Introduction
Compound Kushen injection, made from Sophora
flavescens and Rhizoma Heterosmilacis Japonicae, is a
kind of commonly-used compound in traditional Chinese medicine
prescriptions, and its active ingredients mainly include matrine,
oxymatrine, saponin and oxysophocarpine, with effects of removing
pathogenic heat from the blood and toxic material from the body,
clearing heat and promoting diuresis, removing stasis and relieving
pain (1). Current studies have shown
that compound Kushen injection can affect the normal cycle of tumor
cells or vascular endothelial growth factors (VEGFs), thus inducing
cell apoptosis, inhibiting tumor cell proliferation and tumor
metastasis, improving immune level in the body of cancer patients,
and increasing physical condition and life quality (2). Therefore, compound Kushen injection has
been widely used in the clinical treatment of tumors in China, such
as ovarian, gastric, liver, breast and esophageal cancers (3,4). However,
the exact antitumor mechanism of compound Kushen injection remains
unclear at present. This experiment aimed to further verify the
antitumor effect of compound Kushen injection and reveal its
mechanism in inhibiting tumor growth, so as to provide a scientific
basis for its clinical application.
Materials and methods
Experimental animals and grouping
A total of 40 specific pathogen-free (SPF)
BALB/cA-nu nude mice (20 male, 20 female), 4 weeks old, weighing
18–22 g, were purchased from the Laboratory Animal Center of
Chinese Academy of Medical Sciences. The mice were kept in cages
with controlled temperature, light cycles and humidity (24°C and
12/12 light cycles, 60±10%) and had free access to food and water.
Mice were divided into model group (n=10), low-dose compound Kushen
injection group (n=10), medium-dose compound Kushen injection group
(n=10) and high-dose compound Kushen injection group (n=10) by
using a random number table. The study was approved by the Ethics
Committee of the First Affiliated Hospital of Qiqihar Medical
University (Qiqihar, China).
Main reagents
The following reagents were used: human hepatoma
HepG2 cells (cat. no. CL-0103; Procell Life Science &
Technology Co., Ltd., Wuhan, China) (5), total ribonucleic acid (RNA) extraction
kits (Shanghai Chaoyan Biotechnology Co., Ltd., Shanghai, China),
rabbit anti-mouse α-smooth muscle actin (α-SMA) and cluster of
differentiation 31 (CD31) monoclonal antibodies (at dilution of
1:600 and 1:100, respectively; cat. nos. 19245 and 77699,
respectively; both from Cell Signaling Technology, Inc., Danvers,
MA, USA), Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS; both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The rest of the reagents were analytically pure
reagents made in China.
Main instruments
The following instruments were used: ultra-clean
bench (Air Tech, Suzhou Purification Equipment Co., Ltd., Suzhou,
China), precision electronic balance (Adventurer Precision; OHAUS
Corp., Parsippany, NJ, USA), upright fluorescence microscope
(Olympus Corp., Tokyo, Japan), reverse transcription-polymerase
chain reaction (RT-PCR) instrument (Biometra, Göttingen, Germany),
dual-vertical protein electrophoresis apparatus (Beijing Liuyi
Instrument Factory, Beijing, China), ultraviolet spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
microplate reader (Thermo Fisher Scientific, Inc.).
Establishment of liver cancer model of
nude mice and treatment
HepG2 cells in the logarithmic growth phase were
collected, washed twice with phosphate-buffered saline (PBS),
stained with trypan blue and counted. After the cell concentration
was adjusted to 1×107/ml, 0.2 ml (2×106
cells) of cell suspension were taken by using a syringe, and
inoculated into subcutaneous tissues on the back of nude mice. The
life changes and tumor growth were observed every day; the long
diameter (L) and short diameter (W) of tumor were measured every 3
days, and the tumor volume was calculated according to the formula
V = L × W2 × 0.52. When the tumor volume reached 0.5
cm3 (the baseline level before treatment), 200, 400 and
600 µl of compound Kushen were injected into the mice of the
low-dose, medium-dose and high-dose compound Kushen injection
groups, respectively, for 3 consecutive days; while 400 µl normal
saline was injected into the mice of the model group. There were no
significant differences in body weight, tumor volume and time of
subcutaneous cell inoculation among groups of mice. At 9 days after
treatment, the mice of each group were executed, and the tumors
were taken and weighed. The tumor inhibition rate was calculated;
α-SMA and CD31 were detected via immunohistochemistry, and
microvessel density (MVD) and vascular maturity index (VMI) were
also detected.
