Introduction
Cholangiocarcinoma arises from malignantly
transformed bile duct epithelium. Despite only accounting for 10%
of all forms of primary hepatobiliary cancer, its prevalence has
increased in recent decades (1).
Hilar cholangiocarcinoma, also known as Klatskin tumors, represents
60–70% of all cholangiocarcinoma cases and constitutes a major
surgical challenge as it is characteristically located in the
vicinity of pivotal structures (2).
Currently, surgical resection offers the best
treatment outcome, however prognosis remains dismal with a 5-year
survival rate of 9–28% (3). The
subset of patients with hilar cholangiocarcinoma, however, are not
suitable for resection due to advanced tumor stage or the presence
of underlying liver disease, including primary sclerosis
cholangitis (PSC) (4). Orthotopic
liver transplantation (OLT) has been proposed as an alternative to
treat patients with unresectable hilar cholangiocarcinoma (5), as it may increase the resection margin
and circumvent subsequent liver failure that occurs due to
insufficient hepatic portions or co-morbidity (6). However, interest in OLT has waned due to
its high recurrence rate, and patient prognosis following OLT
remains poor (7,8); therefore, more efficient therapeutic
strategies are required (9).
Neoadjuvant chemoradiation plus OLT, which has been
accomplished by the Mayo Clinic, demonstrates promising results
regarding patient prognosis, with a 5-year survive rate of ~82%
(10). Encouraged by these outcomes,
the United Network of Organ Sharing/Organ Procurement and
Transplantation Network approved the allocation of a standard model
of end-stage liver disease exception score for patients with hilar
cholangiocarcinoma who undergo this treatment modality (11).
The neoadjuvant chemoradiotherapy method, also known
as the ‘Mayo protocol’, is currently regarded as having therapeutic
applications for hilar cholangiocarcinoma. However, its efficacy
and safety have been questioned as only a few treatment centers
have investigated its effects and all studies have involved <300
patients (12). This is attributed,
at least in part, to protocol complexity and completion difficulty,
and the involvement of multiple departments.
