Introduction
Osteosarcoma has become one of the most common types
of bone cancer (1,2). The clinical treatment for osteosarcoma
has little effect on amputated patients due to pulmonary metastases
(3). Although surgery and neoadjuvant
chemotherapy has improved the 5-year survival rate to ~60%
(4), distant metastasis may occur due
to poor responses to chemotherapy. Therefore, understanding the
detailed mechanisms involved in osteosarcoma development is
important.
MicroRNAs (miRs) are highly conserved, non-coding
RNA molecules that serve central roles in multiple
cancer-associated signaling pathways, including invasion and
metastasis, through post-transcriptional regulation of genes
(5–8).
miRs are able to recognize the 3′-untranslated regions (3′-UTRs) of
target genes and result in their degradation or destabilization
through the RNA-induced silencing complex. miR-885-5p has been
reported to be upregulated in pancreatic cancer and breast cancer
(9,10). In breast cancer, miR-885 targets the
3′-UTR of E2F transcription factor 1 to downregulate its
expression, thereby regulating the proliferation of human breast
cancer MCF-7 cells (10). Previous
work has demonstrated that miR-885-5p may be upregulated in
osteosarcoma tissues (11); however,
the function of miR-885-5p in osteosarcoma is yet to be
elucidated.
The Wnt/β-catenin signaling pathway serves as a key
oncogenic pathway in multiple cancer types, including osteosarcoma
(12). β-catenin is a key component
of the Wnt/β-catenin signaling pathway, and is re-localized from
the cytosol to the nucleus upon Wnt ligand stimulation, thereby
regulating gene expression (13). A
number of previous studies have suggested that Wnt/β-catenin may
regulate various downstream targets, including c-Myc proto-oncogene
(c-myc), cyclin D1 and mitogen-activated protein kinase 8 (MAPK8),
which regulate migration, cell proliferation and stemness (14–16).
Notably, a previous study has demonstrated that miR-885 may
interact with β-catenin in bovine semitendinosus and masseter
muscles (17). However, whether
miR-885-5p is able to regulate β-catenin in osteosarcoma cells
requires further investigation.
Although previous work reported that miR-885-5p may
be upregulated in osteosarcoma tissues (11), the detailed mechanism of action of
miR-885-5p remains unknown. The aim of the present study was to
verify whether miR-885-5p displays abnormal expression levels in
osteosarcoma and to further decipher the molecular mechanism of
miR-885-5p in osteosarcoma.
In the present study, it was demonstrated that
miR-885-5p was downregulated in osteosarcoma tissues and cell
lines. miR-885-5p expression levels were closely associated with
tumor size, Tumor-Node-Metastasis (TNM) stage and lymph node
metastasis, in addition to the prognosis of osteosarcoma.
Furthermore, β-catenin, a key component of the Wnt signaling
pathway, was demonstrated to be a target of miR-885-5p.
Additionally, miR-885-5p suppressed cell proliferation, migration
and invasion through regulation of β-catenin. These results
suggested that miR-885-5p may serve key roles in osteosarcoma
development, and may be a novel target for molecular therapy.
Materials and methods
Human tissue samples
Eighty-five pairs of osteosarcoma tissue samples and
adjacent non-cancerous tissue samples were collected from patients
who underwent osteosarcoma without preoperative systemic therapy at
Jingjiang Hospital of Chinese Medicine (Jingjiang, China) between
April 2012 and October 2016. Following surgical removal, all tissue
samples were immediately frozen in liquid nitrogen. All patients
gave consent and all human tissue experiments were approved by the
Ethics Committee of Jingjiang Hospital of Chinese Medicine.
Cell lines and cell culture
Human osteosarcoma MG-63 and U2OS cells, and the
osteoblast cell line hFOB1.19, were purchased from the American
Type Culture Collection (Manassas, VA, USA). The cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% penicillin and streptomycin. All cells
were maintained at 37°C in a humidified incubator with 5%
CO2.
Cell transfection
The empty vector (pcDNA3.1) and the plasmids
containing β-catenin were purchased from YouBio (Hunan, China).
miR-885-5p mimics and inhibitors were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). A total of 5×105
cells were transfected with 2.5 µg plasmid or mimic control (Mi-C),
inhibitor-control (In-C) or miR-885-5p mimics/inhibitors using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
primer sequences were as follows: miR-885-5p mimic forward,
5′-UCCAUUACACUACCCUGCCUCU-3′ and reverse,
5′-AGGCAGGGUAGUGUAAUGGAUU-3′; mimic control forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGATT-3′; miR-885-5p inhibitor,
5′-AGAGGCAGGGUAGUGUAAUGGA-3′; inhibitor control,
5′-CAGUCUUUUGUGUGUACAA-3′. Following transfection for 48 h, the
cells were harvested and used for further experiments.
