Introduction
Over the past 20 years, the overall survival of
patients with esophageal cancer has remained poor (1). More than two-thirds of patients who
undergo radical resection of this type of cancer will eventually
succumb as a result of relapse and distant metastasis (2). Esophageal squamous cell carcinoma
(ESCC) accounts for the majority of cases (>90%) of esophageal
cancer in Asia (3), and the 5-year
survival is 15–20% (4). The
detection of circulating tumor cells (CTCs) in the peripheral
circulation has been demonstrated to serve as a prognostic factor
that may offer novel strategies for cancer treatment (5). Studies have reported that the detection
of high CTCs by the CellSearch® system in metastatic
breast cancer is associated with poor overall survival (6–8). The
CellSearch® system has been authorized by the US Food
and Drug Administration for the follow up of patients with breast,
colonic and prostate metastases. However, this system has its
limits; direct and indirect methods have been proposed for
detecting CTCs, but these methods vary in specificity, sensitivity
and cost (9–16). Among the direct methods, detecting
CTCs according to the size of the epithelial tumor cells has been
associated with good specificity and sensitivity, and has performed
well in esophageal cancer (17). A
low cost technique, it allows for the cytomorphological analysis
and characterization of CTCs. Although there has been research into
the clinical significance of CTCs detected in patients with ESCC,
isolation by size of epithelial tumor cells is still not widely
used, and the detection of CTCs is not closely associated with
surgical or neoadjuvant therapies (18,19). As
a preliminary study for the clinical trial no. NCT03005314
(ClinicalTrials.Gov
ID)/ChiCTR-OON-17010807 (Chinese Clinical Trial Registry),
isolation by size of epithelial tumor cells technology was used in
the present study to isolate CTCs and circulating tumor microemboli
(CTM) from patients with ESCC who had undergone surgery with
curative intent.
Patients and methods
Patients
A total of 55 patients with ESCC were enrolled in
this single institution study conducted at the Second Hospital of
Shandong University (Jinan, China) between July 2016 and June 2017.
The study was approved by the Ethics Committee of the Second
Hospital of Shandong University. Where blood samples were obtained,
patients provided informed consent. Blood samples from 20 healthy
volunteers were used as controls. All the patients who enrolled in
the study underwent esophagectomy with 2- or 3-field lymph node
dissection.
Peripheral blood samples
Peripheral blood samples were drawn from the median
cubital vein into a tube with K2-EDTA, followed by adequate mixing.
The first 2 ml of blood was discarded to prevent epithelial
contamination, and the remaining 5 ml was immediately processed
(within 2 h). Blood samples were harvested in the morning, 1–3 days
prior to surgery and 7 days post-surgery.
Surgical procedure
All patients underwent curative thoracic
esophagectomy and lymphadenectomy. Patients underwent either right
or left thoracotomy, and the thoracoscopic and laparoscopic
approaches were encouraged. Additionally, patients who underwent
cervical anastomosis (McKeown) (20)
or thoracic anastomosis (Ivor-Lewis) (21) were accepted. The incised margin was
≥5 cm from the superior border of the tumor.
The range of lymphadenectomy included the
periesophageal lymph nodes, subcarinal lymph nodes, left and right
recurrent laryngeal nerve lymph nodes, hilar lymph nodes, and
lesser omentum (specifically the left gastric vessel region) and
any suspicious lymph nodes next to the common hepatic arteries, and
cervical lymph nodes were selectively dissected according to tumor
location and ultrasound examination. Jian-Hui et al
(22) established that the log odds
of positive lymph nodes (LODDS) exhibited improved prognostic
performance compared with either the number of lymph node
metastases (LNMs) or the positive lymph node ratio (LNR) in
patients with gastric cancer. Cao et al (23) considered the LODDS a more accurate
index compared with the LNMs or LNR for evaluating the survival of
patients undergoing resection for esophageal cancer. LODDS is
classified as follows: LODDS1≤-2.6, −2.6<LODDS2≤-1.5,
−1.5<LODDS3≤-0.5 and LODDS4>-0.5. As the index increases, the
5-year cancer-specific survival decreases.