Tumor inhibition rate
The tumor inhibition rate was calculated using the
formula: Tumor inhibition rate (%) = (tumor weightmodel
group - tumor weightdrug administration
group)/tumor weightmodel group.
Immunohistochemical detection
After computed tomography (CT) scan, 5 mice of each
group were sacrificed, and tumors were removed and embedded in
paraffin. MVD and α-SMA expression were detected via
immunohistochemical staining, and they were labeled with CD31 and
α-SMA antibodies, respectively. Specific operations are as follows:
α-SMA was incubated with primary and secondary antibodies, dropwise
added with QDs-IgG complex (labeling CD31) and QDs-α-SMA complex
(labeling α-SMA), and were observed under an upright fluorescence
microscope (Nikon Instruments, Inc., Melville, NY, USA).
MVD detection of transplanted
tumor
After 3 fields of view were randomly selected under
high-power lens (×200), the red CD31 fluorescence signal
(CD31-positive marker showing red fluorescence) was used to label
mature and immature vessels. MVD criteria: CD31-positive
endothelial cells showing red fluorescence were clearly separated
from tumor cells, adjacent blood vessels and other connective
tissues. The average microvessel count was taken, and microvessels
in tumor-adjacent normal structure were not counted.
VMI detection of transplanted
tumor
In the same field of view in MVD detection, ImageJ
professional image analysis software (National Institutes of
Health, Bethesda, MD, USA) was used to quantify the expression
levels of CD31 and VEGF, and VMI was calculated (VMI = α-SMA
expression level/CD31 expression level).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
18.0 software (SPSS, Inc., Chicago, IL, USA) was used for the
statistical analysis of all data. t-test was used for measurement
data and the results were presented as mean ± standard deviation
(SD). ANOVA and LSD test were used for the comparison of multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tumor inhibition rate
Compared with that in the model group, the tumor
weight in low-dose, medium-dose and high-dose compound Kushen
injection groups was significantly decreased (P<0.05). With the
increase of compound Kushen injection dose, the tumor mass was
decreased significantly (P<0.05), and the tumor inhibition rate
was obviously increased (P<0.05) (Table I).
 | Table I.Comparison of tumor inhibition rate
among groups. |
Table I.
Comparison of tumor inhibition rate
among groups.
