Introduction
Glioblastoma is a common primary tumor in the
central nervous system, with a 5-year survival rate of below 3%
(1). Glioblastoma is characterised by
rapid growth, high invasion, and strong resistance to radiotherapy
and chemotherapy (2). Although great
progress has been made with the use of modern treatments including
surgery, radiotherapy, and chemotherapy, the survival time of
patients with glioblastoma remains short (3). Therefore, it is necessary to identify
new effective target genes to suppress the development of
glioblastoma.
MicroRNAs (miRNAs/miRs) have been reported to
influence the post-transcriptional reaction by binding with the
3′-UTR of the corresponding genes to result in translational
suppression or degradation (4).
Moreover, increasing miRNAs have been reported to control
tumorigenesis, including cell growth, migration, invasion,
differentiation and apoptosis (5–7).
Additionally, the function of miRNAs is different in various types
of cancer, such as lung adenocarcinoma, osteosarcoma and
nasopharyngeal carcinoma (8–10). Some miRNAs act as tumor suppressors
while others as oncogenes. Many miRNAs have been found to regulate
the progression of glioblastoma. For instance, miR-137 inhibited
the growth of glioblastoma through suppressing EGFR (11). miR-520c inhibited glioma cell
migration and invasion by suppressing TGFBRII (12). miR-595 contributed to cell
proliferation in human glioblastoma by regulating SOX7 expression
(13). Among them, only a slight
effect of miR-7 on glioblastoma has been identified (14,15). In
addition, miR-7 with inhibitory effect has been identified in
various types of cancer, such as colon cancer (16), pancreatic carcinoma (17), non-small cell lung cancer (18) and thyroid papillary cancer (19). Although the effect of miR-7 has been
confirmed repeatedly, the regulatory mechanism of the miR-7/special
AT rich sequence binding protein 1 (SATB1) axis has not been
previously clarified.
This study focused on the change of miR-7-5p
expression in glioblastoma. Moreover, the function of miR-7-5p for
the migration and invasion of glioblastoma was determined. We found
that miR-7-5p repressed cell mobility and invasiveness through
regulation of SATB1 in glioblastoma. These observations may lead to
a new approach in the treatment of glioblastoma.
Materials and methods
Clinical samples and cell culture
Forty-eight glioma specimens and adjacent tissues
were taken from the Yantai Yuhuangding Hospital (Yantai, China).
The patients received no treatment other than surgery, and all
participants provided written informed consent. The tissue was
frozen in liquid nitrogen and then stored at −80°C in a
refrigerator until use. Human glioblastoma cell lines U87 MG ATCC
(male malignant gliomas, ATCC HTB-14), whose origin is unknown, and
U373 MG ATCC (male malignant gliomas, ATCC HTB-17) as mixed
astrocytoma cells were purchased from the ATCC and cultured in DMEM
supplemented with 10% fetal bovine serum (FBS). Normal human
astrocytes (NHAs) were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA). Although the U373 and U87 cells
have been reported to be contaminated or misidentified (20–22), the
use of U373 and U87 cells in this study did not affect the outcomes
of this research. This experiment was approved by the Institutional
Ethics Committee of Yantai Yuhuangding Hospital.
Cell transfection
The miR-7-5p mimic and inhibitor, SATB1 siRNA were
obtained from GenePharma (Shanghai, China) and were transferred
into U87 or U373 cells with Lipofectamine 2000 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturers'
protocols.
RNA extraction and RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA. RT-qPCR was
carried out through the SYBR PCR Master Mix on an ABI PRISM 7900
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) to detect the expressions of miR-7-5p and SATB1.
U6 or GAPDH served as control for miR-7-5p or SATB1. The following
primers were used: miR-7 forward, 5′-AAAACTGCTGCCAAAACCAC-3′ and
reverse, 5′-GCTGCATTTTACAGCGACCAA-3′; SATB1 forward,
5′-CACAGAGGTGTCTTCCGAAATCTA-3′ and reverse,
5′-AAAGCAAGCCCTGAGTTCTGTTA-3′; GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. Amplification reaction protocol was
performed for 35 cycles consisting of 94°C for 45 sec, 95°C for 15
sec, 60°C for 1 min. The miR-7-5p or SATB1 levels were analyzed
using the 2−ΔΔCq method (23).
Dual luciferase assay
The wild-type (wt) 3′-UTR of SATB1 or mutant (mut)
3′-UTR of SATB1 were inserted into the pGL3 promoter vector (Sangon
Biotech, Shanghai, China) for luciferase reporter experiments. The
vector and miR-7-5p mimic were transfected into cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
and 24 h after transfection, Dual-Luciferase Reporter Assay system
(Beyotime Institute of Biotechnology, Beijing, China) was applied
to measure luciferase activity for 30 min.
