Introduction
Research on mevalonate kinase deficiency has
revealed that it may predispose patients to the development of
renal angiomyolipomas (RAMLs) (1).
RAMLs are benign tumors of the kidney, composed of blood vessels,
smooth muscle cells and adipose tissues. RAMLs account for 2.0–6.4%
of all renal tumors. RAML is associated with pain, hematuria,
retroperitoneal hemorrhage and mortality. RAMLs require active
intervention if the tumor size increases with time (2,3). Despite
the benign nature of RAMLs, nephron-sparing surgery is the
preferred treatment modality (4–6).
Renal epithelioid angiomyolipomas (REAs) comprise
approximately 7.7% of RAMLs (7).
There is considerable evidence indicating that REA is a malignant
disease and tumor metastasis usually occurs following surgery
(8). Notably, there is no effective
clinical drug for REA (9,10).
Recently, analysis of the genetic basis of
angiomyolipoma (AML) development has revealed that loss of
heterozygosity in the tuberous sclerosis 2 (TSC2) regions is
present in certain AMLs, with no evidence of other genetic
alterations (11). Therefore, the
phosphoinositide 3-kinase/AKT/mammalian target of rapamycin (mTOR)
pathways have been suggested to be involved in the occurrence and
development of AMLs. Previous research by our group demonstrated
that geranylgeranyl pyrophosphate synthase (GGPPS) deletion may
upset the balance of protein farnesylation and geranylgeranylation
and influence the mTOR signaling pathway (12,13).
Additionally, it has been reported that inhibition of
geranylgeranylation of the small Rho GTPase proteins by statins or
bisphosphonates may block the mTOR pathway and lead to TSC2-null
cell apoptosis (14). Furthermore,
geranylgeranyl pyrophosphate (GGPP) may preserve prostate cancer
cell autophagy and decrease the migration of breast cancer cells
via bisphosphonates (15,16). Therefore, it was hypothesized that the
mevalonate pathway and geranylgeranylation could play an important
role in the mTOR pathway in RAML tumor progression.
GGPPS is a key enzyme in the mevalonate pathway that
is responsible for GGPP synthesis and that utilizes
farnesylpyrophosphate (FPP) to produce GGPP. GGPPS balances the
prenylation of G proteins in cells (17). However, the function of GGPPS in the
progression of RAML is unknown.
The aim of the present study was to distinguish the
different pathogeneses of REA and RAML by evaluating the expression
of GGPPS. A total of 60 cases with clinical and pathological
characteristics of RAMLs were included, and the association between
GGPPS expression and the progression of RAMLs and REAs was
investigated. To validate the association, it was identified in
in vitro studies that GGPPS was upregulated in mouse
TSC2-null cells and inhibition of GGPPS markedly induced apoptosis
of TSC2-null cells by autophagy.
Patients and methods
Patients
A total of 60 patients, including 9 cases with REA
and 51 cases with RAML, were recruited at the Department of
Pathology of Nanjing Drum Tower Hospital (Nanjing, China) from June
2013 to December 2015. RAML, REA and malignant REA were
pathologically diagnosed according to the 2004 WHO classification
(18,19). A total of 48 patients were included in
the follow-up. All specimen collection procedures were approved by
Nanjing Drum Tower Hospital (Nanjing, China). All procedures
involving human participants were in accordance with the ethical
standards of the Independent Ethic Committee of Nanjing Drum Tower
Hospital (Nanjing, China) and with the 1964 Declaration of Helsinki
and its later amendments or comparable ethical standards. Informed
consent was obtained from all individual participants included in
the study.
Immunohistochemistry
All RAML and REA tissue samples were processed
according to standard methods. Briefly, 4-µm-thick slices were
dewaxed in xylene and graded concentrations of alcohol, and
hydrated and washed in PBS. To eliminate interference from blood
cells, endogenous peroxidase was inhibited with 3% hydrogen
peroxide in dH2O for 15 min. Then, heat-mediated antigen
retrieval was performed on the deparaffinized sections using a
citrate buffer (10 mmol/l sodium citrate buffer, pH=6) for 10 min
in a microwave oven prior to incubation with the primary
antibodies, and this process was followed by avidin-biotin blocking
using goat serum (Beyotime Institute of Biotechnology, Haimen,
China). The slices were then immunoassayed with antibodies against
Ki-67 (1:400; cat. no. RM-9106-S) and Melanoma (gp100) Ab-1 (clone
HMB45; 1:80; cat. no. MS-364-S; both from NeoMarkers, Inc.,
Fremont, CA, USA), SMA (ASM-1; dilution, 1:250; cat. no. SMA-L-CE;
Leica Biosystems Newcastle Ltd., Newcastle, UK), Desmin (clone D33;
1:100; cat. no. M0760) and S100 (dilution, 1:5,000; cat. no. S100),
Melan A (clone A103; dilution, 1:100; cat. no. IS63330-2) both from
Dako; Agilent Technologies, Inc. Santa Clara, CA, USA), human GGPPS
(E1; 1:200; cat. no. 14944-1-AP; ProteinTech Group, Inc., Chicago,
IL, USA) and p-S6ser235/236 (1:200; cat. no. 4858; Cell Signaling
Technology, Inc., Danvers, MA, USA). Then, the slices were
incubated with a bio-free horseradish peroxide-labeled polymer from
an EnVision plus detection system (cat. no. K500711-2; Dako;
Agilent Technologies, Inc.) for 1 h at room temperature. The
positive immunoreactions were revealed with diaminobenzidine
solution, and in the negative control samples, the primary
antibodies were replaced with non-immune goat serum (cat. no.