The current case aims to build on the findings of
the Mayo Clinic and describes a patient with unresectable hilar
cholangiocarcinoma, who underwent brachytherapy and
chemoradiotherapy followed by OLT, and remains disease-free after 8
months.
Case report
A 53-year-old woman presented with yellow skin and
urine upon admittance to the Department of Hepatobiliary Surgery,
Nanjing Drum Tower Hospital, Affiliated Hospital of Nanjing
University Medical School (Nanjing, China) on March 18, 2013.
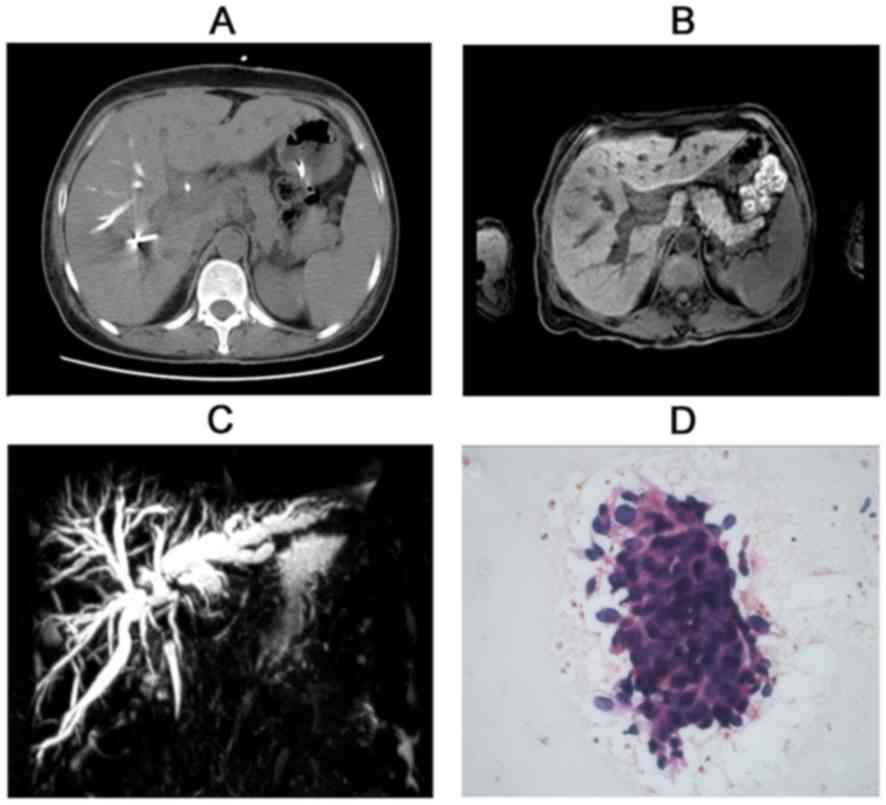
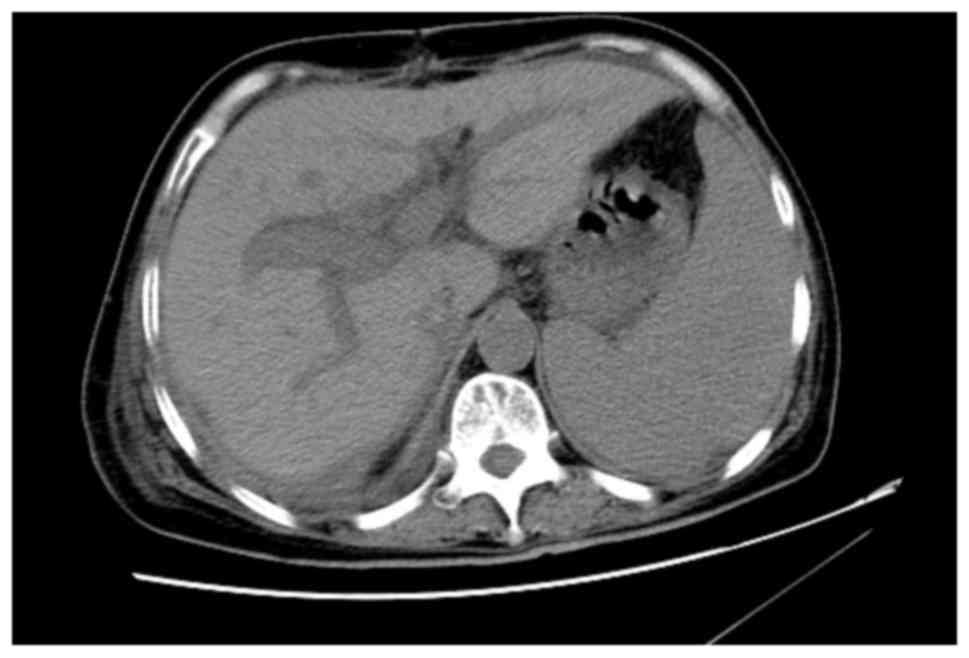
Computed tomography (CT) and magnetic resonance imaging (MRI)
revealed a hilar mass with left and right hepatic duct and vessel
involvement (Figs. 1A and B).
Subsequent magnetic resonance cholangiopancreatography exhibited
disruption of the hepatic bile duct (Fig.
1C). The total bilirubin carbohydrate antigen (CA)19-9 levels
were 479.6 µmol/l and 1,044 U/ml, respectively, which were beyond
the healthy ranges (3–25 µmol/l and 0–37 U/ml, respectively).
To alleviate jaundice of the patient, two tubes were
placed in the left and right bile duct using the percutaneous
transhepatic drainage (PTCD) technique. The daily drainage volume
was ~300 ml. The patient was classed as having type IV
cholangiocarcinoma (Bismuth classification) (13) with invasion of major vessels;
therefore, a radical, major hepatectomy was considered to be
unfeasible. Inspired by the success of the Mayo clinic, it was
decided that neoadjuvant therapy plus OLT was the best treatment
option.
Chest and abdominal MRI, contrast-enhanced CT, a
bone scan and positron emission tomography (PET)-CT were employed
to rule out local lymph node and distant metastasis. Repetitive
endoscopic retrograde cholangiopancreatography (ERCP) was performed
to obtain positive results by brush cytology, which indicated the
presence of malignant cells consistent with the patient diagnosis
(Fig. 1D). Notably, the patient had
developed a number of severe, but non-lethal complications,
including acute cholangitis, liver abscess and biliary-pleural
fistula, due to repeated ERCP and cholangiography (Fig. 2A). Administering anti-infection,
drainage and nutrition support alleviated these symptoms (Fig. 2B).
