were transfected with Lipofectamine 2000 (cat. no. 11668-027;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The pc pcDNA3.1 (+) plasmid was used as a
negative control of PTEN plasmid to transfect the lung cancer cells
for overexpression of PTEN, and the vector already contained the
shRNA sequence. A total of 0.5 µg PTEN shRNA or control shRNA were
transfected using Lipofectamine 2000 in 24-well plates for 48, 72
and 96 h. An MTT assay was performed following 48, 72 or 96 h of
transfection. Cell cycle determination via flow cytometry was
performed 24 h following transfection. A549 cells or SK-MES-1 cells
were transfected with pcDNA3.1-PTEN plasmid and control plasmid for
48 h. A total of 0.5 µg of PTEN recombinant plasmid and control
plasmid were transfected using Lipofectamine 2000 in 24-well plates
for 48 h. The expression of total caspase-3, cleaved caspase-3,
poly ADP ribose polymerase (PARP) and cleaved PARP was assessed
using western blotting.
MTT assay
An MTT assay was used to determine the viability of
NSCLC cells. Briefly, BEAS-2B and H460 cells, A549 and SK-MES-1
cells were transfected with PTEN-shRNA or negative control shRNA
for 48, 72 or 96 h. Cells were subsequently incubated at 37°C with
20 µl of MTT (5 mg/ml) for 4 h and the purple crystals were
dissolved in dimethylsulfoxide for 15 min. A total of 150 µl/well
was transferred into 96-well plates and the absorbance was measured
using a microplate reader at 490 nm (iMark Microplate Reader;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The inhibition rate
was calculated using Microsoft Excel software 2016 (Microsoft
Corporation, Redmond, WA, USA).
Antibodies
Rabbit monoclonal anti-PTEN antibody (cat. no.
ab32199), rabbit polyclonal anti-caspase-3 antibody (cat. no.
ab44976) and rabbit polyclonal anti-active caspase-3 antibody (cat.
no. ab2302) were purchased from Abcam (Cambridge, UK). Rabbit
anti-PARP antibody (cat. no. 9542) was obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal anti-cleaved
PARP antibody (cat. no. ab4830) was obtained from Abcam and mouse
monoclonal anti-β-Actin antibody (cat. no. sc-47778) was purchased
from Santa Cruz Biotechnology, Inc. The Anti-SKP2 antibody
(ab19877) was purchased from Abcam. The secondary antibodies
included goat anti-rabbit IgG H&L horseradish peroxidase (HRP)
(cat. no. ab6721; Abcam) and HRP-conjugated goat anti-mouse IgG
(cat. no. sc-2005; Santa Cruz Biotechnology, Inc.).
Western blot analysis
The cell lysates were prepared using
immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentration determination
method BCA was performed and 20 µg/lane of total protein was added
and subsequently separated by 10% SDS-PAGE. Proteins were
transferred onto nitrocellulose membranes, which were subsequently
blocked with 5% bovine serum albumin (Thermo Fisher Scientific,
Inc.) for 30 min at room temperature. The membrane was incubated
with the indicated primary and secondary antibodies. The primary
antibody was diluted to 1:1,000 and incubated at 4°C overnight. The
secondary antibody was diluted to 1:10,000 and incubated at 37°C
for 1 h. Membranes were washed three times for 5 min with 1X
Tris-buffered saline + Tween 20 buffer between each step. The bands
were visualized using enhanced chemiluminescence reagents (Pierce;
Thermo Fisher Scientific, Inc.). The grey values of the bands were
determined and calculated by Image J software (version 1.48,
National Institutes of Health, Bethesda, MD, USA).
Cell cycle analysis
Cell cycle analysis was performed using propidium
iodide staining and flow cytometry analysis (Propidium Iodide Flow
Cytometry kit; cat. no. ab139418, Abcam). A total of
3×106 NSCLC cells were collected by centrifugation at
300 × g for 10 min at room temperature. The medium was discarded
and cells were washed twice with ice-cold PBS. Cells were
subsequently fixed in 70% ethanol at 4°C overnight, washed twice
with PBS and centrifuged at room temperature at 800 × g for 5 min.
Cells were resuspended in PBS containing 1 mg/ml RNase A (Abcam)
for 30 min at 37°C. Propidium iodide (50 µg/ml) was added into the
cell suspension at 4°C for 15 min and flow cytometry analysis was
performed using a BD LSRFortessa X-20 flow cytometer (BD
Biosciences, San Jose, CA, USA).
Statistical analysis
Data were analyzed using SPSS v.13 (SPSS, Inc.,
Chicago, IL, USA) and are presented as the mean ± standard
deviation. Independent samples were analyzed using independent
sample t-tests and multiple comparisons were analyzed using ANOVA
followed by Tukey's test. P≤0.05 was considered to indicate a
statistically significant difference.