Luciferase reporter assay
The β-catenin 3′-UTR sequences were subcloned into
the pGL3-basic luciferase reporter vector (Promega Corporation,
Madison, WI, USA). A total of ~1.0×105 MG-63 and U2OS
cells were seeded into 12-well plates and co-transfected with
β-catenin 3′-UTR, Renilla and pre-NC or miR-21 mimics using
Lipofectamine® 2000. Following transfection for 24 h,
the luciferase reporter assay was performed using the
Dual-Luciferase Reporter kit (Promega Corporation), according to
the manufacturer's protocol. The relative luciferase activity was
normalized to Renilla luciferase activity. Each independent
experiment was performed at least three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissue samples and cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. A total of 2 µg
RNA was used as a template to produce cDNA using the QuantScript RT
kit (Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocol. The reverse transcription conditions were
as follows: 5 min at 25°C, 30 min at 42°C and 5 min at 85°C. SYBR
Green (Roche Diagnostics GmbH, Mannheim, Germany) was used to
perform a qPCR assay using an ABI PRISM 7500 sequence detection
system. The qPCR conditions were as follows: 5 min at 98°C,
followed by 33 cycles of denaturation at 98°C for 30 sec, annealing
at 56°C for 30 sec and extension at 72°C for 30 sec. The relative
expression of mRNA was calculated by normalizing target gene
expression to that of the internal control, using the
2−∆∆Cq formula (18). Each
independent experiment was performed at least three times. The
primer sequences were as follows: c-myc forward,
5′-GATTCTCTGCTCTCCTCGAC-3′ and reverse,
5′-TTCTTCCTCATCTTCTTGTTCC-3′; mmP7 forward,
5′-CGAGACTTACCGCATATTACAG-3′ and reverse,
5′-AGTTAATCCCTAGACTGCTACC-3′; survivin forward,
5′-CGCATCTCTACATTCAAGAACTG-3′ and reverse,
5′-TCCTTGAAGCAGAAGAAACAC-3′; β-catenin forward,
5′-GCTGGAATTCTTTCTAACCTC-3′ and reverse,
5′-TCCAACAGTAGCCTTTATCAG-3′; GAPDH forward,
5′-TGATGACATCAAGAAGGTGG-3′ and reverse,
5′-TTACTCCTTGGAGGCCATGT-3′.
Western blotting
Total protein was extracted from cells using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) and the protein concentration was
measured using a bicinchoninic acid assay. A total of ~50 µg
protein/lane was separated by SDS-PAGE on a 10% gel, and
subsequently transferred onto a nitrocellulose filter membrane. The
membranes were blocked with 5% skimmed milk at room temperature for
1 h, and incubated with primary antibodies overnight at 4°C.
Following washing with PBS containing 0.1% Tween-20 (PBST) three
times at room temperature, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature. Finally, the membranes were washed with PSBT
three times at room temperature, and the specific bands were
visualized via chemiluminescence using an enhanced
chemiluminescence kit (GE Healthcare, Chicago, IL, USA). The
antibodies used were as follows: Anti-β-catenin (1:2,000; cat. no.
ab32572), anti-β-actin (1:4,000; cat. no. ab8227), HRP-conjugated
anti-rabbit antibody (1:5,000; cat. no. ab6721) and HRP-conjugated
anti-mouse antibody (1:5,000; cat. no. ab6789) (all Abcam,
Cambridge, UK).
Target prediction
Putative target genes of miR-885-5p were predicted
by bioinformatics analysis using the MirTarget tool on the miRDB
database (http://www.mirdb.org).
Wound healing assay
Wound healing assays were performed to investigate
the effect of miR-885-5p on cell migration. In brief,
~5×105 transfected MG-63 or U2OS cells were seeded into
6-well plates. Once the cells had reached 90–100% confluence, a 20
µl pipette tip was used to scratch a line into the cell monolayer.
Detached cells were removed by washing with PBS, and the cells were
maintained in serum-free DMEM at 37°C. The relative distance of
cell migration was measured via the width of the wound at 0 and 48
h following the scratch, under a light microscope (magnification,
×20). Each independent experiment was performed at least 3
times.
Transwell invasion assay
Transwell invasion assays were performed to
investigate the effect of miR-885-5p on cell invasion. In brief,
Transwell chambers were coated with 80 µl Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA), and maintained at 37°C for 30 min. A
total of ~2×105 transfected MG-63 or U2OS cells in 300
µl serum-free medium were seeded into the upper chamber. The lower
chamber was filled with 500 µl medium containing 10% FBS. Following
incubation for 24 h, cells were stained with 0.5% crystal violet at
room temperature for 10 min and the cells on the surface of upper
chamber were removed gently using a cotton swab. The invaded cells
were counted under a light microscope (magnification, ×20), in 5
random fields. Each independent experiment was performed at least 3
times.
Colony formation assay
Colony formation assays were performed in order to
determine the effect of miR-885-5p on cell proliferation. Briefly,
~3×104 transfected MG-63 or U2OS cells in serum-free
DMEM were seeded into 6-well plates. At 14 days later, the colonies
were fixed with 4% paraformaldehyde at room temperature for 10 min
and stained with 0.5% crystal violet at room temperature for 10
min. The number of colonies (>50 cells) was counted under a
light microscope (magnification, ×20). Each independent experiment
was performed at least 3 times.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assays was performed in order to determine the
effect of miR-885-5p on cell proliferation. Briefly,
~3×103 transfected MG-63 or U2OS cells were seeded into
96-well plates and cultured in 200 µl DMEM supplemented with 10%
FBS. A total of 20 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was added at 0, 24, 48 and 72 h after seeding.
Following incubation at 37°C for 1 h, the absorbance of each well
was measured at 450 nm using a microplate reader. Each independent
experiment was performed at least three times.