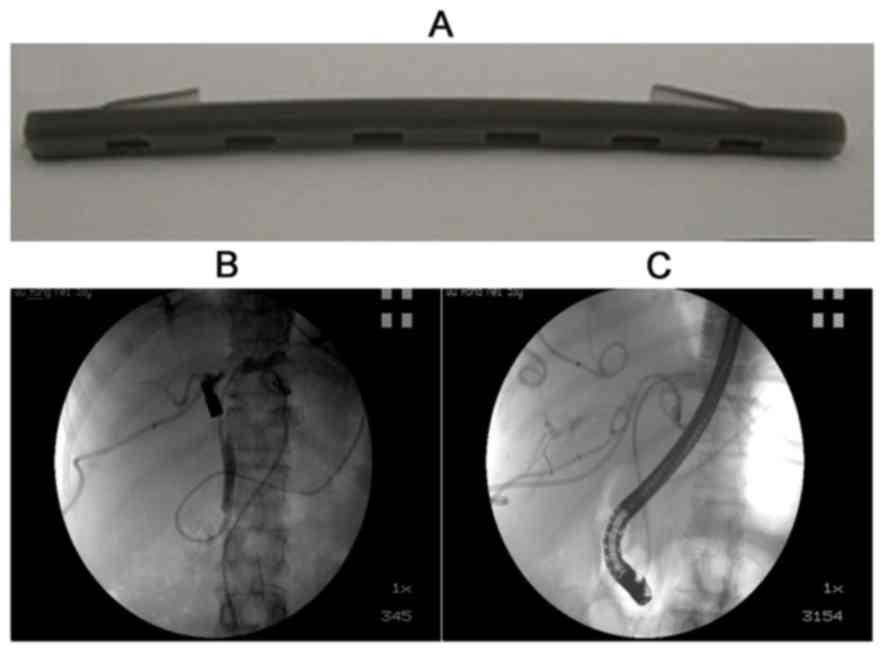
I-125 radioactive plastic stents were used to
perform brachytherapy (Fig. 3A). Each
stent was composed of a drainage tube with a stab at each end,
which had a stabilizing effect. Two opposite channels were made in
the wall of stents paralleling the drainage lumen, with an
irradiation window in the lateral wall of the channel. The channel
diameter was slightly smaller than that of the radioactive seeds,
as were the length and width of the irradiation windows. This
immobilizes the radioactive seeds and improve the effectiveness of
radiation treatment. The radioactive I-125 seeds (Shanghai Xinke
Pharmaceutical Co., Ltd., Shanghai, China) were 4.5 mm long and 0.8
mm thick, with a half-life of 60.1 days.
Nasobiliary radiography was performed to determine
the extent of bile duct involvement (Fig.
3B). A total of 11 radioactive seeds were loaded into the
channel in sequence and the ERCP procedure was performed to put
stents in the appropriate location on May 7, 2013 (Fig. 3C). Following ~1 month, external beam
radiotherapy was administrated at a targeted dose of 30 Gy (30
fractions of 1.5 Gy twice a day). Concomitantly, intravenous
capecitabine was provided at 1.0 g for 2 weeks, and repeated until
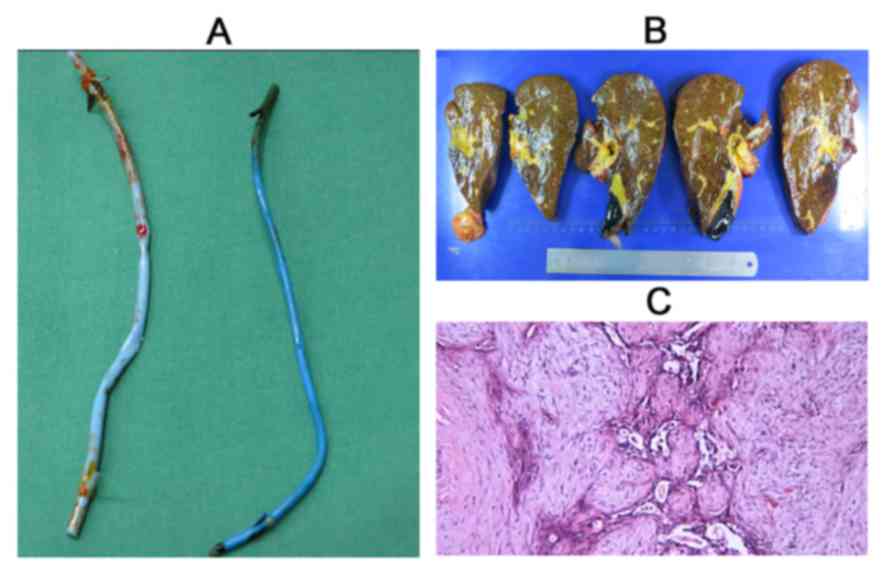
14 days post-transplantation. On July 26, 2013, the patient
received a liver allograft from a deceased donor. However, the
patient underwent laparoscopic exploration at the beginning of
liver transplantation to assess for lymph node and distant
metastases, which were negative. A standard OLT was performed with
no major incidents (Figs. 4A and B).