Results
PTEN levels are low in NSCLC cell
lines
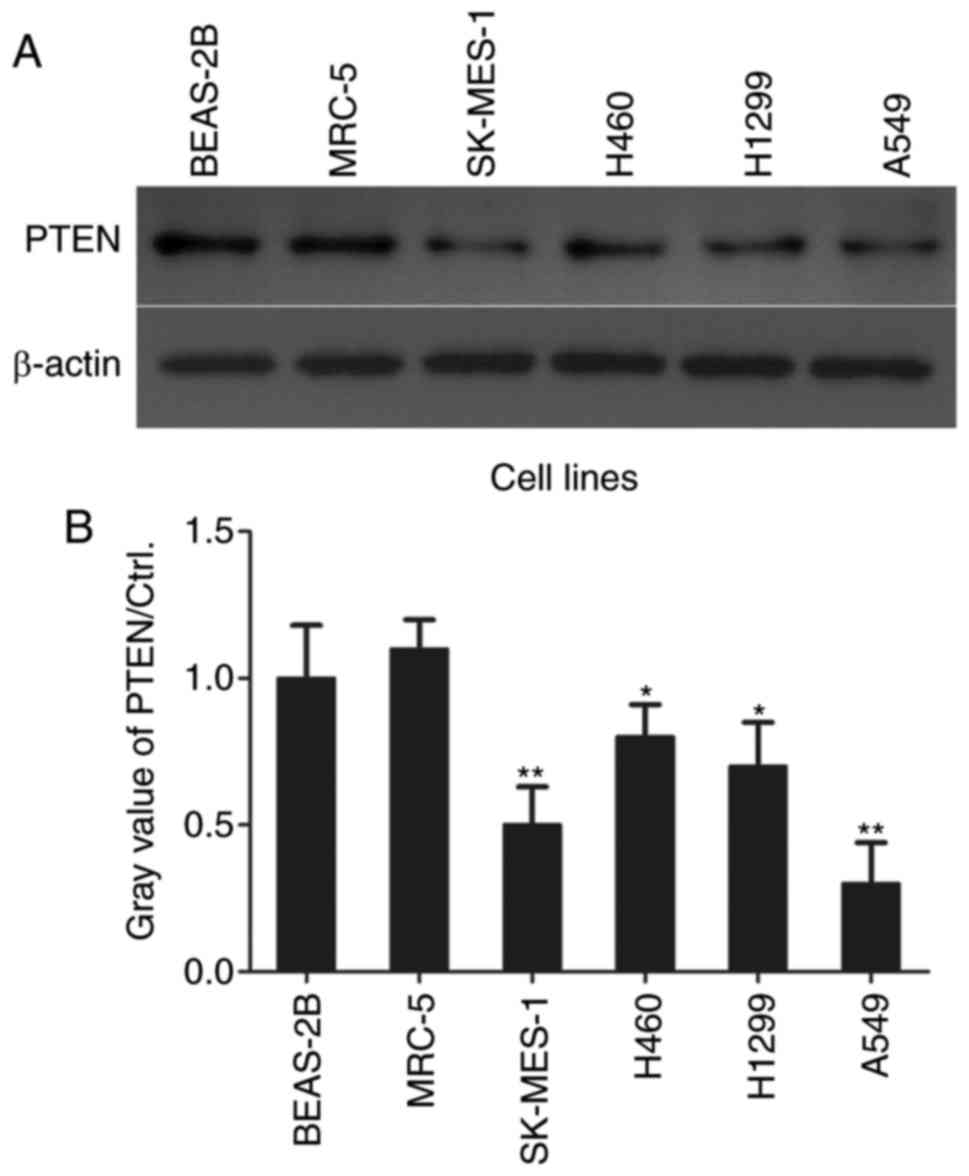
In order to investigate the role of PTEN in NSCLC
cell proliferation, PTEN levels in NSCLC cells were assessed using
western blotting (Fig. 1A). PTEN
levels were significantly decreased in lung adenocarcinoma A549
(P<0.01) and H1299 (P<0.05) cell lines, lung squamous cell
carcinoma cell line SK-MES-1 (P<0.01) and lung large cell
carcinoma H460 cells (P<0.05) compared with the normal control
BEAS-2B and MRC-5 cell lines (Fig.
1B).
PTEN knockdown increases the viability
of BEAS-2B and H460 cells
The effect of PTEN on viability in BEAS-2B and H460
cells was assessed using western blotting (Fig. 2). PTEN was significantly downregulated
in cells transfected with PTEN-shRNA (P<0.01; Fig. 2B). Additionally, cell viability was
determined using an MTT assay and the results demonstrated that
cell viability was significantly increased in PTEN-shRNA
transfected cells compared with cells transfected with control
shRNA and untreated cells (P<0.05; Fig. 2C). Furthermore, western blotting was
performed to assess the effects of PTEN knockdown on caspase-3 and
PARP expression. As indicated in Fig. 2D
and E, transfection with PTEN shRNA significantly decreased the
levels of cleaved caspase-3 and cleaved PARP in BEAS-2B cells and
H460 cells (P<0.01). These results demonstrated that PTEN
knockdown may inhibit cell apoptosis of lung cancer cells.