Statistics
SPSS software version 21.0 (IBM Corp., Armonk, NY,
USA) was used for all statistical analyses. Data are presented as
the mean ± standard deviation. The Kaplan-Meier method followed by
the log-rank test was used to analyze the association between
miR-885-5p expression levels and survival rate. The significance of
the differences between two groups was analyzed by Student's t-test
and the difference between multiple groups was analyzed by one-way
analysis of variance followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Downregulation of miR-885-5p predicts
a poorer outcome in patients with osteosarcoma
To identify the function of miR-885-5p in
osteosarcoma, the expression levels of miR-885-5p were analyzed in
85 osteosarcoma samples and adjacent non-cancerous tissue samples
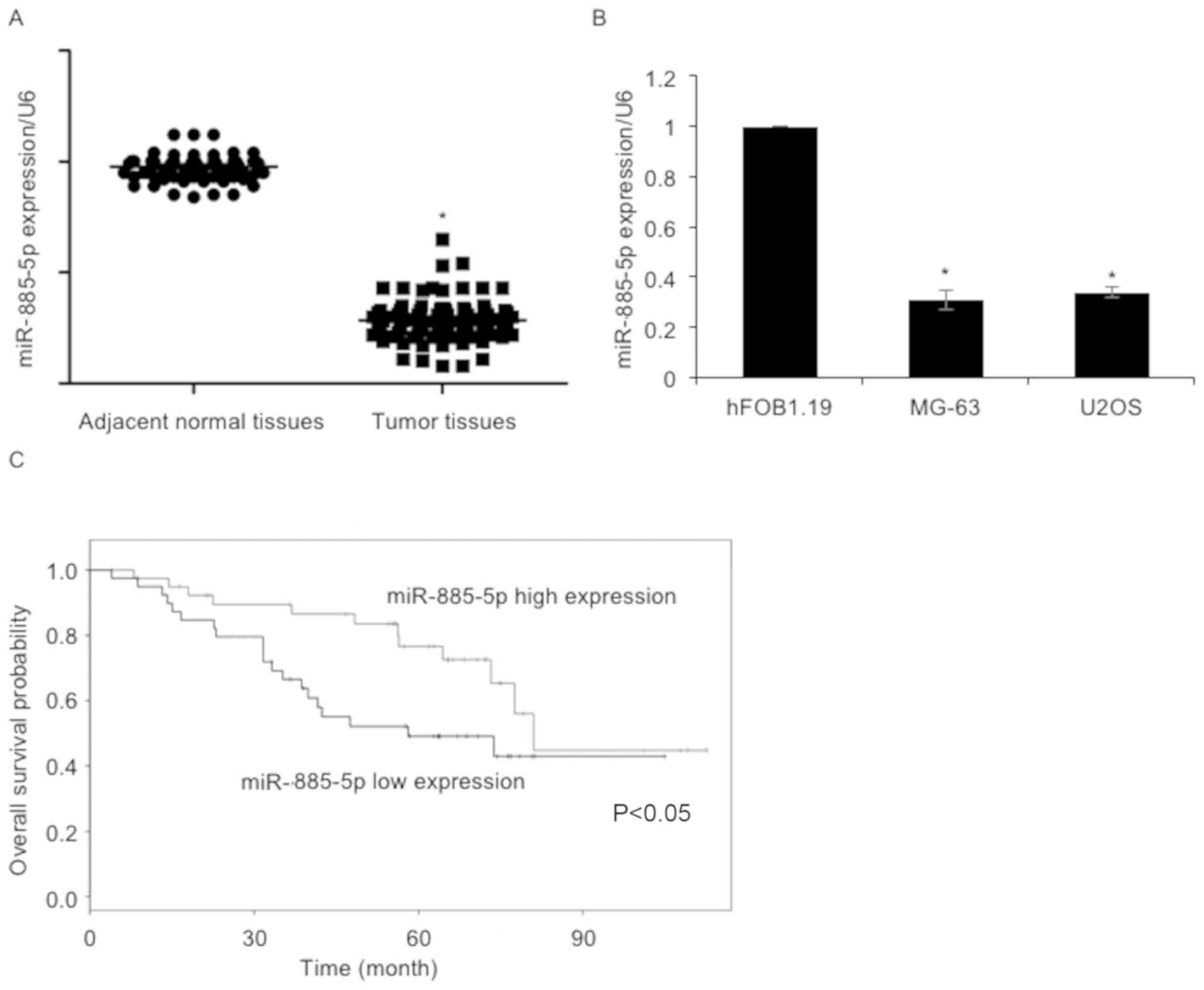
using RT-qPCR. As presented in Fig.
1A, the expression levels of miR-885-5p in osteosarcoma tissue
samples were reduced compared with adjacent non-cancerous tissues.
The expression levels of miR-885-5p in osteosarcoma cell lines,
including MG-63 and U2OS, were also determined. The osteoblast cell
line hFOB1.19 was used as a control group. The results revealed
that miR-885-5p was significantly downregulated in MG-63 and U2OS
cells, compared with hFOB1.19 cells (Fig.
1B). To further decipher the role of miR-885-5p in
osteosarcoma, the association between miR-885-5p expression levels
and patient clinical information was analyzed. The results
suggested that miR-885-5p expression was negatively associated with
tumor size, TNM stage and lymph node metastasis (Table I). Additionally, Kaplan-Meier survival
analysis followed by the log-rank test suggested that patients with
low levels of miR-885-5p had a poorer overall survival compared
with those with high miR-885-5p expression (Fig. 1C). The results of the present study
indicated the importance of miR-885-5p in osteosarcoma.
 | Table I.Clinicopathological variables in 85
patients with osteosarcoma. |
Table I.