| Group | n | Tumor mass (g) | Tumor inhibition rate
(%) |
|---|
| Model group | 10 | 1.65±0.44 |
|
| Low-dose
compound | 10 |
1.41±0.21a | 14.55 |
| Kushen injection
group |
| Medium-dose
compound | 10 |
1.25±0.15a,b | 24.24b |
| Kushen injection
group |
| High-dose
compound | 10 |
1.06±0.11a–c | 35.76b,c |
| Kushen injection
group |
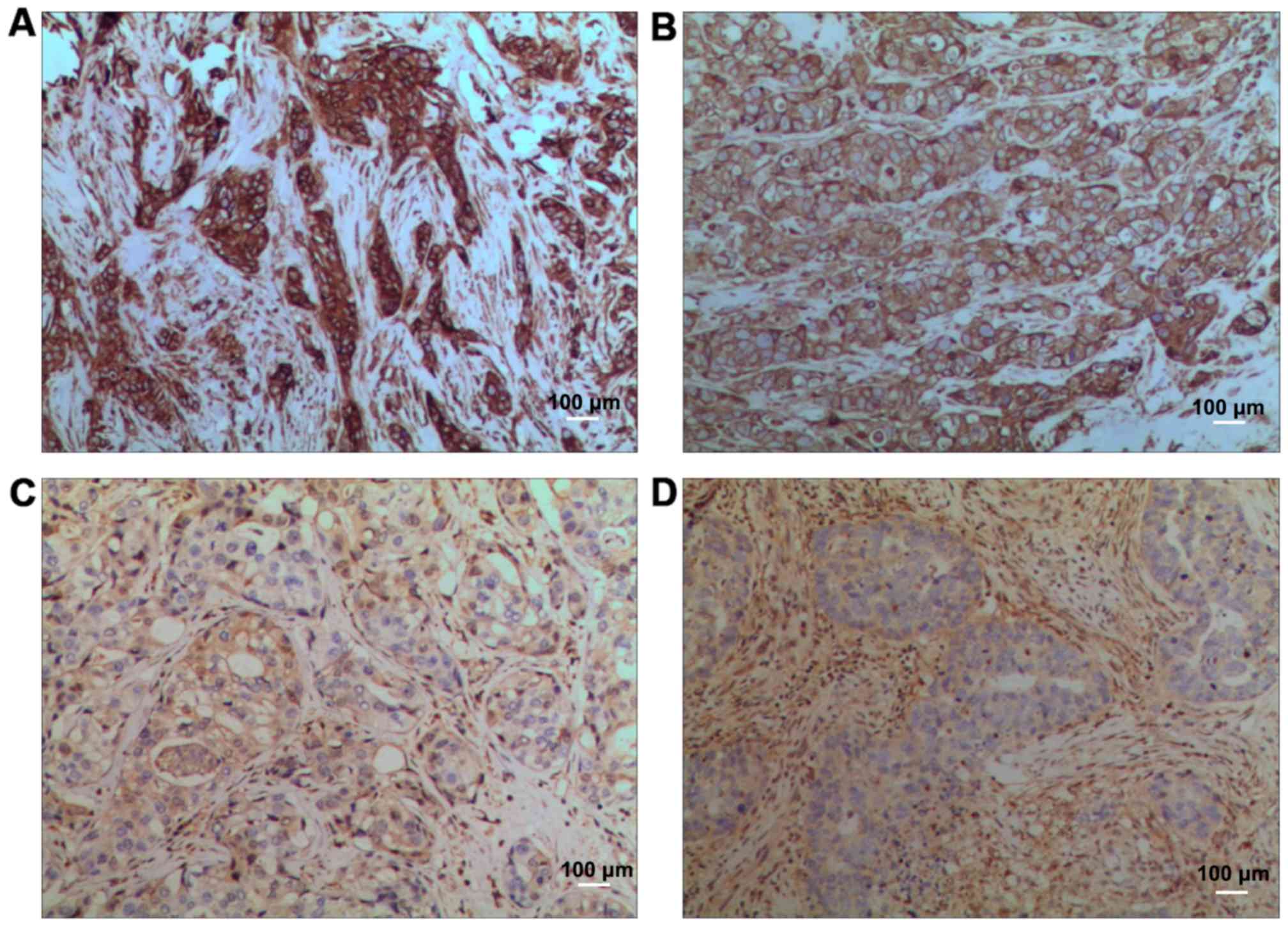
Morphological changes
In model group, the nuclei were large and deeply
stained, there were many mitotic figures, and more small blood
vessels could be seen. In low-dose compound Kushen injection group,
the number of mitotic figures was slightly decreased, and the
vascular distribution was also reduced. In medium-dose compound
Kushen injection group, the number of mitotic figures was
decreased, and the vascular distribution was also significantly
reduced. In high-dose compound Kushen injection group, the number
of mitotic figures was decreased, and the vascular distribution was
further reduced (Fig. 1).