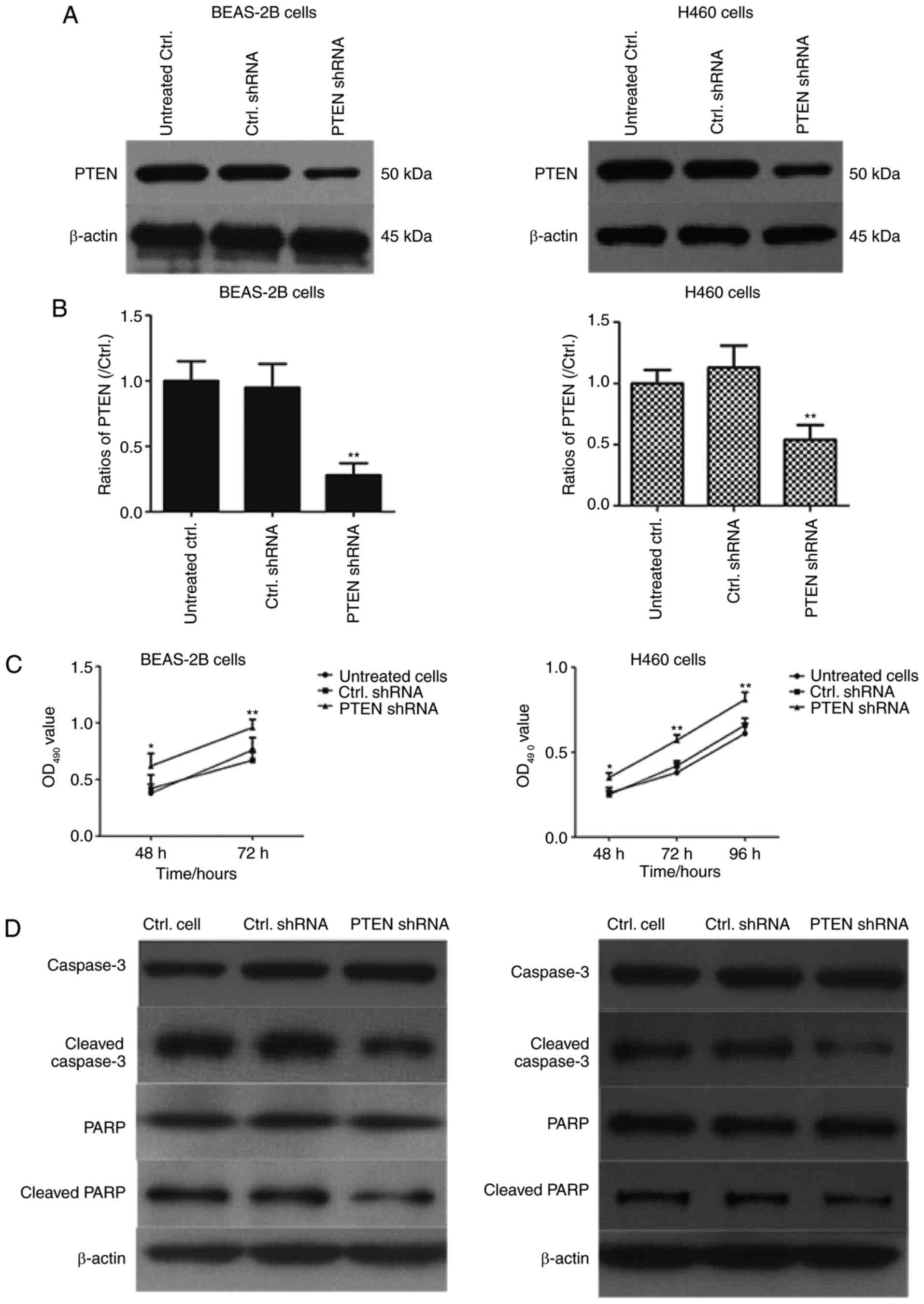 | Figure 2.PTEN knockdown promotes proliferation
in BEAS-2B cells and H460 cells. (A) BEAS-2B cells and H460 cells
were transfected with PTEN-shRNA and control shRNA for 48 h and
PTEN levels were assessed using western blotting and (B) quantified
**P<0.01, compared with Ctrl. shRNA group. (C) The PTEN-shRNA
and Ctrl. shRNA transfected BEAS-2B cells and H460 cells were
cultured for 48, 72 or 96 h and cell viability was determined using
an MTT assay. *P<0.05, between PTEN shRNA and Ctrl. shRNA in 48
h; **P<0.01, between PTEN shRNA and Ctrl. shRNA in 72 h or 96 h;
(D) BEAS-2B cells and H460 cells were transfected with PTEN shRNA
and control shRNA for 48 h and the expression of caspase-3, cleaved
caspase-3, PARP and cleaved PARP was determined using western
blotting. (E) The ratios of Caspase-3, cleaved caspase-3, PARP and
cleaved PARP in BEAS-2B and H460 cells were are presented in
histograms *P<0.05 and **P<0.01 vs. Ctrl. shRNA-transfected
cells. PTEN, phosphatase and tensin homolog 10; sh, short hairpin;