Clinicopathological variables in 85
patients with osteosarcoma.
|
|
| miR-855-5p expression
level |
|
|---|
|
|
|
|
|
|---|
| Variable | No. of patients
(n=85) | Low (n=46) | High (n=39) | P-value |
|---|
| Sex |
|
|
| 0.759 |
| Male | 53 | 28 | 25 |
|
|
Female | 32 | 18 | 14 |
|
| Age, years |
|
|
| 0.358 |
|
<18 | 46 | 27 | 19 |
|
| ≥18 | 39 | 19 | 20 |
|
| Tumor size, cm |
|
|
| <0.01 |
|
<5 | 40 | 13 | 27 |
|
| ≥5 | 45 | 33 | 12 |
|
| TNM stage |
|
|
| 0.039 |
| I–II | 42 | 18 | 24 |
|
|
III–IV | 43 | 28 | 15 |
|
| Metastasis |
|
|
| 0.002 |
|
Yes | 44 | 31 | 13 |
|
| No | 41 | 15 | 26 |
|
miR-885-5p targets β-catenin in
osteosarcoma cells
Previous studies have reported that miRNAs may
suppress protein expression by directly binding to RNA. The miRDB
database was used to discover the potential targets of miR-885-5p,
and it was suggested that β-catenin may be a candidate target. To
further verify whether miR-885-5p may regulate β-catenin expression
levels, MG-63 and U2OS cells were transfected with miR-885-5p
mimics or miR-885-5p inhibitors. The expression levels of
miR-885-5p were detected by RT-qPCR, which demonstrated that
miR-885-5p was markedly upregulated or downregulated following
transfection with miR-885-5p mimics or miR-885-5p inhibitors,
respectively (Fig. 2A). Subsequently,
the expression levels of β-catenin were determined by RT-qPCR and
western blotting. The results of the RT-qPCR experiments
demonstrated that the ectopic expression of miR-885-5p in MG-63 and
U2OS cells resulted in the downregulation of β-catenin expression,
whereas inhibition of miR-885-5p led to increased expression levels
of β-catenin (Fig. 2B). Additionally,
the protein expression levels of β-catenin were also reduced
following increased expression of miR-885-5p in MG-63 and U2OS
cells, and were upregulated following the inhibition of miR-885-5p
(Fig. 2C). To further detect the