C0265; Beyotime Institute of Biotechnology). Stained slices from
all cases were reviewed by at least two histopathologists, and the
original diagnoses were confirmed. The nuclear immunoreactivity for
Ki-67 and the cytoplasmic staining for HMB45, SMA, S100, Desmin,
GGPPS and p-S6 were evaluated semi-quantitatively according to
staining intensity and the percentage of positive cells. The
percentage of positive tumor cells was graded as follows: 0, none;
1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The immunostaining
intensity was scored as follows: 0, none; 1, week; 2, moderate; and
3, intense. Thus, the total scores for Ki-67, HMB45, SMA, S100,
Desmin, GGPPS, and p-S6 are provided as the sum of the percentage
of positive tumor cells and the immunostaining intensity. The total
scores 0 (−) were negative scores, and 2 (+); 3 and 4 (++); 5–7
(+++) were positive scores (18,20).
Immunofluorescence
Cells were grown on chamber slices (EMD Millipore,
Billerica, MA, USA), harvested 3 days after siRNA transfection
(described below), fixed in 100% cold methanol, blocked in 10% goat
normal serum, 1% BSA and 0.1% Triton X-100/PBS, and incubated with
the following antibodies: LAMP1 (dilution, 1:100; cat. no.
sc-19992; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and LC3
(dilution, 1:100; cat. no. ab167159; Abcam, Cambridge, MA, USA).
Alexa Fluor 488- (1:200; cat. no. ab150073) or Alexa Fluor
594-conjugated secondary antibodies (1:200; cat. no. ab150140;
Abcam) were used and DNA was stained with DAPI within the mounting
medium (cat. no. ab104139; Abcam).
Cell culture and reagents
TSC2-wt cells and TSC2-null cells were kindly
donated by Dr John Blenis (Weill Cornell Medical College, New York,
NY, USA) and were cultured in Dulbecco's modified Eagle's medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) with 10%
fetal bovine serum (Biological Industries, Bat Haemek, Israel) and
1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). All cells were cultured at 37°C and 5% CO2.
Small interfering RNA (siRNA) oligonucleotides targeting GGPPS were
designed and synthesized by (Nanjing Wisdom Biotech Co., Ltd.,
Nanjing, China). The sequence was as follows: mouse siGGPPS:
5′-GGTGTCCCATCTGTCATTA-3′. The scramble sequence used as a control
was as follows: 5′-TTCTCCGAACGTGTCACGT-3′. For RNA interference
experiments, 1×105 cells/well were seeded in a 6-well
plate, cultured overnight, then transfected with small interfering
(si)RNAs targeting GGPPS and control siRNAs using Lipofectamine
RNAiMAX transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Cells were harvested 3 days after siRNA
transfection for apoptosis detection and immunoblotting.
Cell viability and migration
Assessment of viability, migration of TSC2-wt and
TSC2-null cells using the Real-Time Cell Analyzer (RTCA; ACEA
Biosciences Inc., San Diego, CA, USA). RTCA Software Package 1.2
was used to calibrate the plates. Cells were plated at a density of
1,000/well with fresh medium to a final volume of 200 µl. Cells
were incubated at 37°C and 5% CO2 in the RTCA cradle.
The impedance signals were recorded every 12 h for 6 scans until
the end of the experiment (up to 96 h for proliferation and up to
84 h for migration).
Immunoblotting
Cells were lysed in radioimmunoprecipitation assay
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology,
Shanghai, China) with complete protease inhibitor cocktail and
phosSTOP phosphatase inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland). The protein concentration was detected by the
bicinchoninic acid assay (cat. no. KGPBCA; Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). Each sample contained 30 µg protein per
15 µl and were mixed with loading buffer (cat no. KGP101; Nanjing
KeyGen Biotech Co., Ltd.). Protein extracts were resolved by 10 or
15% SDS-PAGE, transferred to polyvinylidene difluoride (PVDF)
membranes (cat. no. 3010040001, Roche Applied Science, Mannheim,
Germany). Following soaking in PBS with 5% non-fat dry milk for 1 h
at 37°C. The membranes were subsequently incubated at 4°C overnight
with the following primary antibodies: p-mTOR (1:1,000; cat. no.
2971), mTOR (1:1,000; cat. no. 2972), p-P70S6K (1:1,000; cat. no.
9208), P70S6K (1:1,000; cat. no. 2708), S6 (1:1,000; cat. no.
2317), p62 (1:1,000; cat. no. 39749), p-S6 (1:1,000; cat. no.