Isolation by size of epithelial tumor
cells assay
The procedure was performed as previously described
by Vona et al (10). The
filtration module was kindly provided by Wuhan YZY Medical Science
and Technology Co., Ltd. (Wuhan, China). A total of 5 ml whole
blood was diluted to 8 ml with buffer containing 0.2% formaldehyde,
and filtered through an 8 µm membrane. The assay was performed
according to the manufacturers' protocol. The cells were classified
as CTCs if they met ≥4 of the following criteria: i) A markedly
enlarged nucleus (>2–3 calibrated pore sizes); ii) a high
nucleocytoplasmic ratio (ratio >0.8); iii) hyperchromasia and
nonhomogeneous staining; iv) irregularity of the nuclear membrane;
v) anisonucleosis (ratio >0.5) and the presence of three
dimensional sheets; vi) the presence of nuclear chromatin
side-shift or large nucleoli; and vii) the presence of abnormal
mitotic figures. Cells with no cytoplasm were not analyzed. All
images were recorded and reviewed independently by 6
cytopathologists from different institutions, and CTCs were
confirmed by agreement between ≥4 cytopathologists.
Confirmation of CTCs
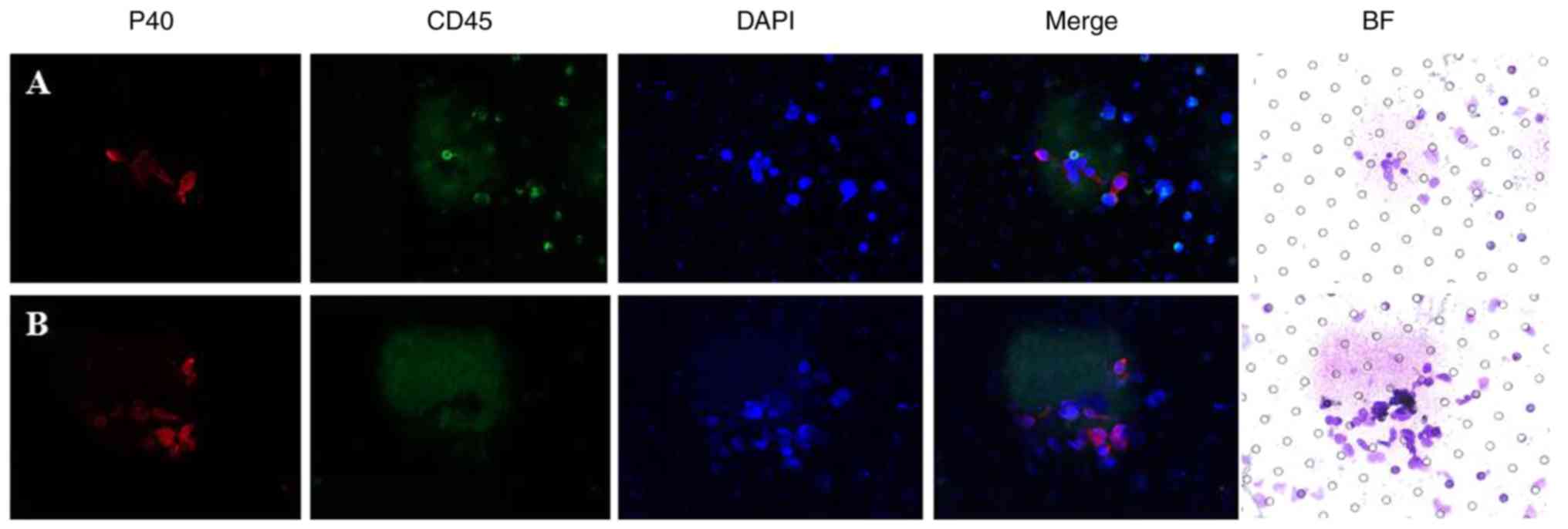
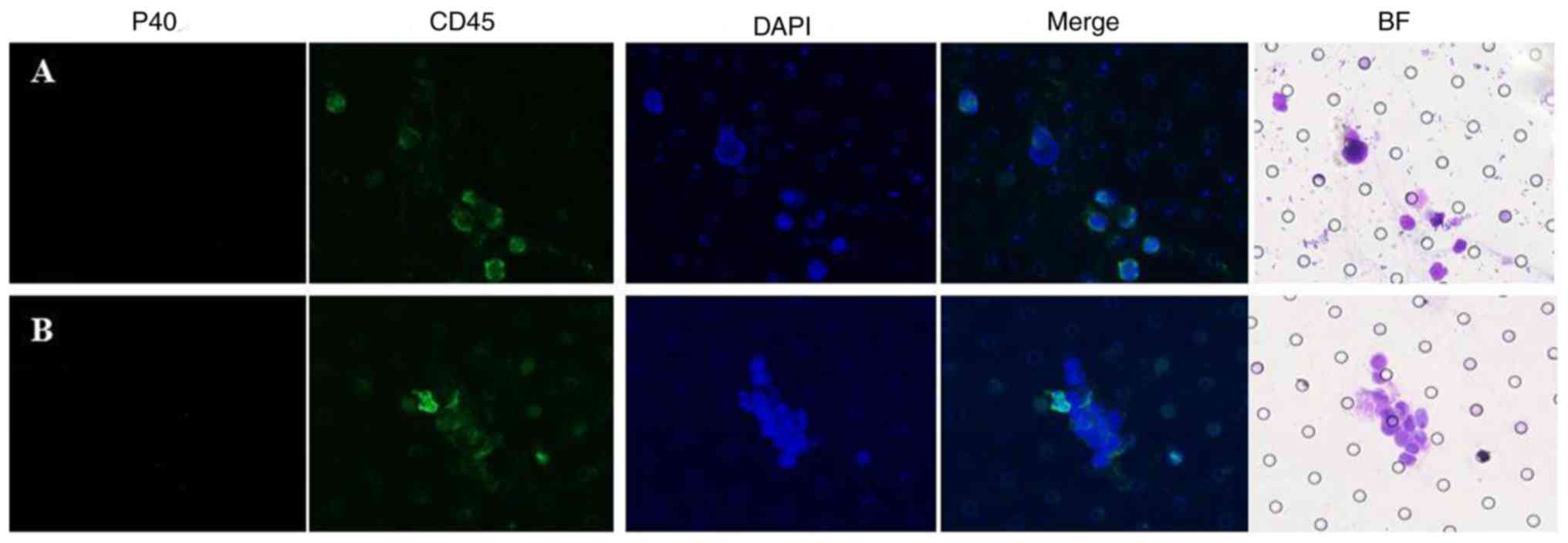
Immunofluorescence staining for cluster of
differentiation (CD)45 and P40 was conducted for preliminary
confirmation. The expression of CD45 was observed to distinguish
between CTCs and leukocytes, particularly megakaryocytes and large
monocytes. The expression of P40 indicated the squamous origin of
cells. Lung squamous cell carcinoma cells were used as a
P40+ control, and the harvested lymphocytes were used as
a CD45+ control. A total of 5 ml/blood sample was
separated by CTC biopsy machine. The cells were fixed for 5 min
with 200 µl paraformaldehyde (2%) added to the filter at room
temperature (18–26°C). The cells were rinsed with PBS for 3×2 min.