interaction between miR-885-5p and β-catenin, luciferase reporter
assays were performed. The relative luciferase activity was
decreased upon co-transfection with miR-885-5p mimics and the
β-catenin-3′-UTR plasmid in MG-63 and U2OS cells (Fig. 2D). Subsequently, the expression levels
of three Wnt/β-catenin target genes, c-myc, matrix
metalloproteinase 7 (MMP7) and survivin, were detected following
miR-885-5p overexpression. As presented in Fig. 2E, ectopic expression of miR-885-5p led
to decreased expression levels of c-myc, mmP7 and survivin,
compared with control groups; however, knockdown of miR-885-5p led
to elevated expression levels of c-myc, mmP7 and survivin, compared
with the control groups (Fig. 2E).
These results demonstrated that β-catenin may be the target of
miR-885-5p in osteosarcoma.
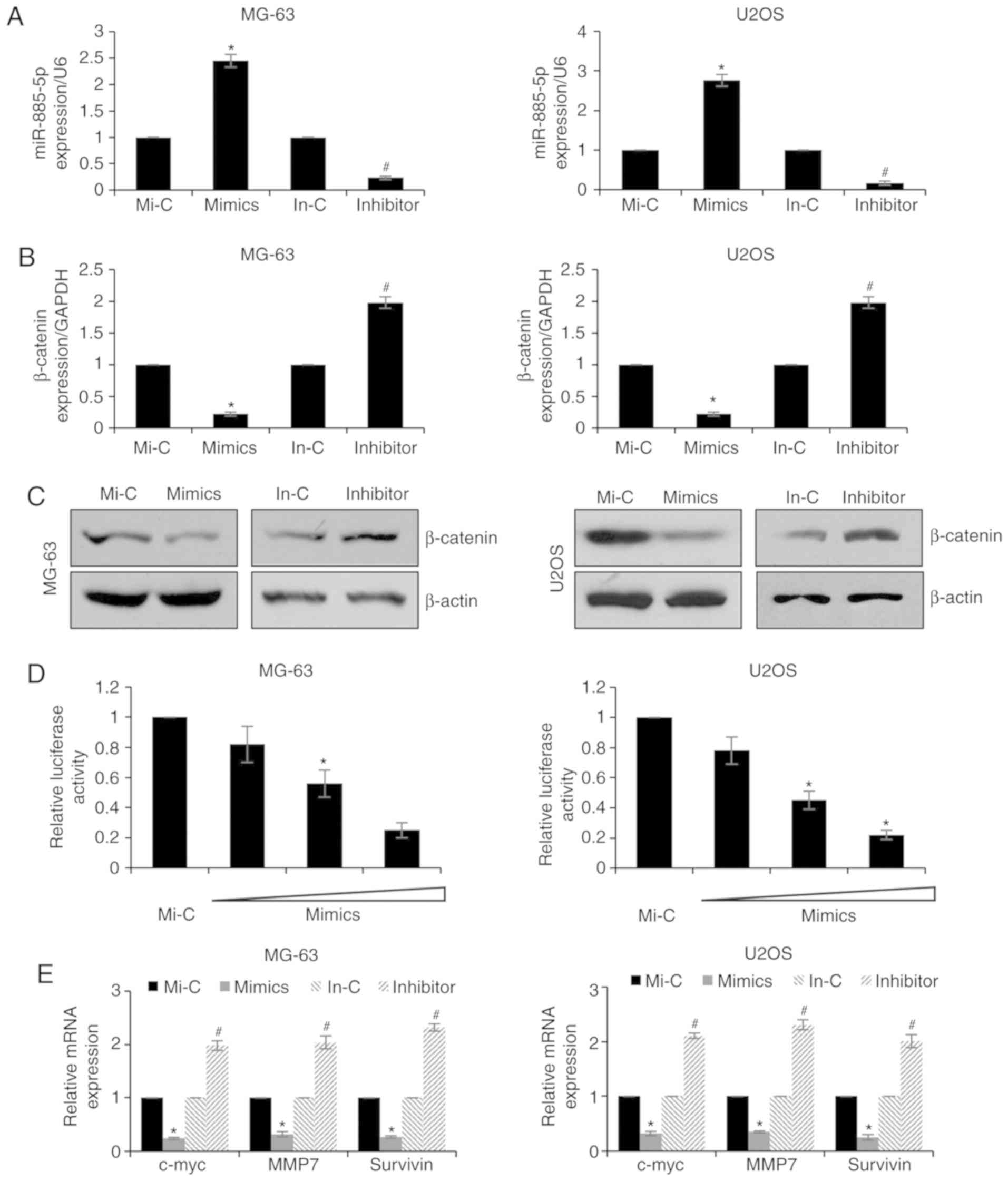 | Figure 2.miR-885-5p targets β-catenin in
osteosarcoma cells. (A) MG-63 and U2OS cells were transfected with
Mi-C, miR-885-5p mimics, In-C or miR-885-5p inhibitor. Following
transfection for 48 h, expression levels of miR-885-5p were
determined by RT-qPCR. (B) MG-63 and U2OS cells were transfected
with Mi-C, miR-885-5p mimics, In-C or miR-885-5p inhibitor. After
transfection for 48 h, the expression of β-catenin was determined
by RT-qPCR and (C) western blotting. (D) MG-63 and U2OS cells were
co-transfected with β-catenin, Renilla and control mimic or
mimics. Following transfection for 24 h, luciferase reporter assay
was performed. (E) MG-63 and U2OS cells were transfected with Mi-C,
miR-885-5p mimics, In-C or miR-885-5p inhibitor. Following
transfection for 48 h, RT-qPCR analysis was performed. *P<0.05
vs. Mi-C; #P<0.05 vs. In-c. miR, microRNA; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; Mi-c,
mimic-control; In-c, inhibitor-control; mmP7, matrix
metalloproteinase 7; c-myc, c-Myc proto-oncogene. |
miR-885-5p inhibits osteosarcoma cell