4858), caspase-3 (1:1,000; cat. no. 9662), cleaved caspase-3
(1:1,000; cat. no. 9661) (all purchased from Cell Signaling
Technology, Inc.). The GGPPS antibody (1:500; cat. no. sc-271679)
was obtained from Santa Cruz Biotechnology, Inc. and the LC3
antibody (1:1,000; cat. no. ab167159) was purchased from Abcam. The
tubulin antibody (1:2,000; cat. no. BS1699) was obtained from
Bioworld Technology, Inc. (St. Louis Park, MN, USA). Membranes were
washed with PBST (PBS + 0.5% Tween-20). Subsequently, the membrane
was incubated at 37°C for 1 h with the horseradish
peroxidase-conjugated secondary antibody. The bands were visualized
using a chemiluminescence procedure (cat. no. WBKLS0500; Merck
KGaA, Darmstadt, Germany) and images were captured using a
ChemiDoc™ XRS imaging system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), according to the manufacturer's
protocols. These were analyzed using Image Lab 5.0 software
(Bio-Rad Laboratories, Inc.).
Flow cytometry
Samples were stained with Annexin V-FITC, PI in
Annexin V binding buffer [Annexin V-FITC/PI Apoptosis Detection kit
(cat. no. BD556547; BD Biosciences, Franklin Lakes, NJ, USA)] for
10 min at room temperature and immediately placed on ice before
analysis on a BD FACSCanto flow cytometer, with the data analyzed
by FlowJo software (Treestar Inc., Ashland, OR, USA). Three time
repeats of this experiment were performed.
Statistical analysis
Data analysis was performed with SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard error of the mean. Student's t-test was performed to
compare groups. Tumor fat content was compared with an
independent-sample non-parametric test and expressed as the median.
The prognostic markers, nuclear atypia, mitosis and necrosis were
compared with a Chi-square test. All tests were two-tailed, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
patients with RAMLs and REAs
According to the presence of epithelioid cells in
RAMLs, 60 patients were divided into two groups. The REA group
consisted of 9 patients (5 males and 4 females) with epithelioid
cells, and the RAML group consisted of 51 patients (9 males and 42
females) without epithelioid cells. The clinicopathological
features of patients are summarized in Table I. The incidence of REAs in all
resected RAMLs was 15%. The mean age of the REA group was
42.67±13.75 years, the mean tumor size was 6.41±2.75 cm (range,
1.50–12.00 cm) and the tumor fat content was 0±5.16%. The mean age
of the RAML group was 47.31±12.84 years (range, 22–74 years), the
mean tumor size was 6.78±6.66 cm (range, 1.50–43.00 cm) and the
tumor fat content was 40±36.86%. The mean tumor fat content of the
RAML group was higher compared with the RAE group (0±5.16%;
P<0.001). Microscopic necrosis was observed in 8% of the RAMLs
and 22% of the REAs.
 | Table I.Detailed clinicopathological
characteristics of the RAMLs and REAs. |
Table I.
Detailed clinicopathological
characteristics of the RAMLs and REAs.
| Variables | RAMLs (n=51) | REAs (n=9) | P-value |
|---|
| Age | 47.31±12.84 | 42.67±13.75 | 0.326 |
| Tumor size
(cm) | 6.78±6.66 | 6.41±2.75 | 0.880 |
| Gender |
|
| 0.002a |
|
Female | 42 (82%) | 4 (44%) |
|
|
Male | 9 (18%) | 5 (56%) |
|
| Fat (%) | 40±36.86 | 0±5.16 |
<0.001b |
| Necrosis | 4 (8%) | 2 (22%) | 0.259 |
| Tumor location |
|
| 1.000 |
|
Left | 23 (45%) | 4 (44%) |
|
|
Right | 24 (47%) | 5 (56%) |
|
|
Both | 4 (8%) | 0 (0%) |
|
| Cell atypia | 5 (9%) | 3 (33%) | 0.238 |
| Mitotic figure | 1 (2%) | 1 (11%) | 0.255 |
| HMB45-positive | 44 (86%) | 7 (78%) | 0.388 |
| Ki67-positive | 49 (96%) | 8 (87%) | 1.000 |
| Melan
A-positive | 43 (84%) | 9 (100%) | 0.571 |
| SMA-positive | 48 (94%) | 8 (87%) | 0.421 |
| S100-positive | 47 (92%) | 2 (22%) |
<0.001c |
|
Desmin-positive | 38 (75%) | 2 (22%) | 0.024a |
| p-S6-positive | 44 (86%) | 7 (78%) | 0.163 |
| GGPPS-positive | 21 (41%) | 8 (89%) | 0.011a |
To further confirm the lesions with epithelioid
cells, the tumor cells were immunoassayed for their pathological
characteristics. REA lesions were negative for S100 (P<0.001)
and Desmin (P=0.024) and significant differences were observed
between the two groups. The immunohistochemical results revealed
positive staining for Melanoma (gp100) Ab-1 (clone HMB45; P=0.388),
Ki-67 (P=1.000), Melan A (P=0.571), SMA (P=0.421) and p-S6
(P=0.163), with no significant differences between the REA and RAML
groups (Table I). The tumor cells in
RAMLs and REAs were positive for Ki-67 (Fig. 1), which was not consistent with
previous findings in which epithelioid angiomyolipomas (EAMLs) were
strongly positive for Ki-67 while RAMLs were completely negative
(21,22).