Immunosuppressive therapy was provided with tacrolimus,
mycophenolate and corticosteroids. Corticosteroids were withdrawn a
week after OLT was completed.
Subsequent specimen pathology revealed an
adenosquamous cell carcinoma, measuring 4×2.5×2.5 cm, with focal
necrosis. The tumor was classified as tumor-node-metastasis (TNM)
stage II (T2bN0cM0) due to neural
and vascular invasion (Fig. 4C).
Following treatment, liver function normalized and physical status
improved significantly (Table I).
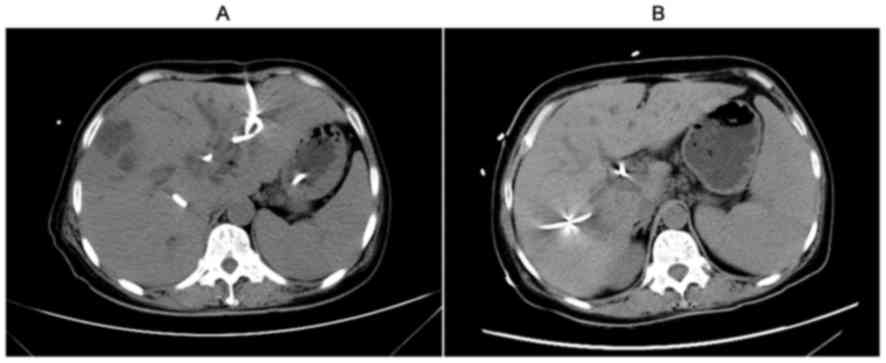
Follow-up tomographic evaluation 8 months post-transplantation
indicated that the liver was normal without tumor recurrence
(Fig. 5). Three chemotherapy cycles
were administered (1.0 mg/m2 capecitabine twice daily) 1
month after OLT. Each chemotherapy cycle was continued for 3
weeks.
 | Table I.Course of serum TB, DB and ALT
post-OLT. |
Table I.
Course of serum TB, DB and ALT
post-OLT.
| Biological
features | Normal range | Prior to OLT | Day 3 post-OLT | Day 9 post-OLT | Day 14 post-OLT | Day 25 post-OLT | Day 39 post-OLT |
|---|
| ALT, U/l | 0–40 | 95.8 | 575.9 | 64.4 | 42.3 | 50.1 | 32.9 |
| AST, U/l | 4–40 | 141.7 | 305.8 | 17.0 | 25.5 | 31.1 | 33.9 |
| ALP, U/l | 40–150 | 345.9 | 63.5 | 73.3 | 182.1 | 269.5 | 85.7 |
| GGT, U/l | 3–50 | 158.1 | 78.4 | 136.3 | 216.5 | 203.1 | 69.7 |
| TB, µmol/l | 3–25 | 175.2 | 39.7 | 38.5 | 30.2 | 18.5 | 11.8 |
| DB, µmol/l | 0–7 | 128.3 | 27.3 | 23.1 | 18.6 | 11.1 | 6.3 |
| Alb, g/l | 65–85 | 35.0 | 33.6 | 33.5 | 29.7 | 30.3 | 36.5 |
Discussion
Although hilar cholangiocarcinoma is a debilitating
disease with poor patient prognosis, there is interest in improving
the efficacy and safety of the currently limited treatment options
available. A number of patients are diagnosed during an advanced
cancer stage, which deprives them of the opportunity to undergo
resection (14). Furthermore, the
survival rate of those eligible to undergo surgical resection
remains low, suggesting that resection is not an acceptable
treatment for hilar cholangiocarcinoma (15–17). It
was previously considered that OLT alone may be an effective method
of treating cholangiocarcinoma, however, it was later
contraindicated due to the high recurrence and low survival rates
accompanying its use. Therefore, reducing the recurrence rate has
become a key objective.