Subsequently, 200 µl methanol was added to the filter and allowed
to stand for 1 min, the filter film was removed, placed on one side
of a glass slide, dried for 4–5 min at room temperature, and
transferred to the center of the slide, where it was mounted with 2
µl adhesive [transparent reagent (BASE BA-7002B) and mountant (BASE
BA-7004)1/4 (Baso Biotechnology Co., Ltd., Wuhan, China)]. A circle
was drawn around the filter film with a PAP pen. The sample was
subsequently treated with 200 µl 0.5% Triton X-100 for 5 min and
rinsed with PBS for 3×2 min. Subsequently, 100 µl 10% goat serum
(Jackson ImmunoResearch Europe, Ltd., Newmarket, UK) in PBS was
added to the filter film and allowed to stand for 30 min at room
temperature; the excess serum was removed. The samples were
incubated at 4°C overnight with 100 µl primary antibody (anti-CD45;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat no. sc-70699;
or anti-P40; Abcam, Cambridge, UK; cat no. ab137691), diluted 1:500
and 1:200, respectively, with 10% goat serum. Thee samples were
rinsed with PBS for 3×3 min, 100 µl secondary antibody (Alexa Fluor
488-conjugated goat anti-rat; cat no. A11006; or Alexa Fluor
647-conjugated goat anti-rabbit; cat no. A21245; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) diluted 1:500 with 10%
goat serum was added, and the slides were incubated for 50 min at
room temperature. Following washing with PBS 3×2 min, the films
were sealed with DAPI and observed by fluorescence microscopy
(magnification, ×40). When images of the slides had been captured,
Wright-Giemsa staining was performed (10,17) for
comparison with the immunofluorescence results. The slides were
stained with 100 µl diff A (Eosin; YZY Medical Science and
Technology Co., Ltd., Wuhan, China; catalog no. YZY-CTC-P100) for 1
min at room temperature. Following rinsing with PBS for 1 min, 100
µl diffB (Methylthioninium Chloride; YZY Medical Science and
Technology Co., Ltd.; catalog no. YZY-CTC-P100) was added for 90
sec at room temperature. The slides were then rinsed with deionized
water three times for 30 sec each time and dried for 30 min at
50°C. Following mounting with permanent mounting medium (Baso
Ultra-Clear Advanced Mounting Resin; Baso Biotechnology Co., Ltd.;
catalog no. BASE BA-7004), the slides were dried for 1 h at 50°C.
Finally, the cells were observed using an optical microscope
(magnification, ×40).
Statistical analysis
Stata 12.0 (StataCorp LP, College Station, TX, USA)
was used for statistical evaluation. Quantitative data are
presented as the mean ± standard deviation. Any associations
between CTC detection and clinicopathological parameters, lymph
node metastasis and surgical procedure were ascertained by
χ2 test or Fisher's exact test. Student's t-tests were
used to analyze continuous variables when samples were from a
population with a normal distribution and homogeneous variance;
otherwise, two-sample Wilcoxon rank-sum tests were used. Cox
proportional hazards regression analysis was employed to evaluate
the clinical factors for survival. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient clinicopathological parameters
and CTC detection
In all, 55 patients were enrolled between July 2016
and June 2017, of which 46 were male and 9 female, with an age
range of 49–86 years. All patients achieved R0 resection (tumor was
resected and no pathological residual was observed) on their first
treatment; 2 patients received neoadjuvant chemotherapy. Baseline
clinical data included age, sex, primary tumor location, tumor
size, differentiation, Tumor (T) stage, lymph node metastasis,
stage, venous invasion, lymphatic invasion and
Tumor-Node-Metastasis (TNM) stage. The associations between the
clinicopathological parameters and CTC detection are displayed in
Table I.
 | Table I.Associations of clinicopathological
parameters with CTC detection. |
Table I.
Associations of clinicopathological
parameters with CTC detection.