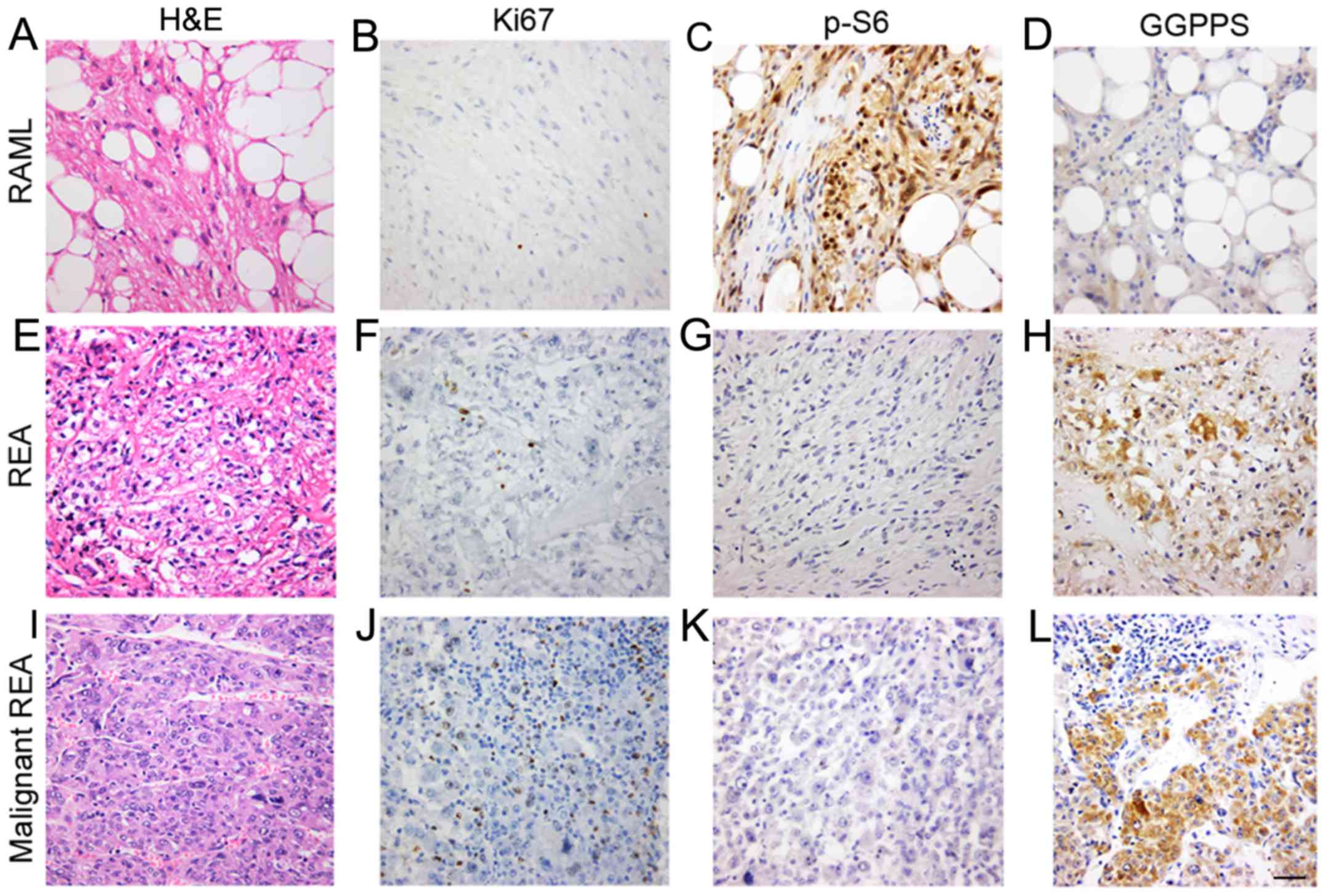 | Figure 1.GGPPS is associated with the
pathological characteristics of REAs. Histological features of the
RAMLs, REAs and malignant REAs. (A) H&E staining and
immunostaining of the RAMLs for (B) Ki-67, (C) p-S6, and (D) GGPPS.
(E) H&E staining and immunostaining of the REAs for (F) Ki-67,
(G) p-S6, and (H) GGPPS. (I) H&E staining and immunostaining of
the malignant REAs for (J) Ki-67, (K) p-S6, and (L) GGPPS. Scale
bar, 50 nm. GGPPS, geranylgeranyl pyrophosphate synthase; RAMLs,
renal angiomyolipomas; REAs, renal epithelioid angiomyolipomas. |
GGPPS is associated with the
pathological characteristics of REAs
To further assess the mTOR pathway activation status
in angiomyolipomas, p-S6 expression was examined in RAML and REA
lesions. The results indicated that 44 were positive and 7 were
negative in terms of immunoreactivity with p-S6 in the RAML group.
In the REA group, 7 cases were p-S6-positive and 2 cases were
p-S6-negative.
Next, GGPPS expression was evaluated in the
cytoplasm of tumor cells and it was revealed that certain REAs
exhibited variable cytoplasm-positive staining (Fig. 1). GGPPS-positive staining primarily
occurred in blood vessels, smooth muscle cells and fat cells. There
was a significant difference between the RAML and REA groups
(P=0.011).
Among the 9 REA cases, 8 were positive for GGPPS and
the mean immunoreactivity was 1.78 (8/9 cases exhibited
immunoreactivities ≥2; Table II).