Ctrl., control; PARP, poly ADP ribose polymerase; OD, optical
density. |
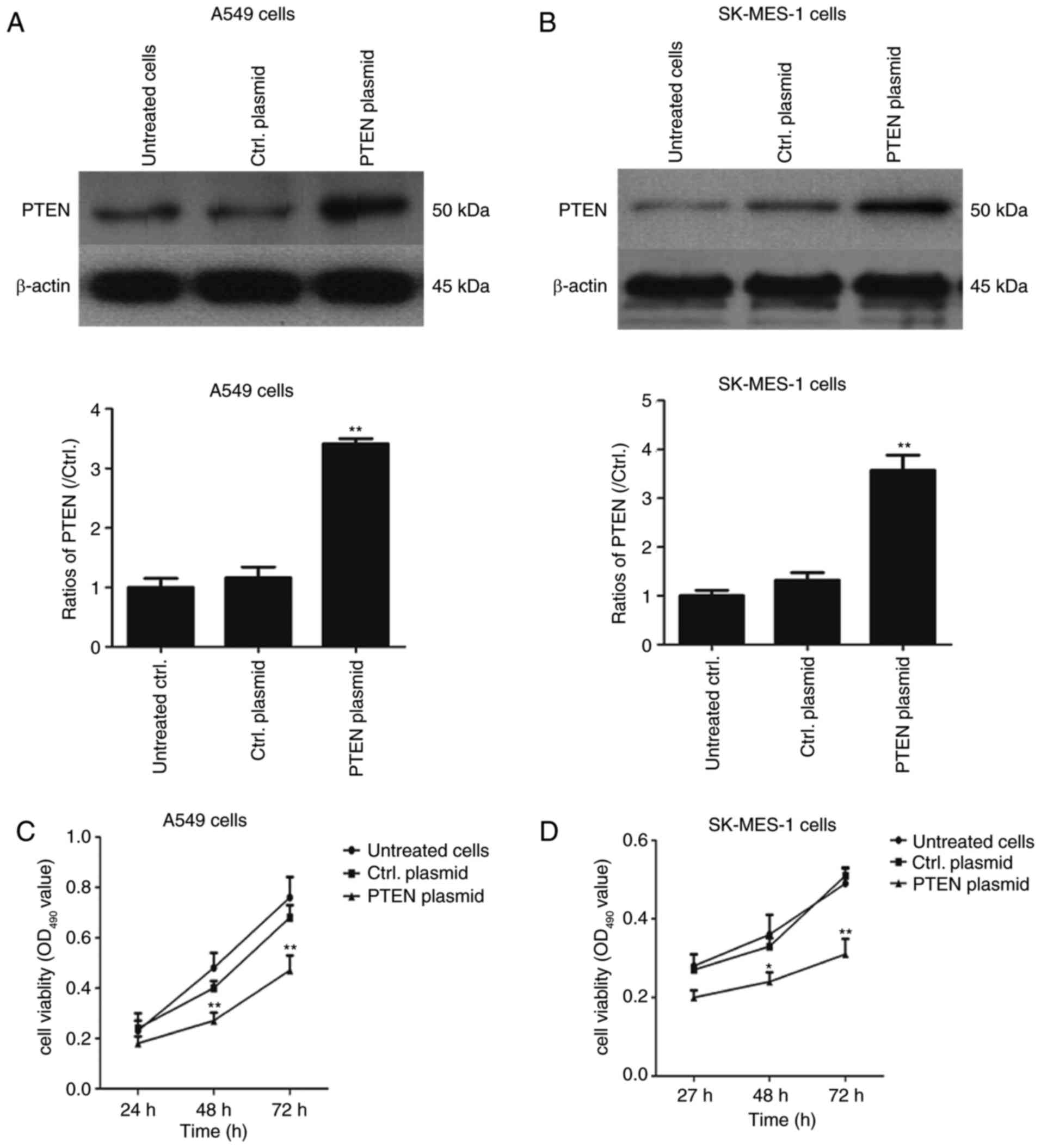
Overexpression of PTEN decreases the
viability of lung cancer cell lines
PTEN was overexpressed in NSCLC cells and the role
of PTEN in cell proliferation was evaluated further. A549 and
SK-MES-1 cells were transfected with pcDNA3.1-PTEN and control
pcDNA3.1 (+) for 24, 48 and 72 h. The expression of PTEN was
determined using western blotting and cell proliferation was
determined using an MTT assay. As illustrated in Fig. 3A and B, the PTEN expression was
significantly increased in PTEN plasmid-transfected A549 cells and
SK-MES-1 cells compared with the control plasmid group (P<0.01),
The proliferation of A549 and SK-MES-1 cells was significantly
suppressed in pcDNA3.1-PTEN overexpressed lung cancer cells
compared with cells transfected with the control plasmid
(P<0.05; Fig. 3C) in a
time-dependent manner. These data suggest that PTEN overexpression
suppresses cell growth in lung cancer cell lines, with PTEN
acting as a tumor suppressor gene.