Transwell migration and invasion
assay
Transwell chambers (Corning Inc., Corning, NY, USA)
were applied to evaluate the migratory and invasive ability of
glioblastoma cells. Transfected cells (5×104) without
FBS were placed in the top chamber on the non-coated membrane, and
then the lower chamber filled with 20% FBS to induce transfected
cells to migrate or invade through the membrane. The cells were
placed in the upper chamber with the coated membrane for the
invasion assay, and were incubated for the migration and invasion
assay for 48 h. The cells were then stained with crystal violet
(Beyotime Institute of Biotechnology) at 37°C for 30 min. The cells
(magnification, ×200) were imaged at random using an inverted
microscope (Olympus Corporation, Tokyo, Japan).
Western blot analysis
Protein samples were obtained using RIPA buffer.
Proteins were separated with 10% SDS-PAGE and then incubated with
5% non-fat milk blocked membranes at room temperature. Next we
incubated the PVDF membranes overnight at 4°C with anti-SATB1
(dilution 1:1,000; rabbit polyclonal; cat. no. ab70004; Abcam,
Cambridge, MA, USA), anti-GAPDH (dilution 1:1,000; mouse
monoclonal; cat. no. 60004-1-Ig; ProteinTech, Wuhan, China) and
subsequently incubated with goat anti-rabbit IgG H&L (HRP)
(dilution 1:3,000; cat. no. ab6721; Abcam) secondary antibody. The
25 µl protein sample was added in the protein loaded per lane.
Protein concentration was calculated using bicinchoninic acid (BCA;
Beyotime Institute of Biotechnology, Shanghai, China). Then,
protein expression levels were measured by ECL detecting system
(Thermo Fisher Scientific, Inc.) using Bio-Rad Image-Lab software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ±
standard deviation. Statistical analyses between two groups were
performed by Student's t-test. Differences among groups were tested
by one-way analysis of variance following by a Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Downregulation of miR-7-5p in human
glioblastoma cell lines and tissues
In order to better understand the regulation process
of miR-7-5p in glioblastoma pathogenesis, we firstly identified
miR-7-5p expression in glioblastoma tissues and cell lines. The
observations showed that miR-7-5p expression in glioblastoma
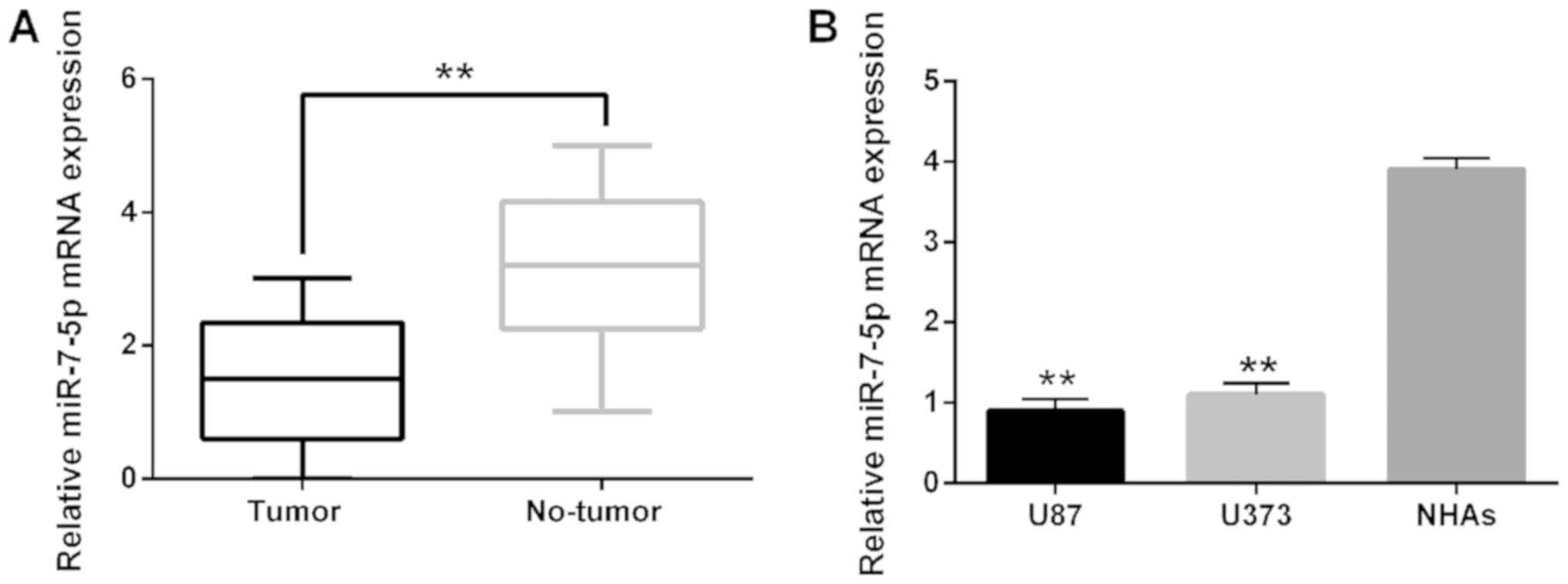
tissues was much lower than that of normal tissues (Fig. 1A). The decreased expression of
miR-7-5p was also identified in U87 and U373 cell lines (Fig. 1B). All these findings indicated that
abnormal expression of miR-7-5p may be related to the progression
of glioblastoma.