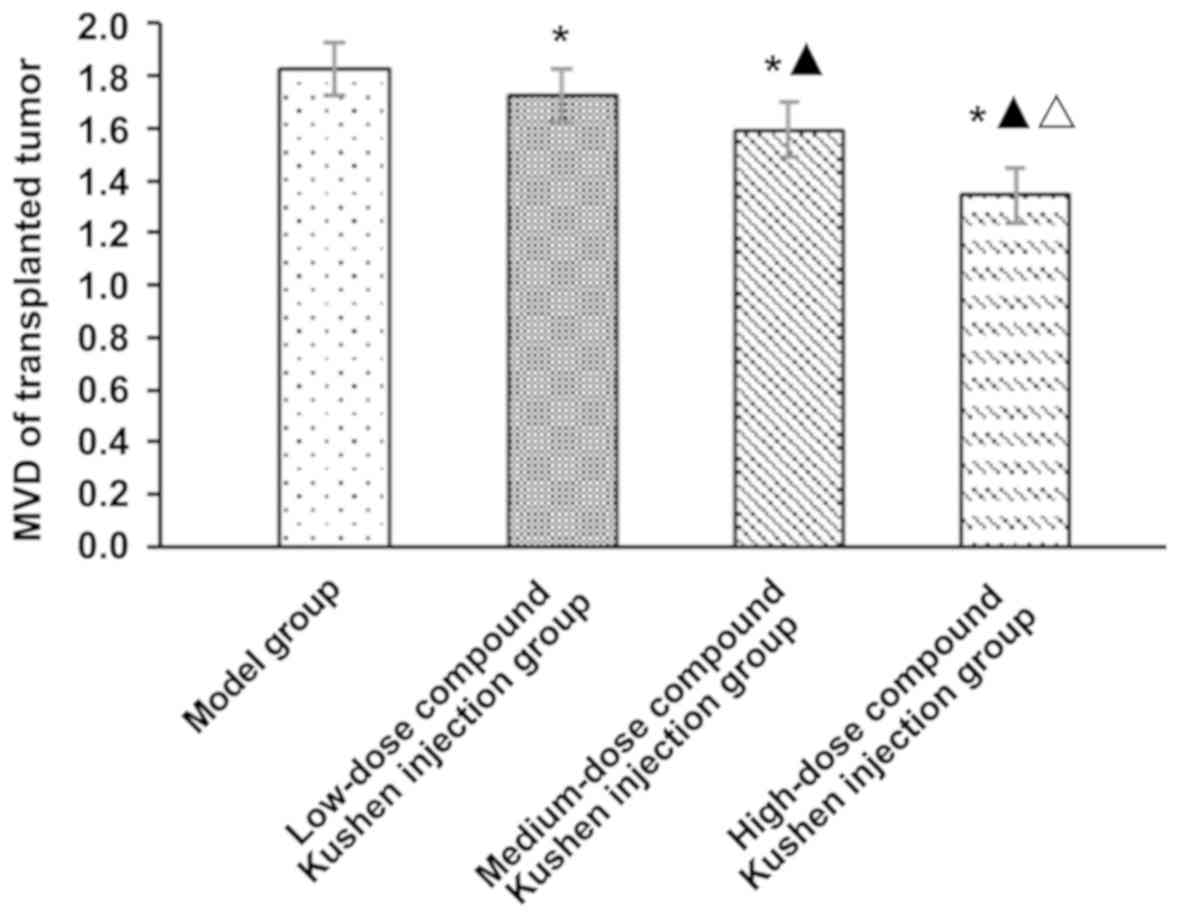
MVD of transplanted tumor
Compared with that in the model group, MVD of
transplanted tumor in low-, medium- and high-dose compound Kushen
injection groups was obviously decreased (P<0.05). With the
increase of compound Kushen injection dose, MVD of transplanted
tumor was decreased significantly (P<0.05) (Fig. 2).