proliferation through regulation of β-catenin
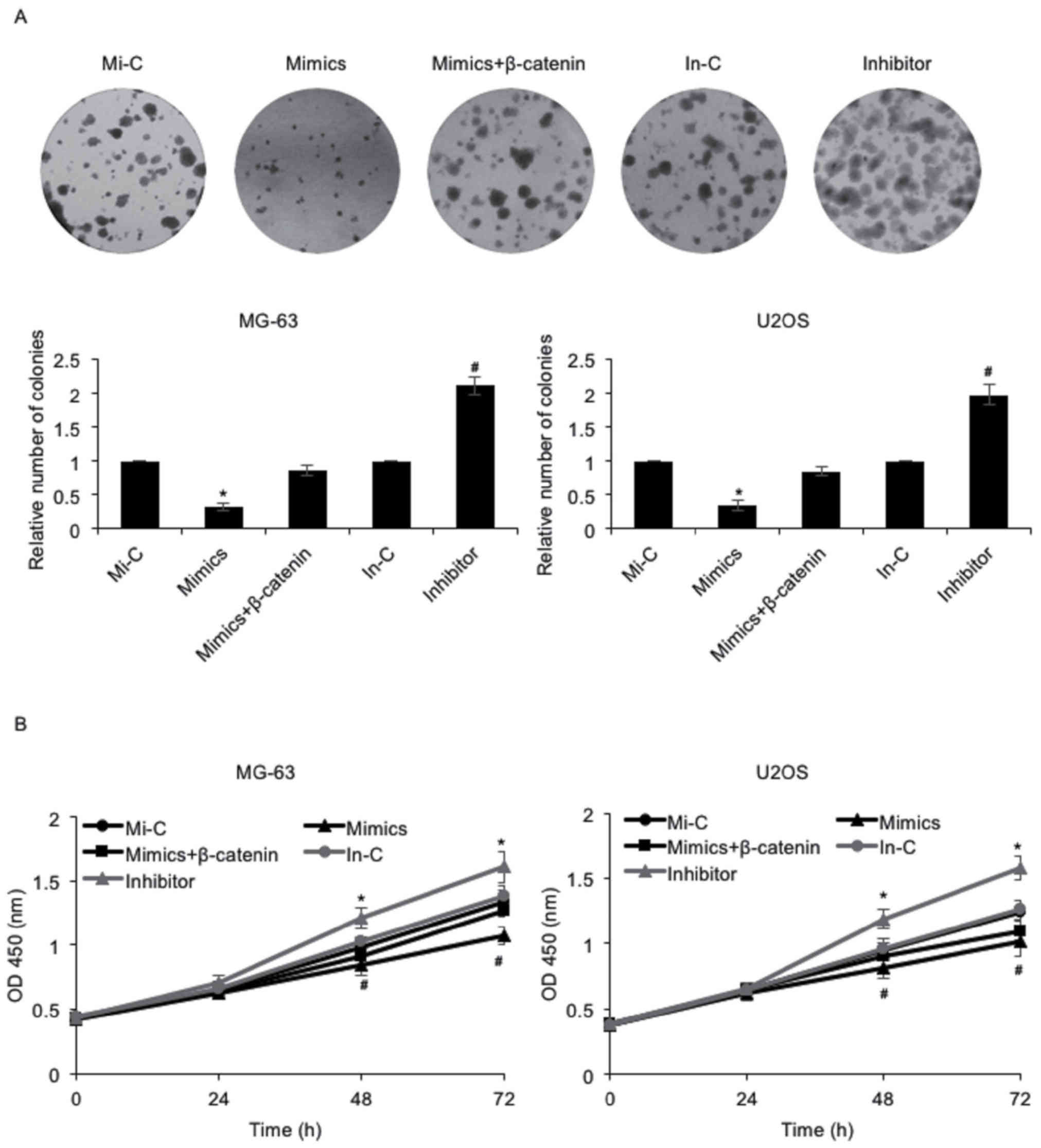
As presented in Table
I, it was reported that miR-885-5p expression was negatively
associated with tumor size. Consequently, the effect of miR-885-5p
on osteosarcoma cell proliferation was investigated using colony
formation and CCK-8 assays. The results of the colony formation
assay indicated that the number of colonies was decreased following
the elevated expression of miR-885-5p in MG-63 and U2OS cells, an
effect that may be offset by simultaneous ectopic expression of
β-catenin; however, the number of colonies was increased when cells
were transfected with miR-885-5p inhibitor (Fig. 3A). Furthermore, the results of the
CCK-8 assay demonstrated that MG-63 cells with miR-885-5p
overexpression grew more slowly compared with those transfected
with Mi-C, an effect that was offset by the simultaneous ectopic
expression of β-catenin (Fig. 3B). By
contrast, MG-63 cells transfected with an miR-885-5p inhibitor grew
more quickly compared with those transfected with an In-C (Fig. 3B). Similar results were observed in
U2OS cells (Fig. 3B). These data
collectively suggested that miR-885-5p may inhibit osteosarcoma
cell proliferation via regulation of β-catenin.
miR-885-5p suppresses the migratory
and invasive capacities of osteosarcoma cells
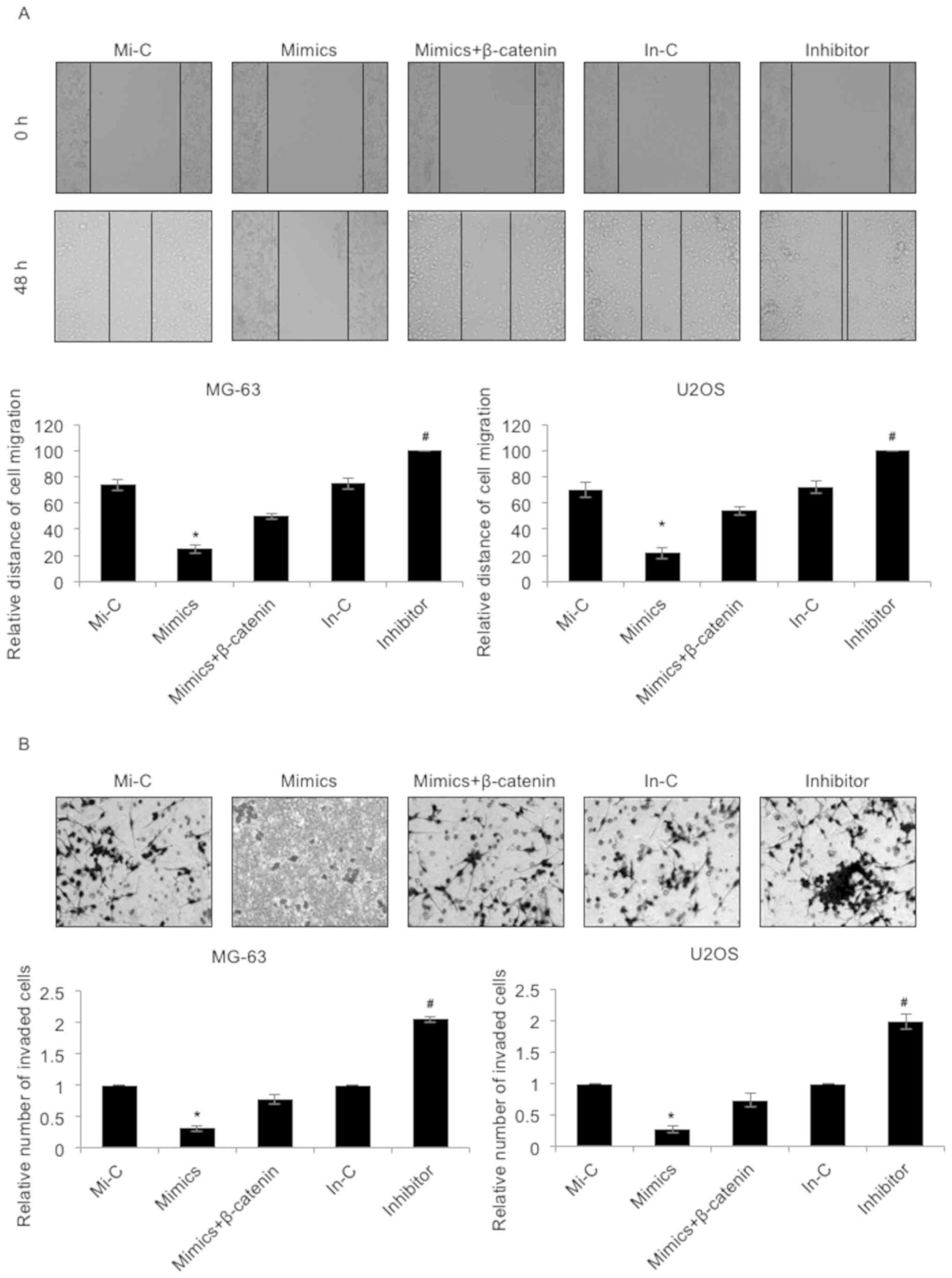
Subsequently, the effect of miR-885-5p on cell
migration and invasion was investigated using a wound healing assay
and a Transwell invasion assay, respectively. As presented in
Fig. 4A, the migratory capacities of
the MG-63 and U2OS cells were markedly downregulated following the
elevated expression of miR-885-5p, an effect that was offset by
simultaneous ectopic expression of β-catenin (Fig. 4A). However, the migratory capacities
of the MG-63 and U2OS cells were notably enhanced when transfected
with miR-885-5p inhibitors (Fig. 4A).