Cell migration and invasion of
glioblastoma are inhibited by miR-7-5p
To further explore the effect of miR-7-5p in
glioblastoma, miR-7-5p mimic or inhibitor was transfected into U87
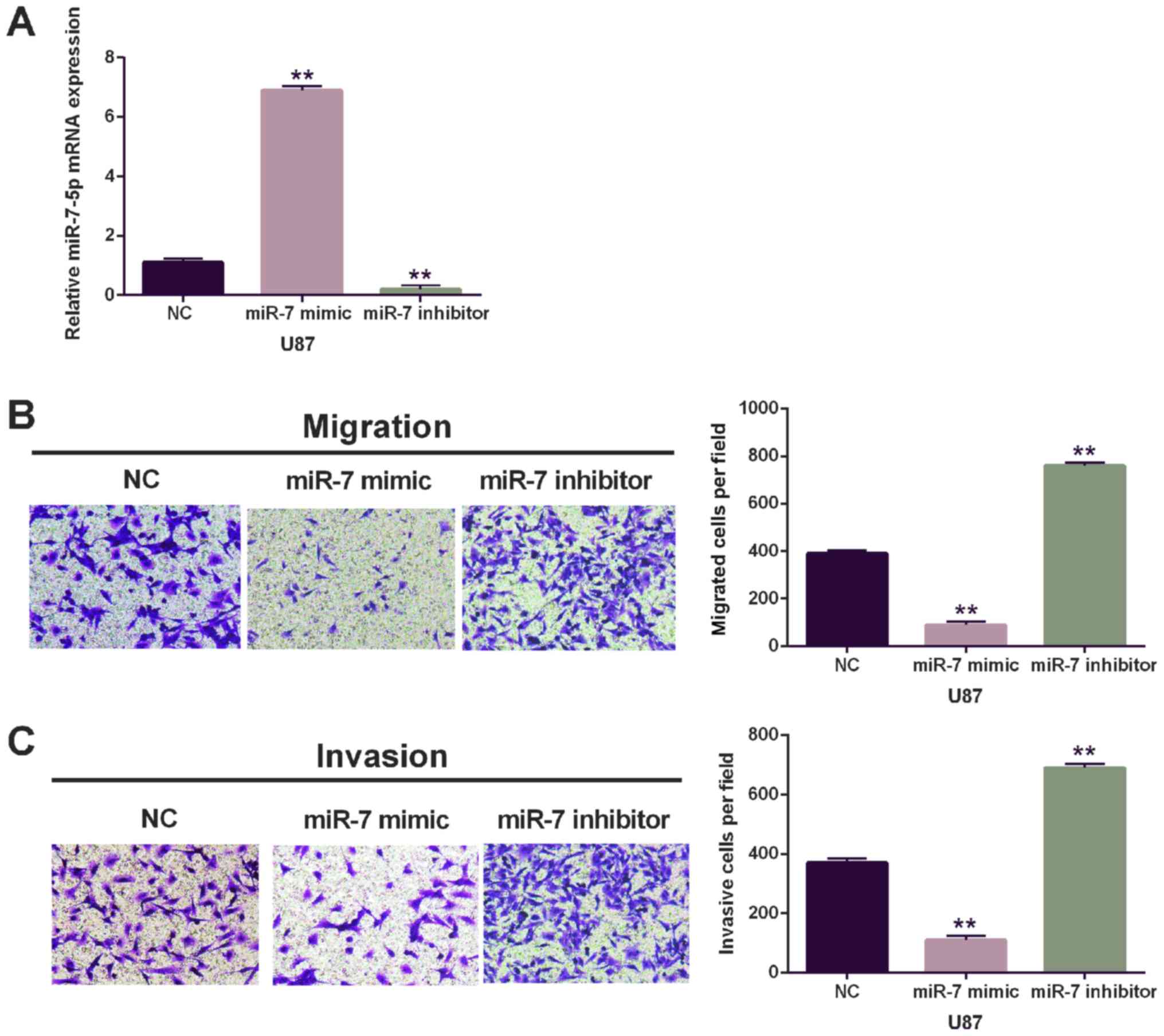
cells. The efficiency of miR-7-5p expression was detected via
RT-qPCR (Fig. 2A). Moreover, the
Transwell assay showed that the migration and invasion in cells
with miR-7-5p mimics were lower than that of miR-NC group (Fig. 2B). On the contrary, the cells with
miR-7-5p inhibitor were higher than that of miR-In-NC for migration
and invasion (Fig. 2C). The data
revealed that miR-7-5p was a tumor suppressive miRNA for
glioblastoma through inhibition of cell migration and invasion.