Among the 9 REA cases, 7 were positive for p-S6 and the mean
immunoreactivity was 1.11 (3/9 cases exhibited immunoreactivities
≥2; Table II). Among the 9 REA
cases, 8 were positive for Ki-67 and the mean immunoreactivity for
Ki-67 was 0.89 (8/9 cases exhibited immunoreactivity=1; Table II). The mean immunoreactivity for
GGPPS was greater compared with p-S6 and Ki-67, and the difference
in immunohistochemical expression of GGPPS between the RAML and REA
groups was significant (P=0.011; Table
I). We also examined the association between GGPPS and clinical
characteristics of renal angiomyolipomas, but did not find a
correlation between GGPPS and patient age, tumor size, and fat
(data not shown).
 | Table II.Detailed clinicopathological
characteristics and clinical outcomes of the REAs. |
Table II.
Detailed clinicopathological
characteristics and clinical outcomes of the REAs.
| Cases | Age | Sex | Maximum diameter
(cm) | Nuclear atypia | Mitotic | Ki-67 | p-S6 | GGPPS | Follow-up
(months) | Outcome |
|---|
| 1 | 43 | M | 12 | + | − | + | + | ++ | 37 | Alive, NED |
| 2 | 43 | M | 5 | − | − | + | + | ++ | 33 | Alive, NED |
| 3 | 36 | F | 4 | − | − | + | ++ | ++ | 33 | Alive, NED |
| 4 | 23 | F | 4 | − | − | − | − | ++ | 28 | Alive, NED |
| 5 | 58 | M | 8 | +++ | +++ | + | − | ++ | 8 | Dead with systemic
metastasis |
| 6 | 44 | M | 6.5 | ++ | − | + | + | − | 24 | Alive, NED |
| 7 | 35 | F | 7.5 | − | − | + | + | ++ | 20 | Alive, NED |
| 8 | 69 | M | 4.3 | − | − | + | ++ | ++ | 11 | Alive, NED |
| 9 | 26 | F | 5 | − | − | + | ++ | ++ | 17 | Alive, NED |
GGPPS is upregulated in TSC2-null
cells and inhibition of GGPPS may induce TSC2-null cell
apoptosis
Immunohistochemical staining indicated that GGPPS
was upregulated in the RAML and REA groups. These results indicated
that activation of the mTOR pathway may induce accumulation of
GGPPS. To ascertain this hypothesis, the protein level of GGPPS was
evaluated in TSC2-wt and TSC2-null cells. A previous study revealed
that TSC2-null tumors had marked p-S6 levels compared to TSC2-wt
cells (23), and our result
concerning p-S6 was consistent with this study. The activation of
mTOR was assessed by p-S6 and p-P70S6K and an increase of GGPPS
protein was observed in TSC2-null cells, which was consistent with
the data from patients (Fig. 2A). The
proliferation of TSC2-null cells was inhibited by GGPPS knockdown,
however TSC2-wt cells were not affected (Fig. 2B). TSC2-wt and TSC2-null cell
migration were both sensitive to GGPPS protein levels (Fig. 2C). Finally, to determine whether
TSC2-null cell proliferation was influenced by cell apoptosis, flow
cytometry analysis was performed. As expected, TSC2-null cells
transfected with siRNA targeting GGPPS exhibited increased
apoptosis in comparison with control cells (Fig. 2D). Since TSC1/TSC2 gene mutant cells
impaired autophagic flux (24), it
was proposed that the inhibition of geranylgeranylation may lead to
apoptosis by autophagy. Thus, GGPPS expression by siRNA for 3 days
in TSC2-null cells was inhibited, which led to the accumulation of
the lysosomal marker LAMP1 and the marker of autophagosome LC3
(Fig. 2E). Notably, increased cleaved
caspase-3 was also detected following inhibition of GGPPS in
TSC2-null cells and the mTOR signaling pathway was inhibited
leading to autophagy of TSC2-null cells (Fig. 2F). Collectively, these data
demonstrated that GGPPS activity was required to promote
proliferation of TSC2-null cells in vitro.