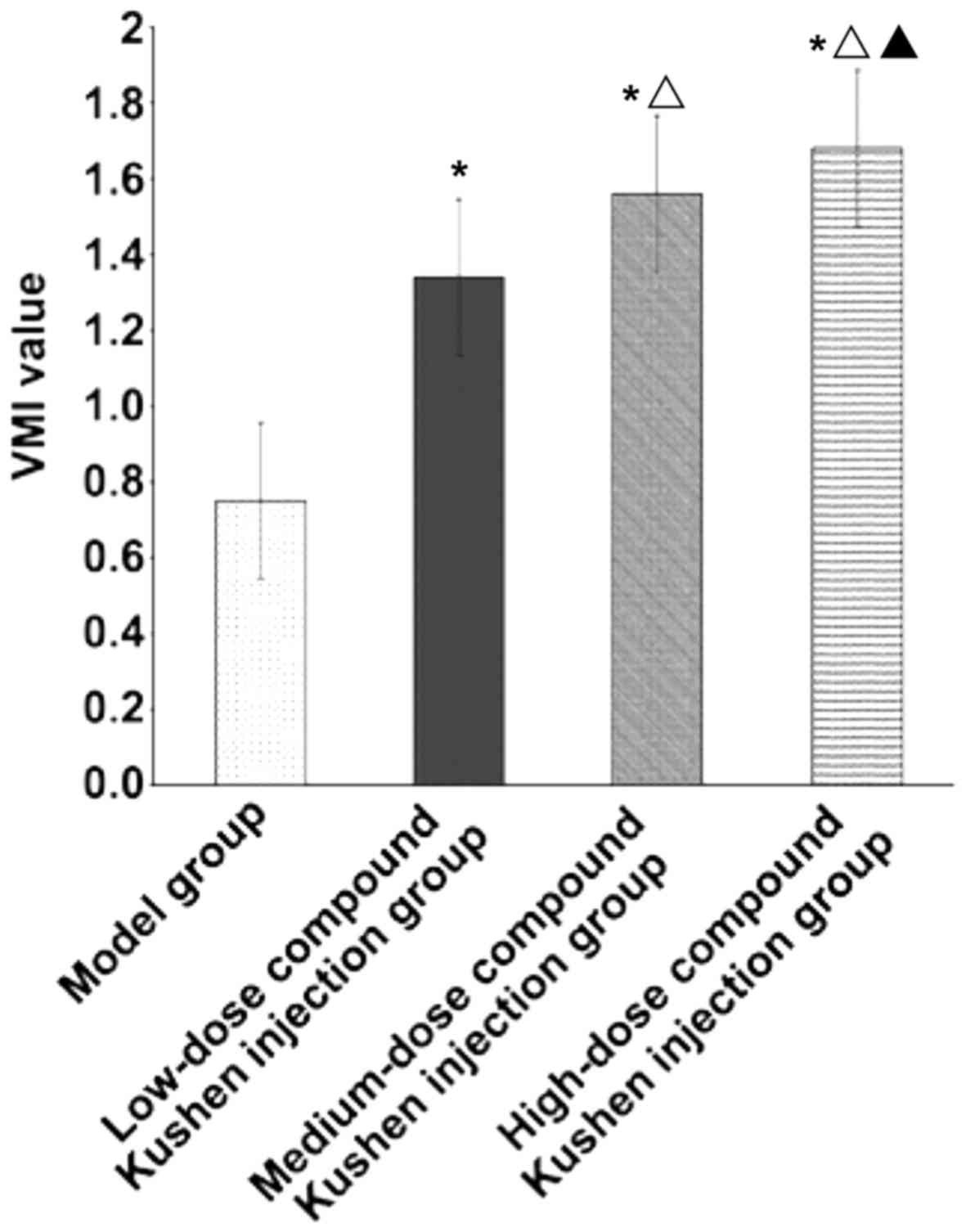
VMI of transplanted tumor
Compared with that in the model group, VMI of
transplanted tumor in low-dose, medium-dose and high-dose compound
Kushen injection groups was obviously increased (P<0.05). With
the increase of compound Kushen injection dose, VMI of transplanted
tumor was increased significantly (P<0.05) (Fig. 3).