Furthermore, the effect of miR-885-5p on the invasion of
osteosarcoma was evaluated by Transwell invasion assay. The
invasive ability of MG-63 and U2OS cells exhibited a similar cell
migration trend; the invasive capacities of the MG-63 and U2OS
cells were significantly downregulated following the elevated
expression of miR-885-5p, an effect that was offset by the
simultaneous ectopic expression of β-catenin (Fig. 4B). However, the invasive capacities of
the MG-63 and U2OS cells were notably upregulated when transfected
with miR-885-5p inhibitors (Fig. 4B).
These results suggested that miR-885-5p suppressed the migratory
and invasive capacities of osteosarcoma cells.
Discussion
miRNAs have been reported to be dysregulated in a
number of cancer types and have been suggested to serve crucial
roles in tumors by activating oncogenes or suppressing tumor
suppressor genes (5,19,20).
Although previous work demonstrated that miR-885-5p may be
upregulated in osteosarcoma tissues (11), the detailed mechanism of action of
miR-885-5p remains unknown. The present study reported that
miR-885-5p was downregulated in osteosarcoma tissues and cell
lines. The results of the present study are contrary to those of
previous studies, which reported that miR-885-5p was upregulated in
pancreatic cancer and breast cancer (9,10). This
suggests that miR-885-5p may serve alternative functions in
different parts of the human body or in different cancer types.
Furthermore, although a previous report indicated that miR-885-5p
was upregulated in osteosarcoma (11), the number of tissue samples used was
not sufficient. The present study detected the expression levels of
miR-885-5p in osteosarcoma cell lines, demonstrating that
miR-885-5p was downregulated in osteosarcoma tissues and cell
lines.
The Wnt/β-catenin signaling pathway has been
reported to promote cell survival and mobility. Furthermore,
activation of the Wnt/β-catenin signaling pathway improves
resistance to drug-induced apoptosis. Previous reports indicated
that β-catenin inhibition is able to prevent chemoresistance by
suppressing O6-methylguanine-DNA methyltransferase activation
(21). Additionally, miR-320 is able
to suppress β-catenin expression in prostate cancer (22). Ma et al (23) demonstrated that the Wnt/β-catenin and
neurogenic locus notch homolog protein signaling pathways may
regulate the sensitivity of osteosarcoma cells to chemotherapy. The
present study demonstrated that β-catenin may be a target of
miR-885-5p. Additionally, the downstream targets of the
Wnt/β-catenin signaling pathway, including c-myc, mmP7 and
survivin, were also regulated by miR-885-5p. miR-885-5p may
regulate the proliferation, migration and invasion of cancer cells
by targeting β-catenin. However, whether miR-885-5p is able to
suppress osteosarcoma cancer cell proliferation and metastasis
in vivo requires further investigation.
It has been hypothesized that overexpression of
miR-885-5p in osteosarcoma may suppress tumor development through
inhibition of the Wnt/β-catenin signaling pathway. Furthermore,
miR-885-5p has been reported to target cyclin-dependent kinase 2
and minichromosome maintenance complex component 5, activate
cellular tumor antigen p53, and inhibit neuroblastoma proliferation
and survival (24). Although previous
work indicated that miR-885-5p was significantly upregulated in
liver metastasis and colorectal cancer, and activated
epithelial-to-mesenchymal transition (25), it was hypothesized that miR-885-5p may
interact with alternative proteins and serve different functions in
liver metastasis and colorectal cancer.
In summary, the present study revealed that
miR-885-5p was downregulated in osteosarcoma tissues and cell
lines, and downregulation of miR-885-5p was closely associated with
tumor size, TNM stage and lymph node metastasis. Furthermore,
downregulation of miR-885-5p predicted a poor prognosis. In
osteosarcoma cells, miR-885-5p suppressed cell proliferation,
migration and invasion through inhibition of β-catenin, a key
component of the Wnt signaling pathway. Thus, miR-885-5p may be a
potential therapeutic target for the treatment of osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and GCH conceived and designed the work. YL, GCH,
ZLB, and WQT constructed expression plasmids, prepared proteins and
performed experiments. YL, GCH, ZLB, and WQT analyzed the data.