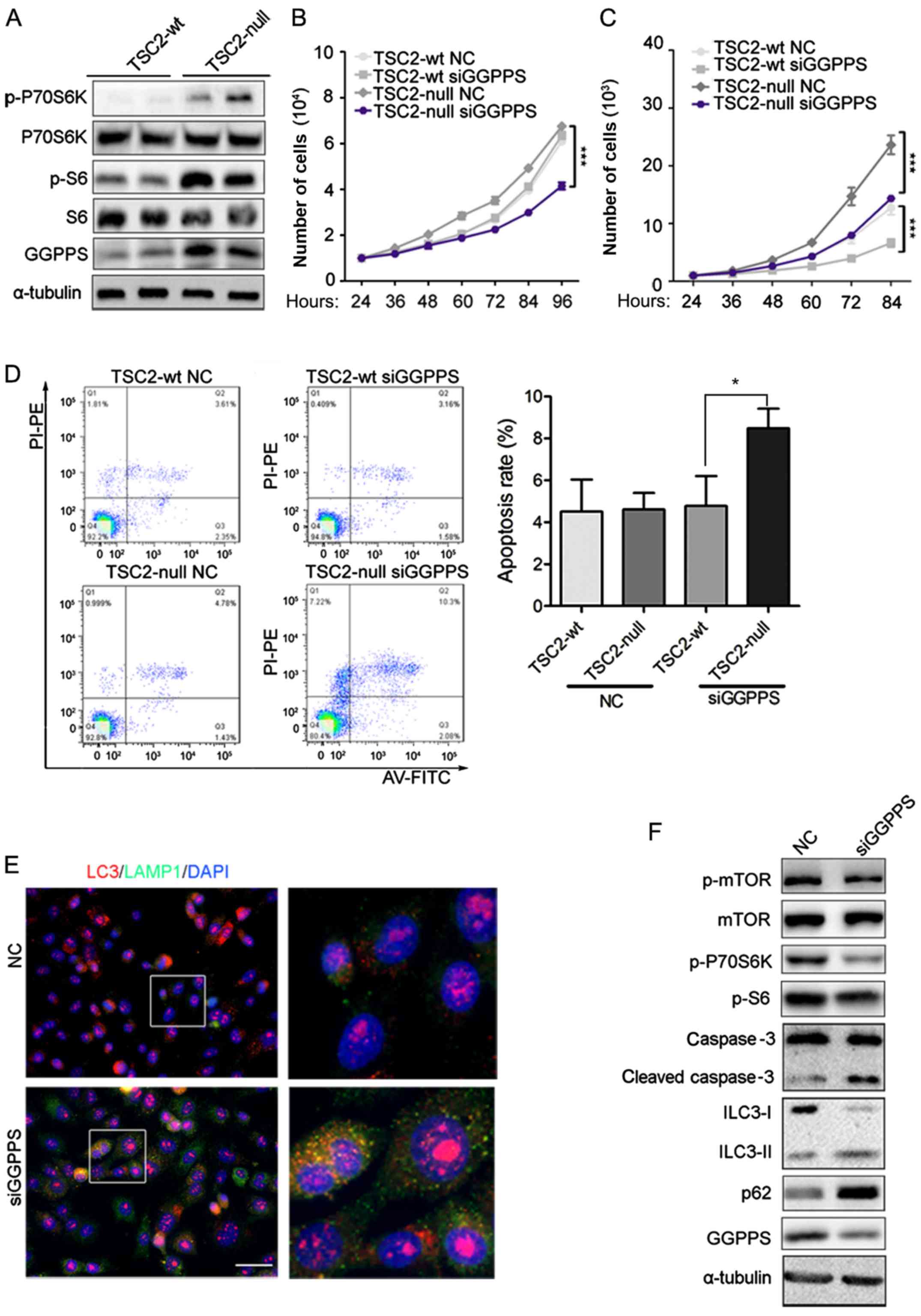 | Figure 2.GGPPS is upregulated in TSC2-null
cells and inhibition of GGPPS induces TSC2-null cell apoptosis. (A)
Expression of GGPPS, p-S6, S6, p-P70S6K, P70S6K was analyzed by
immunoblotting in TSC2-wt and TSC2-null cells. (B) Proliferation of
TSC2-wt and TSC2-null cells treated with siRNA (NC, siGGPPS) for 96
h was analyzed by RTCA, ***P<0.001. (C) Migration of TSC2-wt and
TSC2-null cells treated with siRNA (NC, siGGPPS) for 96 h was
analyzed by RTCA, ***P<0.001. (D) Apoptosis of TSC2-wt and
TSC2-null cells treated with siRNA (NC, siGGPPS) for 72 h was
analyzed by flow cytometry. *P<0.05, as indicated. (E) LC3 and
LAMP1 protein detection in TSC2-null cells treated with siRNA (NC,
siGGPPS) for 3 days was analyzed by immunofluorescence. Scale bar,
50 nm. (F) Expression of mTOR signaling pathway proteins was
analyzed by immunoblotting in TSC2-null cells treated with siRNA
(NC, siGGPPS) for 3 days. GGPPS, geranylgeranyl pyrophosphate
synthase. |
Discussion
The present immunohistochemical findings regarding
p-S6 provide further evidence that the mTOR pathway is activated in
RAMLs. It was also identified that GGPPS and p-S6 were expressed in
RAML lesions, however GGPPS was markedly upregulated only in REA
lesions. The differential expression of GGPPS between RAMLs and
REAs indicated that the pathogeneses of RAML and REA are
different.
It has been suggested that the mTOR signaling
pathway plays an important role in AML progression and AMLs are
usually associated with mutations in TSC2 (25,26). The
current observation of p-S6 expression in cysts was consistent with
previous research. However, p-S6 expression could not be used to
distinguish RAML and REA.
Upregulation of GGPPS in REAs was observed in the
present study, which indicated that GGPPS may contribute to the
occurrence and progression of REAs. GGPP produced by GGPPS is
essential for the geranylgeranylation of small Rho GTPases,
including RhoA, Rac1, and Cdc 42 (27,28). Based
on our previous study, GGPPS was primarily expressed in the
cytoplasm of hepatocellular carcinomas and it was expressed at
relatively high levels in HCC patients with cirrhosis (29). In addition, tumor stage, vessel
invasion and tumor recurrence were closely correlated with GGPPS in
terms of the diagnoses of pathological characteristics.
Furthermore, Patel et al demonstrated that the activation of
small Rho GTPases played a key role in the epithelial-mesenchymal
transition (EMT) of renal epithelial cells. EMT of renal epithelial
cells led to metastasis, resulting in poor clinical outcomes
(30,31). In summary, these results indicated
that GGPPS may be involved in epithelioid cell differentiation and
may further aggravate RAMLs.