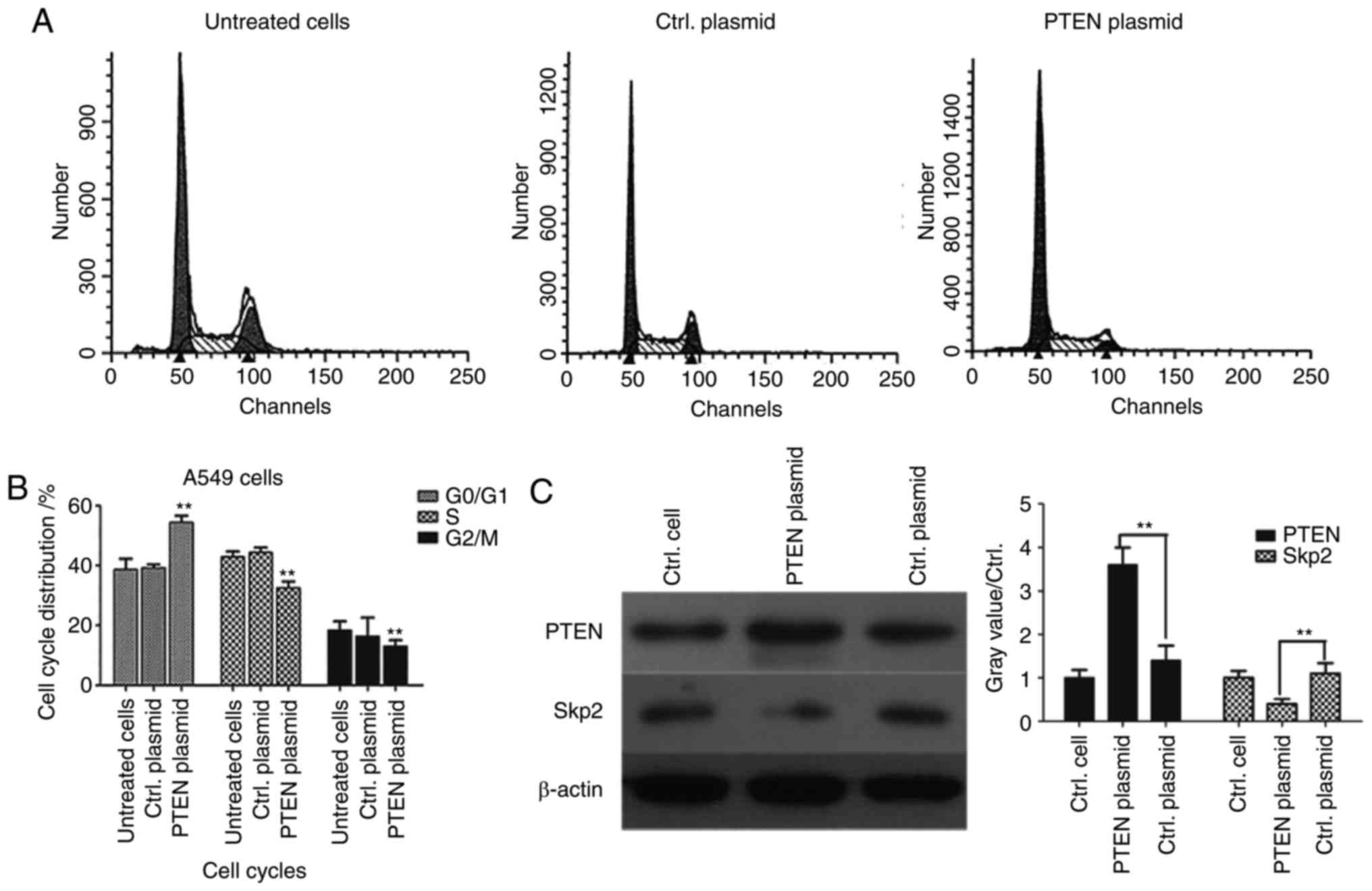
Overexpression of PTEN downregulates
Skp2 expression and induces cell-cycle arrest in
G0/G1 phase
It has previously been reported that PTEN signaling
regulates the assembly of the Skp, Cullin, F-box containing complex
(SCF-Skp2) at the G1/S cell cycle transition (22,23).
However, to the best of our knowledge, it is unclear whether Skp2
expression is deregulated in lung cancer cells. In order to further
clarify the molecular mechanism of PTEN, flow cytometry was used
for cell-cycle analysis in PTEN-overexpressed A549 cells.
G0/G1 phase arrest was significantly induced
(P<0.01) and the proportion of cells in S phase was
significantly decreased in the PTEN-overexpressed group compared
with the control plasmid group (P<0.01), suggesting that PTEN
may regulate signaling pathways associated with cell cycle
progression (Fig. 4B). In order to
detect the associations between PTEN and Skp2, western blotting was
performed and the results demonstrated that overexpression of PTEN
significantly decreased the expression level of Skp2 (P<0.01;
Fig. 4C), which suggested that Skp2
expression was likely regulated by PTEN in lung cancer cells.
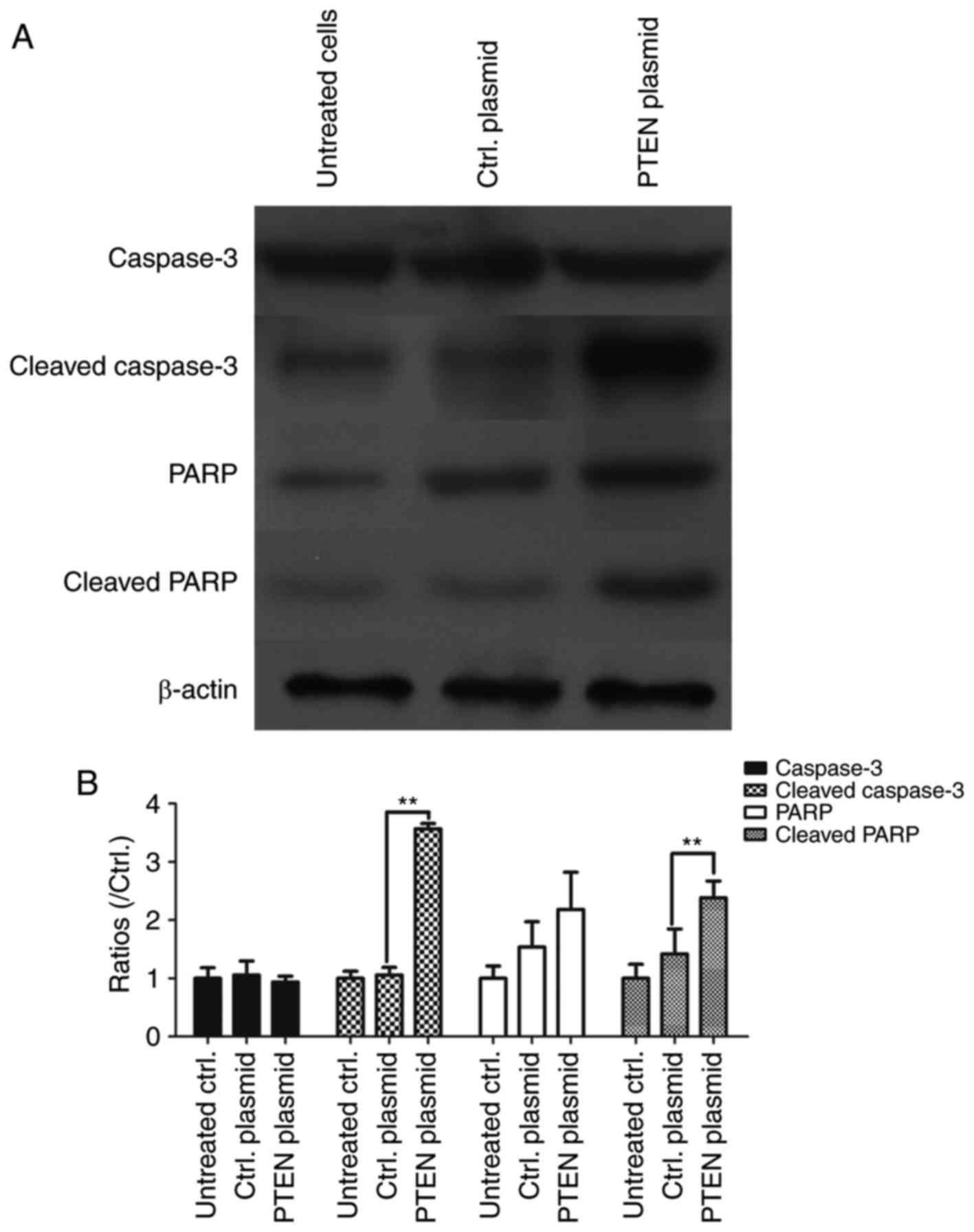
Overexpression of PTEN activates
caspase-3 and promotes the cleavage of PARP
In order to assess whether PTEN regulates apoptosis
in NSCLC cells, it was overexpressed in A549 cells and levels of
cleaved caspase-3 and cleaved PARP were detected using western
blotting. PTEN overexpression in A549 cells significantly
upregulated cleaved caspase-3 expression (P<0.01), which
suggests that PTEN overexpression contributes to the activation of
caspase-3 (Fig. 5). The levels of
PARP and cleaved PARP was also assessed using western blotting and
the results demonstrated that PARP, the substrate of the activated
caspase-3, was cleaved into the active form in PTEN overexpressing
cells (P<0.01 vs. Ctrl. plasmid; Fig.
5). These results suggest that overexpression of PTEN increases
cell apoptosis in NSCLCs.
Discussion
Lung cancer comprises malignant lung tumors
characterized by uncontrolled cell growth (24). NSCLC is the primary type of lung
cancer and accounts for ~85% of all lung cancer cases (25). It has previously been reported that