Discussion
Compound Kushen injection has been widely used in
the treatment of cardiovascular and rheumatic diseases as well as
viral hepatitis (2). In addition,
compound Kushen injection has a broad-spectrum of antitumor
effects, which can not only enhance antitumor and chemoradiotherapy
sensitization, and reduce relevant side-effects of
chemoradiotherapy, but also exert effects of hemostasis, analgesia
and regulation of the body immunity (6). In this study, the effects of compound
Kushen injection on pathology and angiogenesis of tumor tissues in
mice with liver cancer were analyzed, so as to clarify the
antitumor mechanism of compound Kushen injection.
Results of this study showed that with the increase
of compound Kushen injection dose, the tumor mass was significantly
decreased (P<0.05), and the tumor inhibition rate was
significantly increased (P<0.05), indicating that compound
Kushen injection can significantly inhibit the growth of
transplanted tumor in nude mice in a dose-dependent manner.
Compound Kushen injection is extracted and prepared by two
medicinal materials: Sophora flavescens and Rhizoma
Heterosmilacis Japonicae. Oxymatrine (molecular formula:
C15H24N2O2) is one of
the main active ingredients of Sophora flavescens, and it
belongs to quinolizidine alkaloid, which is colorless crystal and
easily soluble in water with relative molecular mass of 264.4
(7). Modern pharmacological studies
have shown that oxymatrine has a wide range of pharmacological
effects, such as antivirus, antitumor, anti-hypertension, heart
strengthening, antipyresis and analgesia, inhibition of myocardial
ischemia and infarction, asthma relieving, anti-arrhythmia,
sterilization, anti-inflammation, sedation and hypnosis, and
anti-allergy (8,9). In Rhizoma Heterosmilacis
Japonicae, flavonoids and triterpenes have extensive
anti-inflammatory, detoxification and antitumor effects (10).
Anti-angiogenic therapy is a therapeutic method to
prevent and/or reduce angiogenesis in diseased tissues. Currently,
commonly-used anti-angiogenic drugs in clinic, such as bevacizumab,
recombinant human endostatin injection and imatinib, have achieved
varying degrees of effects, bringing new hope for the clinical
treatment of tumors (11). Studies
have shown that anti-angiogenic therapy combined with surgery,
thermal therapy and chemoradiotherapy is more conducive to
improving the curative effect (12).
The results of this study showed that with the increase of compound
Kushen injection dose, the mitotic figure of tumor cells and
vascular distribution in tumor tissues is reduced, thereby
inhibiting tumor cell division and proliferation, suggesting that
compound Kushen injection may play an antitumor effect through
anti-angiogenesis.
Further analysis showed that with the increase of
compound Kushen injection dose, MVD of transplanted tumor is
significantly decreased (P<0.05), but VMI was significantly
increased (P<0.05). MVD can reflect the rates of changes in
tumor cell components and tumor vascular components, while VMI can
quantitatively analyze the changes in vascular maturity and is more
representative in the microvascular functional status than MVD
(13,14). Therefore, compound Kushen injection
may inhibit tumor angiogenesis to reduce tumor blood supply,
thereby inhibiting tumor growth. Some studies have shown that
compound Kushen injection can reduce the expression of VEGF. VEGF,
as an important regulator of angiogenesis, plays an important role
in regulating neovascularization in endothelial cells in tumor
blood vessels, so when its level is reduced, angiogenesis is
limited, and tumor blood supply is reduced, thus inhibiting tumor
growth (15).
In conclusion, compound Kushen injection can reduce
angiogenesis in tumor tissues and plays a key role in inhibiting
tumor growth. Therefore, anti-angiogenesis may be one of the
important mechanisms of compound Kushen injection in inhibiting
tumor growth.
Acknowledgements
Not applicable.