|
|
| Pre-surgery, n | Post-surgery,
n |
|---|
|
|
|
|
|
|---|
| Variable | All | CTC-positive | P-value | CTC-positive | P-value |
|---|
| Age |
|
| 0.606 |
| 0.864 |
|
≥60 | 34 | 17 |
| 17 |
|
|
<60 | 21 | 12 |
| 10 |
|
| Sex |
|
| 0.721 |
| 0.469 |
|
Male | 46 | 25 |
| 24 |
|
|
Female | 9 | 4 |
| 3 |
|
| Location |
|
| 0.825 |
| 0.645 |
|
Upper | 7 | 4 |
| 3 |
|
|
Middle | 25 | 14 |
| 14 |
|
|
Lower | 23 | 11 |
| 10 |
|
| Size, cm |
|
>5 | 16 | 11 | 0.127 | 11 | 0.062 |
| ≤5 | 39 | 18 |
| 16 |
|
|
Differentiation |
|
| 0.188 |
| 0.936 |
|
Well | 3 | 2 |
| 1 |
|
|
Moderate | 13 | 4 |
| 6 |
|
|
Poor | 37 | 21 |
| 19 |
|
|
Other | 2 | 2 |
| 1 |
|
| T stage |
|
| 0.584 |
| 0.193 |
|
Tis | 1 | 0 |
| 0 |
|
| T1 | 4 | 2 |
| 0 |
|
| T2 | 4 | 3 |
| 3 |
|
| T3 | 42 | 21 |
| 22 |
|
| T4 | 4 | 3 |
| 2 |
|
| Venous
invasion |
|
| 0.205 |
| 0.322 |
|
Positive | 15 | 10 |
| 9 |
|
|
Negative | 40 | 19 |
| 18 |
|
| Lymphatic
invasion |
|
| 0.475 |
| 0.295 |
|
Positive | 9 | 6 |
| 6 |
|
|
Negative | 46 | 23 |
| 21 |
|
| TNM stage |
|
| 0.051 |
| 0.148 |
| I | 3 | 2 |
| 0 |
|
| II | 18 | 5 |
| 8 |
|
|
III | 32 | 21 |
| 19 |
|
| IV | 1 | 1 |
| 0 |
|
| Combination |
|
| 0.017 |
| 0.163 |
|
I+II | 21 | 7 |
| 8 |
|
|
III+IV | 33 | 22 |
| 19 |
|
The overall CTC detection rate was approximately
52.7% preoperatively and 49.1% postoperatively (data not shown).
The CTC and CTM are presented in Fig.
1. No significant differences were observed for CTC positivity
in terms of age, sex, location, size, differentiation, T stage,
venous invasion, or lymphatic invasion prior to or following
surgery. However, a difference in CTC positivity prior to surgery
was observed for TNM stage (P=0.051). Additionally, following the
combination of stages I and II, and stages III and IV, a
significant difference was observed despite 2 out of 3 stage I
patients being positive for CTCs, which was considered to be a
sampling error (P=0.017).
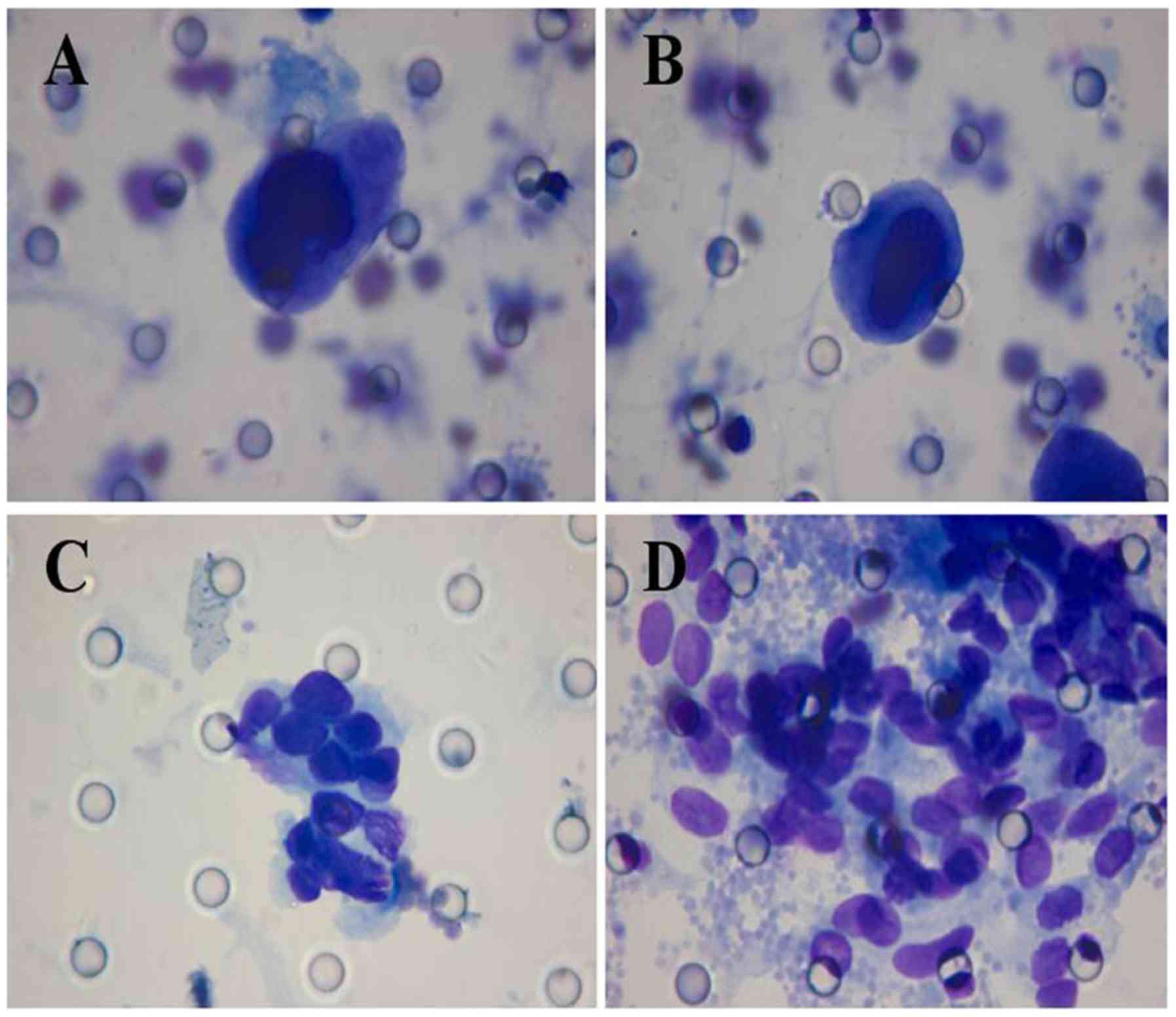 | Figure 1.Morphological analysis of CTCs/CTM in
patients with esophageal squamous cell carcinoma. Giemsa stain,
Diff-Quik staining method (magnification, ×1,000), isolated by
CTCBIOPSY®. (A) The diameter of the CTC was >40 µm,
the shape of the CTC was irregular, the nuclear membrane was
thickened, the nucleus was stained deeply, and a large amount of