SATB1 was the direct target of
miR-7-5p in glioblastoma
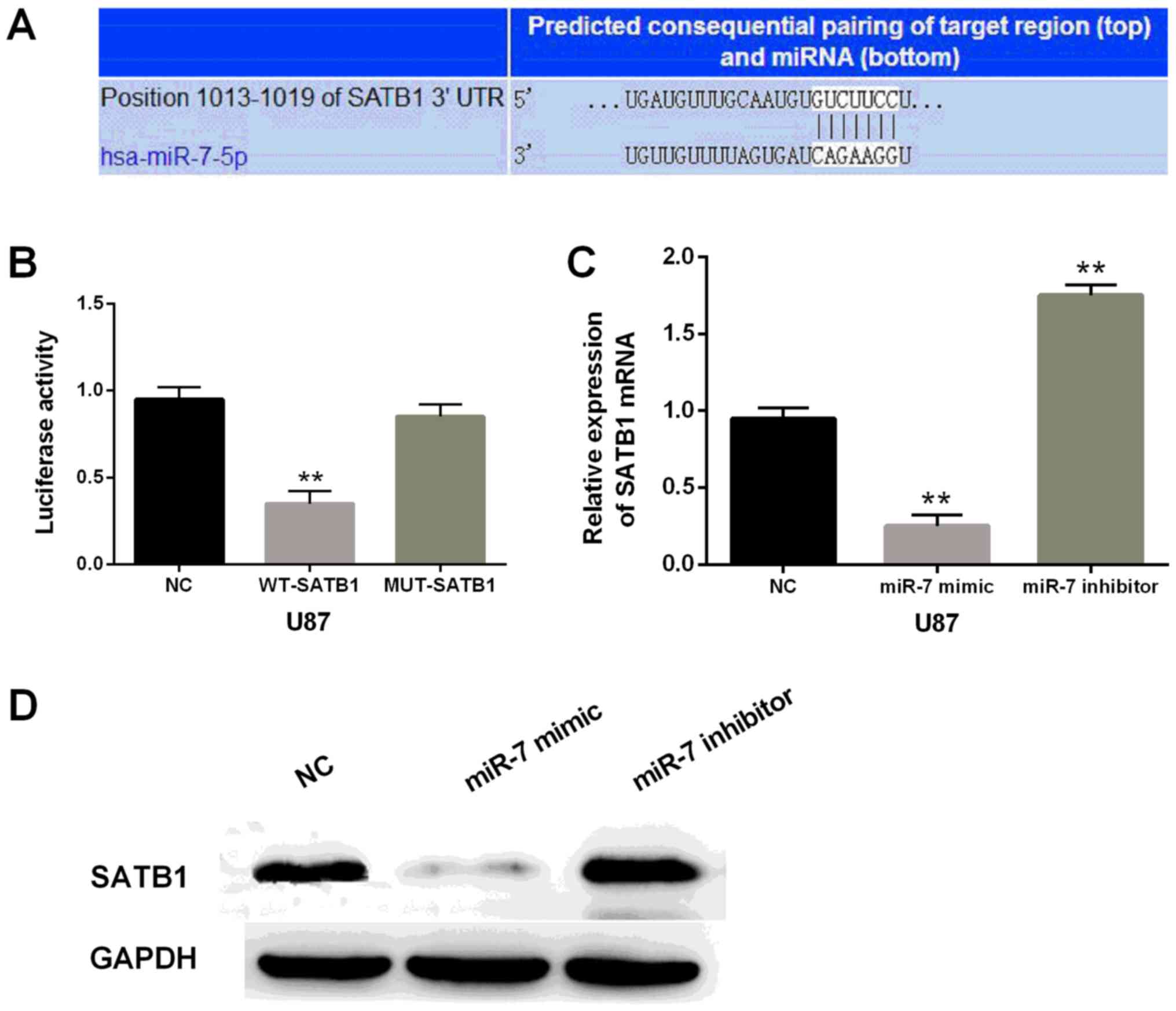
Through TargetScan database (http://www.targetscan.org/vert_71/), SATB1 was
identified as one of the target genes of miR-7-5p (Fig. 3A). Moreover, we performed the
luciferase reporter assay to verify that miR-7-5p directly targeted
SATB1. Luciferase activity in the cells containing miR-7-5p mimics
and the wild-type of SATB1 was significantly reduced (P<0.01) in
comparison with the control. Interestingly, there was almost no
change in cells containing miR-7-5p mimic and mutant SATB1
(Fig. 3B). According to the
luciferase reporter assay, we deduced that miR-7-5p directly
targeted SATB1 in glioblastoma. The SATB1 expression of U87 cells
containing miR-7-5p mimics or inhibitor was also detected.
Apparently, the mRNA and protein expression levels of SATB1 were
significantly reduced in U87 cells with miR-7-5p mimics and
increased in cells containing miR-7-5p inhibitor in comparison with
the control (Fig. 3C and D). In
brief, miR-7-5p overexpression inhibited SATB1 expression.