Funding
This study was supported by the mandatory scientific
research of Qiqihar Science and Technology Bureau
(SFGG-201415).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and HH contributed to the establishment of the
liver cancer model. HR was responsible for the immunohistochemical
detection. XZ assisted with MVD detection. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Qiqihar Medical University
(Qiqihar, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu L, Zhou Y, Yang Y, Lu F and Fan Y:
Efficacy and safety of Compound Kushen Injection on patients with
advanced colon cancer: A meta-analysis of randomized controlled
trials. Evid Based Complement Alternat Med. 2017:71025142017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang W, You RL, Qin WJ, Hai LN, Fang MJ,
Huang GH, Kang RX, Li MH, Qiao YF, Li JW, et al: Anti-tumor
activities of active ingredients in Compound Kushen injection. Acta
Pharmacol Sin. 36:676–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Wu M, Tian Q, Xie G, Hu Y, Meng Q
and Zhang M: The clinical value of Fufangkushen injection in the
treatment of stomach cancer: A meta-analysis. J Cancer Res Ther. 10
Suppl 1:42–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanju B, Yang L, Hua B, Hou W, Shi Z, Li
W, Li C, Chen C, Liu R, Qin Y, et al: A systematic review and
meta-analysis on the use of traditional Chinese medicine compound
Kushen injection for bone cancer pain. Support Care Cancer.
22:825–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
6
|
Zhao Z, Liao H and Ju Y: Effect of
compound Kushen injection on T-cell subgroups and natural killer
cells in patients with locally advanced non-small-cell lung cancer
treated with concomitant radiochemotherapy. J Tradit Chin Med.
36:14–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao YG, Jing S, Li L, Gao JQ, Shen ZY, Liu
Y, Xing Y, Wu ML, Wang Y, Xu CQ, et al: Antiarrhythmic effects and
ionic mechanisms of oxymatrine from Sophora flavescens.
Phytother Res. 24:1844–1849. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang S, Lian X, Sun M, Luo L and Guo L:
Efficacy of compound Kushen injection plus radiotherapy on
non-small cell lung cancer: A systematic review and meta-analysis.
J Cancer Res Ther. 12:1298–1306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malhotra J, Jabbour SK and Aisner J:
Erratum to current state of immunotherapy for non-small cell lung
cancer. Transl Lung Cancer Res. 6:6122017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo YM, Huang YX, Shen HH, Sang XX, Ma X,
Zhao YL and Xiao XH: Efficacy of Compound Kushen injection in
relieving cancer-related pain: A systematic review and
meta-analysis. Evid Based Complement Alternat Med. 2015:8407422015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olaso E and Vidal-Vanaclocha F: Use of
tumor-activated hepatic stellate cell as a target for the
preclinical testing of anti-angiogenic drugs against hepatic tumor
development. Methods Mol Med. 85:79–86. 2003.PubMed/NCBI
|
|
12
|
Browder T, Butterfield CE, Kräling BM, Shi
B, Marshall B, O'Reilly MS and Folkman J: Antiangiogenic scheduling
of chemotherapy improves efficacy against experimental
drug-resistant cancer. Cancer Res. 60:1878–1886. 2000.PubMed/NCBI
|
|
13
|
Zhao Z, Fan H, Higgins T, Qi J, Haines D,
Trivett A, Oppenheim JJ, Wei H, Li J, Lin H, et al: Fufang Kushen
injection inhibits sarcoma growth and tumor-induced hyperalgesia
via TRPV1 signaling pathways. Cancer Lett. 355:232–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CN, Cheng WF, Chen CA, Chu JS, Hsieh
CY and Hsieh FJ: Angiogenesis of endometrial carcinomas assessed by
measurement of intratumoral blood flow, microvessel density, and
vascular endothelial growth factor levels. Obstet Gynecol.
96:615–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69 Suppl 3:4–10. 2005. View Article : Google Scholar : PubMed/NCBI
|