Surgeons suggested the use of OLT plus Whipple's
operation to eradicate tumors, as the loco-region recurrence rate
is high (18). Unfortunately, this
approach did not significantly improve the poor survival rate, and
tumor recurrence continued to account for the majority of patient
mortalities (19). In an attempt to
achieve a more radical margin, the Starzl group proposed the
concept of cluster-OLT, which involves the removal of all visceral
organs derived from the foregut and part of the colon (20). This aggressive method resulted in
markedly high post-operative mortality and no significant
improvements in the long term survival rate of patients (20).
Neoadjuvant therapy refers to adjuvant
chemoradiation performed prior to surgery. This therapy aims to
shrink inoperable tumors to make surgery more feasible, eradicate
micro metastatic sites and reduce tumor cell viability to minimize
the possibility of metastasis. Neoadjuvant therapy has been widely
used in different types of cancer, including breast (21) and gastrointestinal tumors (22), with great success, and was first
introduced by Nebraska University to treat cholangiocarcinoma in
combination with liver transplantation (23). The neoadjuvant protocol consists of
biliary brachytherapy and intravenous infusion of 5-fluorouracil
until transplantation. In the long-term, 45% of patients that
underwent this procedure were reported to be tumor-free
post-transplantation, demonstrating significant progress in
cholangiocarcinoma prognosis (23).
The Mayo Clinic pioneered a neoadjuvant study by
extensively investigating its clinical applications. In 2005, the
study presented promising data with 1–3- and 5-year patient
survival rates of 92, 82 and 82%, respectively, following
neoadjuvant therapy plus OLT, which were markedly higher than the
survival rates of patients with liver cancer following
transplantation (10). The
neoadjuvant protocol differed from that of Nebraska University by
including external beam irradiation (10). The Mayo Clinic has published a series
of articles concerning neoadjuvant plus liver transplantation for
cholangiocarcinoma. In this ongoing study, patients with hilar
cholangiocarcinoma who underwent neoadjuvant therapy were
recommended for liver transplantation (24). Furthermore, Wu et al (18) conducted clinical research to determine
if neoadjuvant plus OLT-Whipple therapy was able to improve patient
prognosis. A total of 6 patients were enrolled and administered
radiation only, including external beam irradiation and
brachytherapy, prior to transplantation. Excellent results were
achieved overall, with 5 patients recurrence-free at 10.1 years
post-treatment, although 1 patient succumbed at 55 months
post-transplant due to an unrelated cause (18). These results implied that a more
aggressive approach of liver transplantation may improve long-term
survival rates, provided that post-operative lethal complications
are avoided.
The aforementioned studies focused on early stage
hilar cholangiocarcinoma and patients with a clinical stage beyond
II were excluded from liver transplantation. However, other
previous studies have investigated possible therapeutic strategies
to improve the prognosis of patients with advanced hilar
cholangiocarcinoma. Researchers from the University of California,
Los Angeles, investigated whether neoadjuvant therapy plus
transplantation may be more beneficial to patients with advanced
hilar cholangiocarcinoma compared with surgical resection alone
(25). Locally advanced hilar
cholangiocarcinoma was defined as follows: A tumor size >3 cm;
invasion beyond the wall of the bile duct to the gallbladder, liver
and either ipsilateral branches of the hepatic artery or the portal
vein; or metastasis to the regional lymph nodes. Notably,
neoadjuvant and adjuvant therapies in the liver transplantation
group resulted in a 47% 5-year recurrence-free survival rate,
whereas patients who underwent resection alone had all succumbed to
the disease 5 years on (25).
Therefore, evidence from numerous studies suggests that neoadjuvant
therapy plus OLT may be the optimal treatment for hilar
cholangiocarcinoma, even for patients with an advanced stage of the
disease (26–28).