Preclinical studies have demonstrated that
inhibiting the mevalonate pathway with drugs, bisphosphonates and
statins leads to a decrease in tumor size (32–34).
However, the effects and exact mechanism of the mevalonate pathway
in AML has not yet been identified. To the best of our knowledge,
this is the first study to investigate GGPPS in AMLs. It was
hypothesized that the association of GGPPS with established
histopathological risk factors and biochemical functions indicated
that the mevalonate pathway may contribute to AML disease
progression. To validate this hypothesis, the expression of GGPPS
protein was evaluated in TSC2-null cells. It was revealed that the
activation of mTOR may induce the accumulation of GGPPS. It was
also determined that inhibition of GGPPS could induce autophagy and
apoptosis.
In conclusion, immunohistochemical assays were
performed to confirm the diagnosis of REA. The tumor cells were
positive for HMB45, Ki-67, SMA, Melan A, p-S6 and GGPPS and
negative for S100 and Desmin. Furthermore, increased GGPPS
expression levels were observed in REAs and TSC2-null cells, which
indicated that the mevalonate pathway and geranylgeranylation may
be involved in disease progression. The limitation of our study is
that our hypothesis was only validated in mouse cell lines
TSC2-null and TSC2-wt. In future studies, further confirmation that
the mevalonate pathway is involved in the formation of RAML in a
renal angiomyolipoma cell line (SV7tert PDGF tumor-1) is required.
Inhibitors of the mevalonate pathway or geranylgeranylation should
also be screened for the treatment of RAML and
lymphangioleiomyomatosis.
Acknowledgements
The authors would like to thank the Department of
Hepatobiliary Surgery of the Affiliated Drum Tower Hospital of
Nanjing University Medical School (Nanjing, China) for the patient
samples and advice on pathology evaluation.
Funding
The present study was supported by the Chinese
National Science Foundation (nos. 31371373 and 31771572), the
Nature Science Foundation of Jiangsu Province (no. BK20151395), and
the Open Fund of State Key Laboratory of Natural Medicines (no.
SKLNMKF201811). Support was also received by the Fundamental
Research Funds for the Central Universities (no. 021414380330).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JC and BX conceived and designed the study. DDZ and
QC collected the data and wrote the manuscript. JY interpreted the
patient data regarding the renal angiomyolipomas, YLQ performed the
patient data collection, KL and QS performed the histological
examination of the kidney of patients by H&E staining. YDQ and
CJL contributed to the design of the project and extensive
discussions. YZ and LF critically analyzed the manuscript for
important intellectual content and provided technical assistance.
MLJ and DCY obtained the human renal agiomyolipoma samples. SSL
contributed to data analysis of flow cytometry and signaling
pathway. All authors read and approved the final manuscript.
Ethics approval and patient consent
All specimen collection procedures were approved by
Nanjing Drum Tower Hospital (Nanjing, China). All procedures
involving human participants were in accordance with the ethical
standards of the Independent Ethic Committee of Nanjing Drum Tower
Hospital and with the 1964 Declaration of Helsinki and its later
amendments or comparable ethical standards. Informed consent was
obtained from all individual participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bader-Meunier B, Florkin B, Sibilia J,
Acquaviva C, Hachulla E, Grateau G, Richer O, Farber CM, Fischbach
M, Hentgen V, et al: Mevalonate kinase deficiency: A survey of 50
patients. Pediatrics. 2010:e152–e159. 2011. View Article : Google Scholar
|
|
2
|
Nelson CP and Sanda MG: Contemporary
diagnosis and management of renal angiomyolipoma. J Urol.
168:1315–1325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamakado K, Tanaka N, Nakagawa T,
Kobayashi S, Yanagawa M and Takeda K: Renal angiomyolipoma:
Relationships between tumor size, aneurysm formation and rupture.
Radiology. 225:78–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boorjian SA, Frank I, Inman B, Lohse CM,
Cheville JC, Leibovich BC and Blute ML: The role of partial
nephrectomy for the management of sporadic renal angiomyolipoma.
Urology. 70:1064–1068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soulen MC, Faykus MH Jr, Shlansky-Goldberg
RD, Wein AJ and Cope C: Elective embolization for prevention of
hemorrhage from renal angiomyolipomas. J Vasc Interv Radiol.
5:587–591. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yip SK, Tan PH, Cheng WS, Li MK and Foo
KT: Surgical management of angiomyolipoma: Nephron-sparing surgery
for symptomatic tumour. Scand J Urol Nephrol. 34:32–35. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aydin H, Magi-Galluzzi C, Lane BR, Sercia
L, Lopez JI, Rini BI and Zhou M: Renal angiomyolipoma:
Clinicopathologic study of 194 cases with emphasis on the
epithelioid histology and tuberous sclerosis association. Am J Surg
Pathol. 33:289–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varma S, Gupta S, Talwar J, Forte F and
Dhar M: Renal epithelioid angiomyolipoma: A malignant disease. J
Nephrol. 24:18–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wolff N, Kabbani W, Bradley T, Raj G,
Watumull L and Brugarolas J: Sirolimus and temsirolimus for
epithelioid angiomyolipoma. J Clin Oncol. 28:e65–e68. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bissler JJ, McCormack FX, Young LR, Elwing
JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J,
et al: Sirolimus for angiomyolipoma in tuberous sclerosis complex
or lymphangioleiomyomatosis. N Engl J Med. 358:140–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan CC, Chung MY, Ng KF, Liu CY, Wang JS,
Chai CY, Huang SH, Chen PC and Ho DM: Constant allelic alteration
on chromosome 16p (TSC2 gene) in perivascular epithelioid cell
tumour (PEComa): Genetic evidence for the relationship of PEComa
with angiomyolipoma. J Pathol. 214:387–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu N, Shen N, Wang X, Jiang S, Xue B and
Li C: Protein prenylation and human diseases: A balance of protein
farnesylation and geranylgeranylation. Sci China Life Sci.