ZLB, YL and GCH wrote the paper. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All patients provided their consent and all human
tissue experiments were approved by the Ethics Committee of
Jingjiang Hospital of Chinese Medicine (Jingjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salah S, Ahmad R, Sultan I, Yaser S and
Shehadeh A: Osteosarcoma with metastasis at initial diagnosis:
Current outcomes and prognostic factors in the context of a
comprehensive cancer center. Mol Clin Oncol. 2:811–816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Subbiah V and Kurzrock R: Phase 1 clinical
trials for sarcomas: The cutting edge. Curr Opin Oncol. 23:352–360.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toiyama Y, Takahashi M, Hur K, Nagasaka T,
Tanaka K, Inoue Y, Kusunoki M, Boland CR and Goel A: Serum miR-21
as a diagnostic and prognostic biomarker in colorectal cancer. J
Natl Cancer Inst. 105:849–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng X, Wu J, Pan C, Wang H, Ying X, Zhou
Y, Yu H, Zuo Y, Pan Z, Liu RY and Huang W: Genetic and epigenetic
down-regulation of microRNA-212 promotes colorectal tumor
metastasis via dysregulation of MnSOD. Gastroenterology.
145:426–436, e421-e426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cekaite L, Rantala JK, Bruun J, Guriby M,
Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe
RA and Skotheim RI: miR-9, −31, and −182 deregulation promote
proliferation and tumor cell survival in colon cancer. Neoplasia.
14:868–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schultz NA, Dehlendorff C, Jensen BV,
Bjerregaard JK, Nielsen KR, Bojesen SE, Calatayud D, Nielsen SE,
Yilmaz M, Holländer NH, et al: MicroRNA biomarkers in whole blood
for detection of pancreatic cancer. JAMA. 311:392–404. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang Y, Lu DL, Li JP, Yu CX, Zheng DL,
Huang X, Wang ZY, Hu P, Liao XH and Zhang TC: Myocardin inhibits
estrogen receptor alpha-mediated proliferation of human breast
cancer MCF-7 cells via regulating MicroRNA expression. IUBMB Life.
68:477–487. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Wang D, Xiong J, Chen L and Huang
J: MicroRNA-33a-5p suppresses growth of osteosarcoma cells and is
downregulated in human osteosarcoma. Oncol Lett. 10:2135–2141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai Y, Cai T and Chen Y: Wnt pathway in
osteosarcoma, from oncogenic to therapeutic. J Cell Biochem.
115:625–631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mora-Blanco EL, Mishina Y, Tillman EJ, Cho
YJ, Thom CS, Pomeroy SL, Shao W and Roberts CW: Activation of
beta-catenin/TCF targets following loss of the tumor suppressor
SNF5. Oncogene. 33:933–938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YJ, Wei ZM, Meng YX and Ji XR:
Beta-catenin up-regulates the expression of cyclinD1, c-myc and
mmP-7 in human pancreatic cancer: Relationships with carcinogenesis
and metastasis. World J Gastroenterol. 11:2117–2123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trierweiler C, Blum HE and Hasselblatt P:
The transcription factor c-Jun protects against liver damage
following activated beta-catenin signaling. PLoS One. 7:e406382012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muroya S, Taniguchi M, Shibata M, Oe M,
Ojima K, Nakajima I and Chikuni K: Profiling of differentially
expressed microRNA and the bioinformatic target gene analyses in
bovine fast- and slow-type muscles by massively parallel
sequencing. J Anim Sci. 91:90–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao XH, Chen M, Wang Y, Cui PG, Liu SB and
Xu ZY: MicroRNA-21 regulates the ERK/NF-kappaB signaling pathway to
affect the proliferation, migration, and apoptosis of human
melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol
Carcinog. 56:886–894. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bing L, Hong C, Li-Xin S and Wei G:
MicroRNA-543 suppresses endometrial cancer oncogenicity via
targeting FAK and TWIST1 expression. Arch Gynecol Obstet.
290:533–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wickstrom M, Dyberg C, Milosevic J, Einvik
C, Calero R, Sveinbjörnsson B, Sandén E, Darabi A, Siesjö P, Kool
M, et al: Wnt/beta-catenin pathway regulates mgMT gene expression
in cancer and inhibition of Wnt signalling prevents
chemoresistance. Nat Commun. 6:89042015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs
JJ, Gitelis S, O'Keefe RJ, Konttinen YT, Yin G and Li TF:
Inhibition of the Wnt-beta-catenin and Notch signaling pathways
sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res
Commun. 431:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Afanasyeva EA, Mestdagh P, Kumps C,
Vandesompele J, Ehemann V, Theissen J, Fischer M, Zapatka M, Brors
B, Savelyeva L, et al: MicroRNA miR-885-5p targets CDK2 and MCM5,
activates p53 and inhibits proliferation and survival. Cell Death
Differ. 18:974–984. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lam CS, Ng L, Chow AK, Wan TM, Yau S,
Cheng NS, Wong SK, Man JH, Lo OS, Foo DC, et al: Identification of
microRNA 885-5p as a novel regulator of tumor metastasis by
targeting CPEB2 in colorectal cancer. Oncotarget. 8:26858–26870.
2017. View Article : Google Scholar : PubMed/NCBI
|