Diagnosing hilar cholangiocarcinoma remains a
challenge. With regards to the Mayo Clinic protocol, the clinical
diagnosis of hilar cholangiocarcinoma requires the presence of a
malignant-appearing stricture on percutaneous or endoscopic
cholangiography, and at least one of the following: Polysomy on
fluorescent in situ hybridization-16; CA19.9 level >100
U/ml; malignant cytology or histology on transluminal brushings or
biopsy; and identification of a hilar mass on cross-sectional
imaging at the site of the malignant-appearing stricture (29). Based on this procedure, it is often
difficult to distinguish malignant carcinoma from benign lesions,
particularly in the setting of PSC. Nearly half of patients that
underwent the Mayo Protocol did not have pathological confirmation
of carcinoma prior to neoadjuvant therapy and explanted liver
pathological analyses revealed no tumor residue in a number of
patients (29), thus raising the
question as to whether the excellent result achieved in the Mayo
Clinic protocol was attributed to the inclusion of patients with
hilar benign lesions in treatment protocols. The authors explained
that brachytherapy was responsible for dissolving the tumor,
however, this is extremely unlikely as a complete pathological
response with contemporary chemotherapy and radiation has rarely
been reported (30). This phenomenon
was validated in the current study; focal necrosis was observed,
but the tumor did not disappear. An accurate pathological
confirmation of diagnosis pretreatment may therefore be necessary
to improve long-term survival rates of patients. Conventional
percutaneous or endoscopic fine aspiration leads to tumor seeding
and therefore cannot be employed to diagnose hilar
cholangiocarcinoma. Thus, in the current study, ERCP was performed
to obtain brush cytology, which was examined by a pathologist at
the same time. Repeated brush was performed until the pathological
diagnosis was confirmed. This approach improved the sensitivity and
specificity of diagnosis.
Brachytherapy has been included in the majority of
previous neoadjuvant protocols. Nebraska University employed Ir-192
wires for tumor irradiation through percutaneous biliary catheters,
whereas the Mayo Clinic harnessed Ir-192 seeds loaded in the stent
by ERCP (23,29). Brachytherapy was administered as an
intensified radiation method in each strategy, which required
withdrawal after 24 h. The current study used I-125 seeds as a
radiation source, as it was considered that continued and effective
brachytherapy would minimize tumor progression prior to
transplantation. This hypothesis was based on the findings of
previous studies, which demonstrated that treating advanced tumors
around the pancreatic head region using I-125 radioactive stents
resulted in 72.7% of patients having ‘stable disease’ 2 months
after treatment, which is remarkable regarding the high malignancy
and advanced stage of the tumors (31,32).
Consistent with these results, in the current study, the image
monitor and tumor marker profile exhibited no signs of recurrence
in the patient.
Identifying ways to avoid tumor recurrence following
transplantation is an important clinical goal. The majority of
liver transplantation centers include corticosteroid treatment in
their anti-rejection regimen, which is associated with tumor
recurrence and major adverse events. However, a recent
meta-analysis demonstrated that steroid-free tacrolimus-based
immunosuppression was safe and that certain adverse events,
including diabetes and hepatitis C virus recurrence, occur less
frequently in the patients that received corticosteroids following
liver transplantation (33).
Therefore, early withdrawal of steroids may minimize the chance of
recurrence. Rapamycin, a novel immunosuppressive agent, has been
reported to inhibit metastatic tumor growth by anti-angiogenesis
(34). The antitumor properties have
been validated by its effectiveness in treating patients with
hepatic cell carcinoma (HCC) (35).
Compared with conventional tacrolimus, rapamycin significantly
reduces the probability of tumor recurrence (36). Further studies are required to
investigate the effectiveness of rapamycin treatment in hilar
cholangiocarcinoma following liver transplantation.
The experience of the present study highlights a
number of issues to be considered prior to the initiation of
neoadjuvant therapy. Firstly, pathological confirmation of hilar
cholangiocarcinoma must be obtained, as these patients may benefit
from the neoadjuvant protocol. The most effective way of achieving
this is by cytological brush through ERCP. Secondly, brachytherapy
serves a pivotal role in the neoadjuvant protocol. Among the
various methods of brachytherapy, the use of an I-125 radioactive
stent may be the most effective method. Thirdly, in order to reduce
tumor recurrence following liver transplantation, early steroid
withdrawal or steroid-free liver transplantation should be
considered in combination with rapamycin usage in the
post-operative stage.
The present case demonstrates that neoadjuvant plus
OLT may be an effective method of treating hilar
cholangiocarcinoma, particularly in the presence of unresectable
tumors. Following this treatment, the current patient was alive and
disease-free for 8 months. In conclusion, the following issues
should be considered before initiating neoadjuvant therapy. In
light of the complexity and expense, it is necessary to obtain
pathological confirmation of hilar cholangiocarcinoma. Patients
diagnosed with cholangiocarcinoma can benefit from the neoadjuvant
protocol discussed in the present study. Brush cytology using ERCP
is an effective and rapid way of diagnosing cholangiocarcinoma.