Overexpression of SATB1 inversely
reversed the suppressive effect of miR-7-5p in glioblastoma
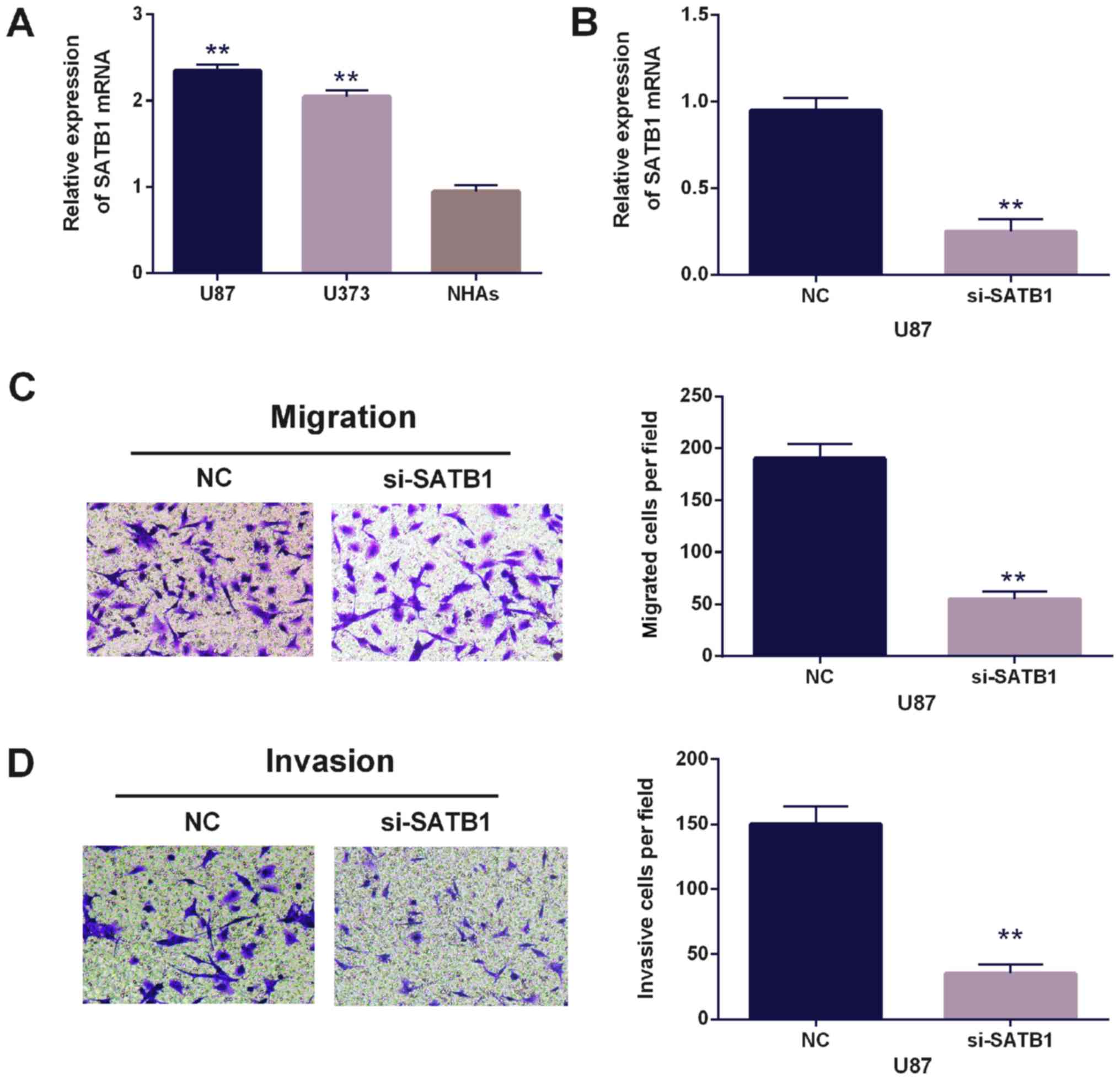
To further confirm the observation that SATB1
promoted cell migration and invasion in glioblastoma, SATB1 siRNA
was used for this investigation. Additionally, the expression of
SATB1 was found to be higher in U87 and U373 cell lines than NHA
cells (the control, Fig. 4A). SATB1
expression in U87 cells with SATB1 siRNA was decreased vs. the
control (Fig. 4B). Migration and
invasion were also impaired following SATB1 siRNA expression
(Fig. 4C and D). The findings
indicated that upregulation of SATB1 in glioblastoma promoted cell
migration and invasion in glioblastoma.