58:328–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu N, Guan S, Chen Z, Yu Y, Xie J, Pan FY,
Zhao NW, Liu L, Yang ZZ, Gao X, et al: The alteration of protein
prenylation induces cardiomyocyte hypertrophy through Rheb-mTORC1
signalling and leads to chronic heart failure. J Pathol.
235:672–685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goncharova EA, Goncharov DA, Li H, Pimtong
W, Lu S, Khavin I and Krymskaya VP: mTORC2 is required for
proliferation and survival of TSC2-null cells. Mol Cell Biol.
31:2484–2498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wasko BM, Dudakovic A and Hohl RJ:
Bisphosphonates induce autophagy by depleting geranylgeranyl
diphosphate. J Pharmacol Exp Ther. 337:540–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin JF, Lin YC, Lin YH, Tsai TF, Chou KY,
Chen HE and Hwang TI: Zoledronic acid induces autophagic cell death
in human prostate cancer cells. J Urol. 185:1490–1496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burke C and Croteau R: Geranyl diphosphate
synthase from Abies grandis: cDNA isolation, functional expression
and characterization. Arch Biochem Biophys. 405:130–136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lopez-Beltran A, Carrasco JC, Cheng L,
Scarpelli M, Kirkali Z and Montironi R: 2009 update on the
classification of renal epithelial tumors in adults. Int J Urol.
16:432–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ooi SM, Vivian JB and Cohen RJ: The use of
the Ki-67 marker in the pathological diagnosis of the epithelioid
variant of renal angiomyolipoma. Int Urol Nephrol. 41:559–565.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato K, Ueda Y, Tachibana H, Miyazawa K,
Chikazawa I, Kaji S, Nojima T and Katsuda S: Malignant epithelioid
angiomyolipoma of the kidney in a patient with tuberous sclerosis:
An autopsy case report with p53 gene mutation analysis. Pathol Res
Pract. 204:771–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goncharova EA, Goncharov DA, Li H, Pimtong
W, Lu S, Khavin I and Krymskaya VP: mTORC2 is required for
proliferation and survival of TSC2-null cells. Mol Cell Biol.
31:2484–2498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smolarek TA, Wessner LL, McCormack FX,
Mylet JC, Menon AG and Henske EP: Evidence that
lymphangiomyomatosis is caused by TSC2 mutations: Chromosome 16p13
loss of heterozygosity in angiomyolipomas and lymph nodes from
women with lymphangiomyomatosis. Am J Hum Genet. 62:810–815. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coombs EJ: Role of mTOR inhibition in the
treatment of patients with renal angiomyolipomas. J Am Assoc Nurse
Pract. 25:588–596. 2013.PubMed/NCBI
|
|
27
|
Sinensky M and Lutz RJ: The prenylation of
proteins. Bioessays. 14:25–31. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konstantinopoulos PA, Karamouzis MV and
Papavassiliou AG: Post-translational modifications and regulation
of the RAS superfamily of GTPases as anticancer targets. Nat Rev
Drug Discov. 6:541–555. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu DC, Liu J, Chen J, Shao JJ, Shen X, Xia
HG, Li CJ, Xue B and Ding YT: GGPPS1 predicts the biological
character of hepatocellular carcinoma in patients with cirrhosis.
BMC cancer. 14:2482014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel S, Mason RM, Suzuki J, Imaizumi A,
Kamimura T and Zhang Z: Inhibitory effect of statins on renal
epithelial-to-mesenchymal transition. Am J Nephrol. 26:381–387.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Konosu-Fukaya S, Nakamura Y, Fujishima F,
Kasajima A, McNamara KM, Takahashi Y, Joh K, Saito H, Ioritani N,
Ikeda Y, et al: Renal epithelioid angiomyolipoma with malignant
features: Histological evaluation and novel immunohistochemical
findings. Pathol Int. 64:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Demierre MF, Higgins PD, Gruber SB, Hawk E
and Lippman SM: Statins and cancer prevention. Nat Rev Cancer.
5:930–942. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hillner BE, Ingle JN, Berenson JR, Janjan
NA, Albain KS, Lipton A, Yee G, Biermann JS, Chlebowski RT and
Pfister DG: American Society of Clinical Oncology guideline on the
role of bisphosphonates in breast cancer. American Society of
Clinical Oncology Bisphosphonates Expert Panel. J Clin Oncol.
18:1378–1391. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pavlakis N and Stockler M: Bisphosphonates
for breast cancer. Cochrane Database Syst Rev. CD003474. 2002.
View Article : Google Scholar : PubMed/NCBI
|
