cytoplasm was present and was stained blue. (B) The diameter of the
CTC was >40 µm, there was a relatively high ratio of nucleus to
cytoplasm, the nucleolus was irregularly shaped and not stained
equally, thick chromosomes were apparent, the nuclear membrane was
thickened, abnormal mitosis of the nucleus was present. (C) CTM was
apparent in 11 CTCs, the nucleoli were irregularly shaped, the
nuclear membranes were thickened and folded, nucleoli were present,
a large volume of cytoplasm was present and appeared pale blue. (D)
the nucleoli CTM were irregularly shaped and different in size, the
nuclear membrane was thickened and folded. CTC, circulating tumor
cell; CTM, circulating tumor microemboli. |
As for the number of CTCs or CTM, another issue was
encountered during detection. There were 18 samples from 6
patients, with three samples from each patient to test for
consistency during repeated detection; all samples were drawn
before 09:00 am on three separate preoperative days under almost
exactly the same conditions. The results are presented in Table II. Although the first detection
results indicate the presence of CTM or CTCs, the numbers were
highly variable, which was considered attributable to the
instantaneous sampling of the peripheral circulation and the
uncertainty of the internal physiological environment. Repeated CTC
detection for clinical stages III and IV is suggested if there is
special consideration of the count; however, further studies are
required to determine whether the mean or the maximal value should
be used.
 | Table II.Samples (n=18) from 6 patients for
CTC detection. |
Table II.
Samples (n=18) from 6 patients for
CTC detection.
|
| 1st detection | 2nd detection | 3rd detection |
|---|
|
|
|
|
|
|---|
| Patient, n | CTM | CTCs | CTM | CTCs | CTM | CTCs |
|---|
| 1 | 4 | – | – | 2 | 18 | 1 |
| 2 | 1 | 1 | – | 1 | – | – |
| 3 | – | – | – | – | – | – |
| 4 | 8 | 2 | 2 | 2 | 15 | 7 |
| 5 | 1 | 4 | 16 | – | 6 | 1 |
| 6 | 4 | 1 | 2 | 3 | – |
|
Systemic inflammatory response,
platelet count and CTC detection
CTC/CTM detection was not significantly associated
with the preoperative neutrophil-to-lymphocyte ratio (NLR), the
preoperative platelet-to-lymphocyte ratio (PLR) or the platelet
count, as displayed in Table III.