Brachytherapy serves a pivotal role in the neoadjuvant protocol. Of
the various methods of brachytherapy, I-125 radioactive stent may
present the best solution. To reduce recurrence following liver
transplantation as much as possible, early steroid withdrawal from
the immunosuppression protocols could be considered in combination
with administration of rapamycin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan SA, Toledano MB and Taylor-Robinson
SD: Epidemiology, risk factors, and pathogenesis of
cholangiocarcinoma. HPB (Oxford). 10:77–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skipworth JR, Keane MG and Pereira SP:
Update on the management of cholangiocarcinoma. Dig Dis.
32:570–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hemming AW, Reed AI, Fujita S, Foley DP
and Howard RJ: Surgical management of hilar cholangiocarcinoma. Ann
Sur. 241:693–702. 2005. View Article : Google Scholar
|
|
4
|
Guthrie CM, Banting SW, Garden OJ and
Carter DC: Segment III cholangiojejunostomy for palliation of
malignant hilar obstruction. Br J Surg. 81:1639–1641. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hassoun Z, Gores GJ and Rosen CB:
Preliminary experience with liver transplantation in selected
patients with unresectable hilar cholangiocarcinoma. Surg Oncol
Clin N Am. 11:909–921. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrowsky H and Hong JC: Current surgical
management of hilar and intrahepatic cholangiocarcinoma: The role
of resection and orthotopic liver transplantation. Transplant Proc.
41:4023–4035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghali P, Marotta PJ, Yoshida EM, Bain VG,
Marleau D, Peltekian K, Metrakos P and Deschênes M: Liver
transplantation for incidental cholangiocarcinoma: Analysis of the
Canadian experience. Liver Transpl. 11:1412–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robles R, Figueras J, Turrion VS, Margarit
C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, López P,
et al: Spanish experience in liver transplantation for hilar and
peripheral cholangiocarcinoma. Ann Surg. 239:265–271. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyer CG, Penn I and James L: Liver
transplantation for cholangiocarcinoma: Results in 207 patients.
Transplantation. 69:1633–1637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rea DJ, Heimbach JK, Rosen CB, Haddock MG,
Alberts SR, Kremers WK, Gores GJ and Nagorney DM: Liver
transplantation with neoadjuvant chemoradiation is more effective
than resection for hilar cholangiocarcinoma. Ann Surg. 242:451–461.
2005.PubMed/NCBI
|
|
11
|
Gores GJ, Gish RG, Sudan D and Rosen CB:
MELD Exception Study Group: Model for end-stage liver disease
(MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver
Transpl (١٢ Suppl ٣). S95–S97. 2006. View
Article : Google Scholar
|
|
12
|
Darwish Murad S, Kim WR, Harnois DM,
Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC,
Schwartz JJ, et al: Efficacy of neoadjuvant chemoradiation,
followed by liver transplantation, for perihilar cholangiocarcinoma
at 12 US centers. Gastroenterology. 143:88–98, e3; quiz e14. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paul A, Kaiser GM, Molmenti EP, Schroeder
T, Vernadakis S, Oezcelik A, Baba HA, Cicinnati VR and Sotiropoulos
GC: Klatskin tumors and the accuracy of the Bismuth-Corlette
classification. Am Surg. 77:1695–1699. 2011.PubMed/NCBI
|
|
14
|
Razumilava N and Gores GJ: Classification,
diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol
Hepatol. 11:13–21.e1; quiz e3-e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Todoroki T, Kawamoto T, Koike N, Takahashi
H, Yoshida S, Kashiwagi H, Takada Y, Otsuka M and Fukao K: Radical
resection of hilar bile duct carcinoma and predictors of survival.
Br J Surg. 87:306–313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klempnauer J, Ridder GJ, von Wasielewski
R, Werner M, Weimann A and Pichlmayr R: Resectional surgery of
hilar cholangiocarcinoma: A multivariate analysis of prognostic
factors. J Clin Oncol. 15:947–954. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakeeb A, Pitt HA, Sohn TA, Coleman J,
Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ and Cameron
JL: Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and
distal tumors. Ann Surg. 224:463–465. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Johlin FC, Rayhill SC, Jensen CS,
Xie J, Cohen MB and Mitros FA: Long-term, tumor-free survival after
radiotherapy combining hepatectomy-Whipple en bloc and orthotopic
liver transplantation for early-stage hilar cholangiocarcinoma.