PTEN functions as a tumor suppressor in a variety of human cancer
types (26,27). Lu et al (21) reported that PTEN inhibits cell
proliferation, promotes cell apoptosis and induces cell cycle
arrest via downregulation of the PI3K/AKT/hTERT pathway in lung
adenocarcinoma A549 cells, which is consistent with the results of
the current study. In the current study, multiple lung cancer cell
lines, including H460, SK-MES-1, H1299 and A549, were used.
Although the levels of PTEN were decreased in lung cancer cell
lines compared with the normal control cells BEAS-2B, there was
still substantial PTEN expression in NSCLC cell lines. It was
probably the limited decrease in PTEN expression, not other
factors, that was the major player for NSCLC progression (21).
However, there are some limitations of the current
study. The current study identified that PTEN plays an important
role in the proliferation and cell cycle progression of lung cancer
cells. Further study would investigate how PTEN regulates the
proliferation or cell cycle of cancer cells, for example, which
specific cancer-related signaling pathways are regulated by PTEN or
how the microRNA expression profiles are changed in the progression
of lung cancer cells. Further studies should be performed in at
least two other lung cancer cells lines, to validate the findings
of the present study.
Levels of PTEN were markedly decreased in lung
cancer cell lines compared with normal control cells. The MTT assay
results in H460 cells revealed that PTEN knockdown promotes the
proliferation of lung cancer cells. However, overexpression of PTEN
in A549 and H460 cells significantly inhibited cell growth. This
was consistent with a previous study by Li et al (13) in which overexpression of PTEN
effectively inhibited proliferation of liver cancer cells and
promoted their apoptosis. Furthermore, in bladder cancer cells,
overexpression of PTEN suppressed growth and induced apoptosis by
inhibiting the expression of surviving (28). It has also been identified that
overexpression of PTEN induces cell growth arrest and apoptosis in
human breast cancer ZR-75-1 cells (29) and human ovarian cancer cells (30). Together, these data suggest that PTEN
functions as a tumor suppressor and may be an effective target for
the regulation of NSCLC cell proliferation.