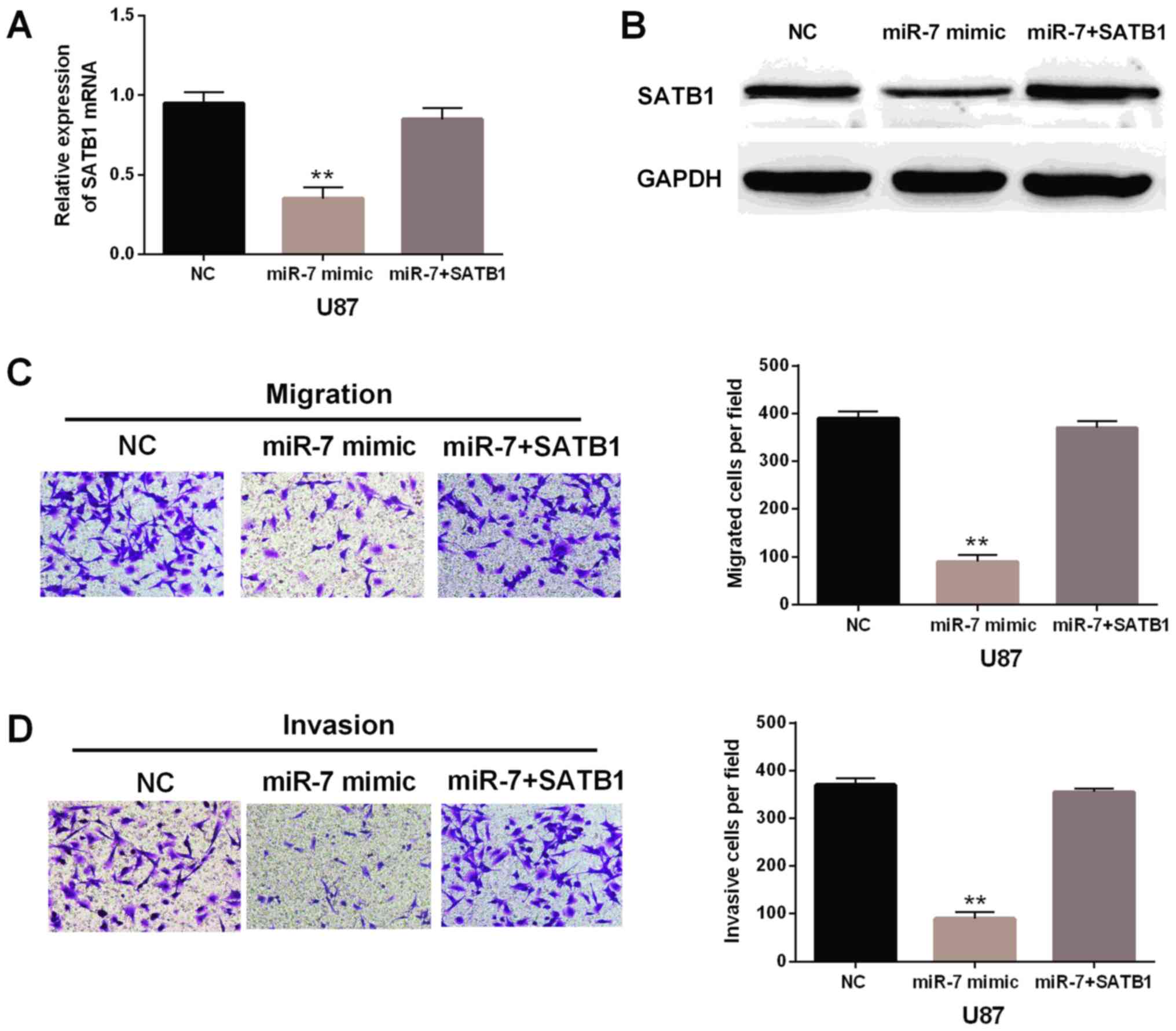
Overexpression of SATB1 was speculated to inversely
reverse the suppressive effect of miR-7-5p. To confirm that, the
negative control or SATB1 expression vector was transfected into
U87 cells which the miR-7-5p overexpressed. The mRNA and protein
expression levels of SATB1 were decreased in cells with miR-7-5p
mimic, whereas little change for SATB1 expression was found in
cells with SATB1 plasmid and miR-7-5p mimic compared with the
control group (Fig. 5A and B).
Importantly, SATB1 overexpression was identified to weaken the
inhibitory effect of miR-7-5p on cell migration and invasion in U87
cells (Fig. 5C and D). These results
indicated that miR-7-5p suppressed cell migration and invasion in
glioblastoma through downregulation of SATB1. The results may
indicate the potential to affect tumorigenesis of glioblastoma.
Discussion
Glioblastoma was reported to develop rapidly and
aggressively accounting for 15.4% of all primary brain tumors and
60–75% of astrocytoma (24). Although
marked progress has been made in understanding the molecular
mechanisms underlying the progression of gliomas, no targeted agent
has exhibited excellent effects on patient prognosis. Therefore, it
is imperative to further understand the pathogenesis of
glioblastoma and to develop new targets for effective
treatment.
In the present study, it was found that miR-7-5p was
downregulated in glioblastoma tissues and cell lines. The
overexpression of miR-7-5p inhibited cell migration and invasion of
glioblastoma, whereas the downregulation of miR-7-5p had the
opposite effect. Moreover, SATB1 was confirmed as a direct target
of miR-7-5p. SATB1 was identified to function as an oncogene in
glioblastoma. Importantly, the interaction between SATB1 and
miR-7-5p was also detected. It indicated that overexpression of
SATB1 could impair the suppressive function of miR-7-5p for cell
motility in glioblastoma. In brief, miR-7-5p inhibited cell
migration and invasion of glioblastoma through the suppression of
SATB1 expression.