Liver Transpl. 14:279–286. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neuhaus P, Jonas S, Bechstein WO, Lohmann
R, Radke C, Kling N, Wex C, Lobeck H and Hintze R: Extended
resections for hilar cholangiocarcinoma. Ann Surg. 230:808–819.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alessiani M, Tzakis A, Todo S, Demetris
AJ, Fung JJ and Starzl TE: Assessment of five-year experience with
abdominal organ cluster transplantation. J Am Coll Surg. 180:1–9.
1995.PubMed/NCBI
|
|
21
|
Zardavas D and Piccart M: Neoadjuvant
therapy for breast cancer. Ann Rev Med. 66:31–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelsen D: Neoadjuvant therapy for
gastrointestinal cancers. Oncol (Williston Park).
7:25-32-411993.
|
|
23
|
Sudan D, DeRoover A, Chinnakotla S, Fox I,
Shaw B Jr, McCashland T, Sorrell M, Tempero M and Langnas A:
Radiochemotherapy and transplantation allow long-term survival for
nonresectable hilar cholangiocarcinoma. Am J Transplant. 2:774–779.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Darwish Murad S, Kim WR, Therneau T, Gores
GJ, Rosen CB, Martenson JA, Alberts SR and Heimbach JK: Predictors
of pretransplant dropout and posttransplant recurrence in patients
with perihilar cholangiocarcinoma. Hepatology. 56:972–981. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong JC, Jones CM, Duffy JP, Petrowsky H,
Farmer DG, French S, Finn R, Durazo FA, Saab S, Tong MJ, et al:
Comparative analysis of resection and liver transplantation for
intrahepatic and hilar cholangiocarcinoma: A 24-year experience in
a single center. Arch Surg. 146:683–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gringeri E, Bassi D, D'Amico FE, Boetto R,
Polacco M, Lodo E, D'Amico F, Vitale A, Boccagni P, Zanus G and
Cillo U: Neoadjuvant therapy protocol and liver transplantation in
combination with pancreatoduodenectomy for the treatment of hilar
cholangiocarcinoma occurring in a case of primary sclerosing
cholangitis: Case report with a more than 8-year disease-free
survival. Transplant Proc. 43:1187–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schule S, Altendorf-Hofmann A, Utess F,
Uteß F, Rauchfuß F, Freesmeyer M, Knösel T, Dittmar Y and
Settmacher U: Liver transplantation for hilar cholangiocarcinoma--a
single-centre experience. Langenbecks Arch Surg. 398:71–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu J, Bai J, Shi X, Zhou J, Qiu Y, Wu Y,
Jiang C, Sun X, Xu F, Zhang Y and Ding Y: Efficacy and safety of
liver transplantation in patients with cholangiocarcinoma: A
systematic review and meta-analysis. Int J Cancer. 130:2155–2163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heimbach JK, Gores GJ, Haddock MG, Alberts
SR, Pedersen R, Kremers W, Nyberg SL, Ishitani MB and Rosen CB:
Predictors of disease recurrence following neoadjuvant
chemoradiotherapy and liver transplantation for unresectable
perihilar cholangiocarcinoma. Transplantation. 82:1703–1707. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kelley RK, Hirose R and Venook AP: Can we
cure cholangiocarcinoma with neoadjuvant chemoradiation and liver
transplantation? Time for a multicenter trial. Liver Transpl.
18:509–513. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Liu JL, Cai ZZ, Lu Z, Gong YF, Wu
HY, Man XH, Jin ZD and Li ZS: A novel approach for treatment of
unresectable extrahepatic bile duct carcinoma: design of
radioactive stents and an experimental trial in healthy pigs.
Gastrointest Endosc. 69:517–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Lu Z, Zou DW, Jin ZD, Liu F, Li SD,
Zhan XB, Zhang WJ, Wu RP, Yao YZ, et al: Intraluminal implantation
of radioactive stents for treatment of primary carcinomas of the
peripancreatic-head region: a pilot study. Gastrointest Endosc.
69:1067–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sgourakis G, Radtke A, Fouzas I, Mylona S,
Goumas K, Gockel I, Lang H and Karaliotas C: Corticosteroid-free
immunosuppression in liver transplantation: a meta-analysis and
meta-regression of outcomes. Transpl Int. 22:892–905. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klintmalm G and O'Farrelly C: Taking the
rap: Multiple effects of blocking mammalian target of rapamycin.
Hepatology. 57:1–3. 2013. View Article : Google Scholar : PubMed/NCBI
|