Cell apoptosis is a process of programmed cell death
that occurs in multicellular organisms (31). There are two major apoptosis signaling
pathways: Extrinsic (death receptor-mediated) and intrinsic
(mitochondrial) (32,33). Caspase-3 is activated in the extrinsic
and intrinsic pathways (34) and is
therefore an effective molecule for inducing cell apoptosis to
treat cancer cells (35). In the
current study, overexpression of PTEN was demonstrated to increase
the level of cleaved caspase-3 in NSCLC cells. In response to
apoptotic signals, cleaved caspase-3, the active form of caspase-3,
cleaves the 116 kDa substrate, PARP, into 85 and 25 kDa fragments
(35). In the current study, the
cleaved 85 kDa fragment of PARP was detected. Furthermore, PTEN
overexpression was observed to increase the percentage of cells in
G0/G1 phase and decrease the number of cells
in S phase, suggesting that PTEN induced
G0/G1 cell cycle arrest. The results of the
current study suggest that PTEN is capable of inducing apoptosis
and may therefore be a potential effective target for gene therapy
in patients with NSCLC.
In conclusion, PTEN suppressed non-small cell lung
cancer cell growth by promoting G0/G1 arrest
and cell apoptosis. Taken togather, the results f the present study
suggest that PTEN may be a potential target gene for gene therapy
in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
The work was supported by Guangdong Provincial
Natural Science Foundation for key doctoral start-up project,
(Grant no. S2013010016379).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL was the guarantor for the integrity of the entire
study and was responsible for study concepts and design. SH
performed the literature research and performed cell cycle
analysis. SM revised the manuscript for critically important
intellectual content. JH undertook the clinical studies, and the
experimental studies were performed by SM. Data acquisition was the
responsibility of LL and LH undertook analysis of the data and
statistical analysis. SC performed the western blotting experiments
and prepared the manuscript. YW performed the MTT assay and
reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lim JS and Soo RA: Nivolumab in the
treatment of metastatic squamous non-small cell lung cancer: A
review of the evidence. Ther Adv Respir Dis. 10:444–454. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi JG, Shao HJ, Jiang FE and Huang YD:
Role of radiation therapy in lung cancer management-a review. Eur
Rev Med Pharmacol Sci. 20:3217–3222. 2016.PubMed/NCBI
|
|
3
|
Condoluci A, Mazzara C, Zoccoli A, Pezzuto
A and Tonini G: Impact of smoking on lung cancer treatment
effectiveness: A review. Future Oncol. 12:2149–2161. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang CL, Yokomise H and Miyatake A:
Clinical significance of the p53 pathway and associated gene
therapy in non-small cell lung cancers. Future Oncol. 3:83–93.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bikkavilli RK, Avasarala S, Van Scoyk M,
Arcaroli J, Brzezinski C, Zhang W, Edwards MG, Rathinam MK, Zhou T,
Tauler J, et al: Wnt7a is a novel inducer of β-catenin-independent
tumor-suppressive cellular senescence in lung cancer. Oncogene.
34:5317–5328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vincenten JP, Smit EF, Grünberg K, Postmus
PE, Snijders PJ, Witte BI, Heideman DA and Thunnissen E: Is the
current diagnostic algorithm reliable for selecting cases for EGFR-
and KRAS-mutation analysis in lung cancer? Lung Cancer. 89:19–26.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li B, Lu Y, Wang H, Han X, Mao J, Li J, Yu
L, Wang B, Fan S, Yu X and Song B: miR-221/222 enhance the
tumorigenicity of human breast cancer stem cells via modulation of
PTEN/Akt pathway. Biomed Pharmacother. 79:93–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun L, Burnett J, Gasparyan M, Xu F, Jiang
H, Lin CC, Myers I, Korkaya H, Liu Y, Connarn J, et al: Novel
cancer stem cell targets during epithelial to mesenchymal
transition in PTEN-deficient trastuzumab-resistant breast cancer.
Oncotarget. 7:51408–51422. 2016.PubMed/NCBI
|
|
10
|
Gu J, Wang D, Zhang J, Zhu Y, Li Y, Chen
H, Shi M, Wang X, Shen B, Deng X, et al: GFRα2 prompts cell growth
and chemoresistance through down-regulating tumor suppressor gene
PTEN via Mir-17-5p in pancreatic cancer. Cancer Lett. 380:434–441.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ping Hai P, Bo Feng T, Li L, Hui Nan Y and
Hong Z: IL-1β/NF-kb signaling promotes colorectal cancer cell
growth through miR-181a/PTEN axis. Arch Biochem Biophys. 604:20–26.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He
BC and Wu K: Oridonin upregulates PTEN through activating p38 MAPK
and inhibits proliferation in human colon cancer cells. Oncol Rep.