There is increasing evidence that miR-7 makes a
contribution to the development of human cancers. Above all, Kefas
et al demonstrated that miR-7 was downregulated and
inhibited the EGFR and the Akt pathway in glioblastoma (25), and miR-7 interfered with the pathways
of PI3K/ATK and Raf/MEK/ERK at the same time to inhibit
glioblastoma growth (26).
Furthermore, miR-7-5p was found to inhibit vascular endothelial
cell proliferation by suppressing RAF1 and be downregulated in
glioblastoma microvasculature (27).
Regarding cell motility, it was reported that the migration and
invasion of glioblastoma cells was inhibited by miR-15b (28), miR-154-5p (29), miR-204 (30) and miR-520c (12) through regulation of IGF1R, PIWIL1,
ATF2 and TGFBRII expression. Nevertheless, whether miR-7-5p
regulated the cell migration and invasion in glioblastoma was
unclear. Our findings indicated that miR-7-5p acted as tumor
suppressor in glioblastoma which was similar to previous findings
and inhibited the cell migration and invasion as well. Furthermore,
miR-7-5p was found to negatively regulate SATB1 expression by
binding with its 3′-UTR.
SATB1, a global genome organizer, was reported to be
upregulated in renal cell carcinoma (31), osteosarcoma (32) and bladder cancer (33). Han et al demonstrated that
SATB1 was involved in the progression and prognosis of glioma
(34). Chu et al proved that
overexpression of SATB1 was related to the development and
progression of glioma (35).
Moreover, it was found that SATB1 was repressed by miR-7 in breast
cancer (36). In the present study,
we found a relationship between miR-7-5p and SATB1 in glioblastoma.
Overexpression of SATB1 could weaken the inhibitory effect of
miR-7-5p on cell migration and invasion in glioblastoma. Our
observation revealed that SATB1 acts as a positive regulator for
tumorigenesis of glioblastoma.
In conclusion, results of the current study
demonstrated that miR-7-5p was downregulated and overexpression of
miR-7-5p inhibited cell migration and invasion in glioblastoma.
Moreover, miR-7-5p inhibited cell migration and invasion in
glioblastoma by suppressing SATB1 expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYY contributed to the study design, data
acquisition and analysis and drafted the manuscript. WK contributed
significantly to data analysis and manuscript preparation. JJ
performed the data analyses. HX assisted in the performance of the
analysis with constructive discussions. WZ contributed to the
conception of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yantai Yuhuangding Hospital (Yantai, China). Signed informed
consent was obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bradshaw A, Wickremsekera A, Tan ST, Peng
L, Davis PF and Itinteang T: Cancer stem cell hierarchy in
glioblastoma multiforme. Front Surg. 3:212016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khosla D: Concurrent therapy to enhance
radiotherapeutic outcomes in glioblastoma. Ann Transl Med.
4:542016.PubMed/NCBI
|
|
3
|
Franceschi S, Mazzanti CM, Lessi F,
Aretini P, Carbone FG, LA Ferla M, Scatena C, Ortenzi V, Vannozzi
R, Fanelli G, et al: Investigating molecular alterations to profile
short- and long-term recurrence-free survival in patients with
primary glioblastoma. Oncol Lett. 10:3599–3606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai MM, Wang CS, Tsai CY, Huang HW, Chi
HC, Lin YH, Lu PH and Lin KH: Potential diagnostic, prognostic and
therapeutic targets of microRNAs in human gastric cancer. Int J Mol
Sci. 17(pii): E9452016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dallaire A and Simard MJ: The implication
of microRNAs and endo-siRNAs in animal germline and early
development. Dev Biol. 416:18–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei S, Zhang ZY, Fu SL, Xie JG, Liu XS, Xu
YJ, Zhao JP and Xiong WN: Hsa-miR-623 suppresses tumor progression
in human lung adenocarcinoma. Cell Death Dis. 8:e28292017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gui ZL, Wu TL, Zhao GC, Lin ZX and Xu HG:
MicroRNA-497 suppress osteosarcoma by targeting MAPK/Erk pathway.
Bratisl Lek Listy. 118:449–452. 2017.PubMed/NCBI
|
|
10
|
Wu RS, Qiu EH, Zhu JJ, Wang JR and Lin HL:
MiR-101 promotes nasopharyngeal carcinoma cell apoptosis through
inhibiting Ras/Raf/MEK/ERK signaling pathway. Eur Rev Med Pharmacol
Sci. 22:150–157. 2018.PubMed/NCBI
|
|
11
|
Zhang Z, Song X, Tian H, Miao Y, Feng X,
Li Y and Wang H: MicroRNA-137 inhibits growth of glioblastoma
through EGFR suppression. Am J Transl Res. 9:1492–1499.
2017.PubMed/NCBI
|
|
12
|
Hu S, Chen H, Zhang Y, Wang C, Liu K, Wang
H and Luo J: MicroRNA-520c inhibits glioma cell migration and
invasion by the suppression of transforming growth factor-β
receptor type 2. Oncol Rep. 37:1691–1697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao Y, Zhang S, Sun S, Zhu J and Xiao Y:
miR-595 targeting regulation of SOX7 expression promoted cell
proliferation of human glioblastoma. Biomed Pharmacother.
80:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Babae N, Bourajjaj M, Liu Y, Van Beijnum
JR, Cerisoli F, Scaria PV, Verheul M, Van Berkel MP, Pieters EH,
Van Haastert RJ, et al: Systemic miRNA-7 delivery inhibits tumor
angiogenesis and growth in murine xenograft glioblastoma.
Oncotarget. 5:6687–6700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Zhang X, Hu S, Zheng M, Zhang J,
Zhao J, Zhang X, Yan B, Jia L, Zhao J, et al: Identification of
miRNA-7 by genome- wide analysis as a critical sensitizer for
TRAIL-induced apoptosis in glioblastoma cells. Nucleic Acids Res.
45:5930–5944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng CY, Zhan YS, Huang J and Chen YX:
MicroRNA-7 suppresses human colon cancer invasion and proliferation
by targeting the expression of focal adhesion kinase. Mol Med Rep.
13:1297–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bi Y, Shen W, Min M and Liu Y: MicroRNA-7
functions as a tumor-suppressor gene by regulating ILF2 in
pancreatic carcinoma. Int J Mol Med. 39:900–906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Q, Mao ZD, Shi YJ, Chen Y, Sun Y,
Zhang Q, Song L and Peng LP: MicroRNA-7 inhibits cell
proliferation, migration and invasion in human non-small cell lung
cancer cells by targeting FAK through ERK/MAPK signaling pathway.
Oncotarget. 7:77468–77481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hua K, Jin J, Zhang H, Zhao B, Wu C, Xu H
and Fang L: MicroRNA-7 inhibits proliferation, migration and
invasion of thyroid papillary cancer cells via targeting CKS2. Int
J Oncol. 49:1531–1540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grens K: Popular tumor cell line
contaminated. The Scientist, Midland, Ontario, 2016. https://www.the-scientist.com/?articles.view/articleNo/46929/title/Popular-Tumor-Cell-Line-Contaminated/Updated.
August 31–2016
|
|
21
|
Gasteiger E: ExPASy Bioinformatics
Resource Portal. 2003, https://web.expasy.org/cellosaurus/CVCL_2219
|
|
22
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Short SC: Survival from brain tumors in
England and Wales up to 2001. Br J Cancer. 99 Suppl 1:S102–S103.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S and
Purow B: MicroRNA-7 inhibits the epidermal growth factor receptor
and the AKT pathway and is down-regulated in glioblastoma. Cancer
Res. 68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu
S, Yu S and Liu X: miR-7 inhibits glioblastoma growth by
simultaneously interfering with the PI3K/ATK and RAF/MEK/ERK
pathways. Int J Oncol. 44:1571–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Liu Y, Li L, Xu Z, Bi B, Wang Y and
Li JY: miR-7-5p is frequently downregulated in glioblastoma
microvasculature and inhibits vascular endothelial cell
proliferation by targeting RAF1. Tumor Biol. 35:10177–10184. 2014.
View Article : Google Scholar
|
|
28
|
Wang J, Liu H, Tian L, Wang F, Han L,
Zhang W and Bai YA: miR-15b inhibits the progression of
glioblastoma cells through targeting insulin-like growth factor
receptor 1. Horm Cancer. 8:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Sun S, Tong X, Ma Q, Di H, Fu T,
Sun Z, Cai Y, Fan W, Wu Q, et al: miRNA-154-5p inhibits cell
proliferation and metastasis by targeting PIWIL1 in glioblastoma.
Brain Res. 1676:69–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song S, Fajol A, Tu X, Ren B and Shi S:
miR-204 suppresses the development and progression of human
glioblastoma by targeting ATF2. Oncotarget. 7:70058–70065. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv C, Bai Z, Liu Z, Luo P and Zhang J:
MicroRNA-495 suppresses human renal cell carcinoma malignancy by
targeting SATB1. Am J Transl Res. 7:1992–1999. 2015.PubMed/NCBI
|
|
32
|
Wang G, Li B, Fu Y, He M, Wang J, Shen P
and Bai L: miR-23a suppresses proliferation of osteosarcoma cells
by targeting SATB1. Tumour Biol. 36:4715–4721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han B, Luan L, Xu Z and Wu B: Expression
and biological roles of SATB1 in human bladder cancer. Tumor Biol.
34:2943–2949. 2013. View Article : Google Scholar
|
|
34
|
Han S, Xia J, Qin X, Han S and Wu A:
Phosphorylated SATB1 is associated with the progression and
prognosis of glioma. Cell Death Dis. 4:e9012013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chu SH, Ma YB, Feng DF, Zhang H, Zhu ZA,
Li ZQ and Jiang PC: Upregulation of SATB1 is associated with the
development and progression of glioma. J Transl Med. 10:1492012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mcinnes N, Sadlon TJ, Brown CY, Pederson
S, Beyer M, Schultze JL, McColl S, Goodall GJ and Barry SC: FOXP3
and FOXP3-regulated microRNAs suppress SATB1 in breast cancer
cells. Oncogene. 31:1045–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|