35:3341–3348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MF, Guan H and Zhang DD: Effect of
overexpression of PTEN on apoptosis of liver cancer cells. Genet
Mol Res. 15:2016.
|
|
14
|
Lotan TL, Wei W, Ludkovski O, Morais CL,
Guedes LB, Jamaspishvili T, Lopez K, Hawley ST, Feng Z, Fazli L, et
al: Analytic validation of a clinical-grade PTEN
immunohistochemistry assay in prostate cancer by comparison with
PTEN FISH. Mod Pathol. 29:904–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xin R, Bai F, Feng Y, Jiu M, Liu X, Bai F,
Nie Y and Fan D: MicroRNA-214 promotes peritoneal metastasis
through regulating PTEN negatively in gastric cancer. Clin Res
Hepatol Gastroenterol. 40:748–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu DY, Li XN, Qi Y, Liu DL, Yang Y, Zhao
J, Zhang CY, Wu K and Zhao S: MiR-454 promotes the progression of
human non-small cell lung cancer and directly targets PTEN. Biomed
Pharmacother. 81:79–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukherjee A and Karmakar P: Attenuation of
PTEN perturbs genomic stability via activation of Akt and
down-regulation of Rad51 in human embryonic kidney cells. Mol
Carcinog. 52:611–618. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bohn BA, Mina S, Krohn A, Simon R, Kluth
M, Harasimowicz S, Quaas A, Bockhorn M, Izbicki JR, Sauter G, et
al: Altered PTEN function caused by deletion or gene disruption is
associated with poor prognosis in rectal but not in colon cancer.
Hum Pathol. 44:1524–1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng Z, Zhang Y, Zhang Z, Yang Y and Song
T: Effect of miR-106b on invasiveness of pituitary adenoma via
PTEN-PI3K/AKT. Med Sci Monit. 23:1277–1285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim HJ, Wang X, Crowe P, Goldstein D and
Yang JL: Targeting the PI3K/PTEN/AKT/mTOR pathway in treatment of
sarcoma cell lines. Anticancer Res. 36:5765–5771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis,
and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT
pathway in lung adenocarcinoma A549 cells. Biomed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jonason JH, Gavrilova N, Wu M, Zhang H and
Sun H: Regulation of SCF(SKP2) ubiquitin E3 ligase assembly and
p27(KIP1) proteolysis by the PTEN pathway and cyclin D1. Cell
Cycle. 6:951–961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mamillapalli R, Gavrilova N, Mihaylova VT,
Tsvetkov LM, Wu H, Zhang H and Sun H: PTEN regulates the
ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1)
through the ubiquitin E3 ligase SCF(SKP2). Curr Biol. 11:263–267.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giannopoulou E, Nikolakopoulos A,
Kotsirilou D, Lampropoulou A, Raftopoulou S, Papadimitriou E,
Theocharis AD, Makatsoris T, Fasseas K and Kalofonos HP: Epidermal
growth factor receptor status and Notch inhibition in non-small
cell lung cancer cells. J Biomed Sci. 22:982015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vesel M, Rapp J, Feller D, Kiss E, Jaromi
L, Meggyes M, Miskei G, Duga B, Smuk G, Laszlo T, et al: ABCB1 and
ABCG2 drug transporters are differentially expressed in non-small
cell lung cancers (NSCLC) and expression is modified by cisplatin
treatment via altered Wnt signaling. Respir Res. 18:522017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Nostrand JL, Brisac A, Mello SS,
Jacobs SB, Luong R and Attardi LD: The p53 target gene SIVA enables
non-small cell lung cancer development. Cancer Discov. 5:622–635.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deben C, Wouters A, de Beeck Op K, van Den
Bossche J, Jacobs J, Zwaenepoel K, Peeters M, Van Meerbeeck J,
Lardon F, Rolfo C, et al: The MDM2-inhibitor Nutlin-3 synergizes
with cisplatin to induce p53 dependent tumor cell apoptosis in
non-small cell lung cancer. Oncotarget. 6:22666–22679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu ZX, Song TB, Li DM, Zhang XT and Wu XL:
Overexpression of PTEN suppresses growth and induces apoptosis by
inhibiting the expression of survivin in bladder cancer cells.
Tumour Biol. 28:9–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Lin G, Wu B, Zhou X and Zhou K:
Overexpression of PTEN induces cell growth arrest and apoptosis in
human breast cancer ZR-75-1 cells. Acta Biochim Biophys Sin
(Shanghai). 39:745–750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan X, Fraser M, Qiu Q and Tsang BK:
Over-expression of PTEN sensitizes human ovarian cancer cells to
cisplatin-induced apoptosis in a p53-dependent manner. Gynecol
Oncol. 102:348–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terra LF, Garay-Malpartida MH, Wailemann
RA, Sogayar MC and Labriola L: Recombinant human prolactin promotes
human beta cell survival via inhibition of extrinsic and intrinsic
apoptosis pathways. Diabetologia. 54:1388–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Zeng F, Liu X, Li Y, Zhou J,
Huang Y, Wang Y, Zhou S, Zhu W, Shu E, et al: Chan-Yu-Bao-Yuan-Tang
induces apoptosis in NSCLC and SCLC cell lines via a
mitochondria-mediated pathway. Xenobiotica. 41:593–602. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim MJ, Kwon SB, Kim MS, Jin SW, Ryu HW,
Oh SR and Yoon DY: Trifolin induces apoptosis via extrinsic and
intrinsic pathways in the NCI-H460 human non-small cell lung-cancer
cell line. Phytomedicine. 23:998–1004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elankumaran S, Rockemann D and Samal SK:
Newcastle disease virus exerts oncolysis by both intrinsic and
extrinsic caspase-dependent pathways of cell death. J Virol.
80:7522–7534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin J, Wang M, Jin C and Qi Q: miR-101
sensitizes A549 NSCLC cell line to CDDP by activating caspase
3-dependent apoptosis. Oncol Lett. 7:461–465. 2014. View Article : Google Scholar : PubMed/NCBI
|