Nonetheless, the platelet count remained closely associated with
CTCs and CTM.
 | Table III.Associations of the NLR, PLR, and
platelet count with CTC detection. |
Table III.
Associations of the NLR, PLR, and
platelet count with CTC detection.
| Item | CTC-positive | CTC-negative | P-value |
|---|
| NLR (median ±
SD) |
3.12±1.54 |
2.96±1.32 | 0.3479 |
| PLR (median ±
SD) | 182.53±96.68 | 178.46±87.73 | 0.4364 |
| Platelet count
(×109/l, median ± SD) | 249.82±68.88 | 224.88±87.71 | 0.1282 |
Lymph node metastasis and CTC
detection
There were no LODDS4 patients in the present study.
A single patient was excluded from the analysis due to a large
difference in the lymph node dissection between the surgical
procedure and the pathological report. There were no significant
differences in CTC positivity between lymph node
metastasis-positive and negative patients prior to or following
surgery. However, there was a significant difference among the
LODDS1, LODDS2 and LODDS3 groups (P=0.033) prior to surgery
(Table IV). CTC positivity
significantly increased from LODDS1 to LODDS2 (P=0.027), but there
was no significant difference between LODDS2 and LODDS3 (P=0.063)
(Table IV).
 | Table IV.Associations of lymph node metastasis
with CTC detection. |
Table IV.
Associations of lymph node metastasis
with CTC detection.
|
|
| Pre-surgery, n | Post-surgery,
n |
|---|
|
|
|
|
|
|---|
| Variable | All | CTC-positive | P-value | CTC-positive | P-value |
|---|
| Lymph node
metastasis |
|
| 0.083 |
| 0.186 |
|
Positive | 33 | 21 |
| 18 |
|
|
Negative | 22 | 9 |
| 8 |
|
| LODDS |
|
| 0.033 |
| 0.918 |
|
LODDS1 | 11 | 5 |
| 6 |
|
|
LODDS2 | 16 | 14 |
| 10 |
|
|
LODDS3 | 5 | 2 |
| 3 |
|
Pre- and postoperative CTC detection
and surgical procedures
Liu et al (24) established a quantitative system for
evaluating the role of CTCs in patients with esophageal cancer who
had undergone surgery. It was postulated that surgery for
esophageal cancer results in tumor cell dissemination and a
significant increase in the number of CTCs in the peripheral blood,
which is associated with the development of metastasis. In the
present study, the number of CTCs detected prior to and following
different surgical procedures was compared: The thoracoscopic and
laparoscopic approach; and left thoracotomy or thoracotomy and
laparotomy (Table V). CTC detection
revealed that: i) CTCs or CTM declined following surgery, or in
particular cases, CTM disappeared entirely; or ii) CTCs or CTM
increased following surgery, or in particular cases, CTM developed
after previously being absent.
 | Table V.Associations of surgical procedures
with CTC detection. |
Table V.
Associations of surgical procedures
with CTC detection.
| Variable | CTCs or CTM
increase | CTCs or CTM
decline | P-value |
|---|
| Thoracoscopic and
Laparoscopic Approach | 9 | 16 | 0.864 |
| Left thoracotomy or
thoracotomy and laparotomy | 5 | 10 |
|
Confirmation of CTCs/CTM
To further confirm the presence of CTCs/CTM,
immunofluorescence staining for CD45 (leukocytes) and P40 (cells of
squamous epithelial origin) was conducted on portions of the
samples. The P40+/CD45− phenotype was
confirmed in CTCs and CTM (Fig. 2).
In particular cases, cells suspected to be CTCs or CTM were
confirmed to be leukocytes (Fig.
3).
Survival analysis
Cox proportional hazards regression analysis was
performed under the condition of inadequate follow up time. A total
of 9 parameters was analyzed, including sex, age, preoperative CTC
detection, postoperative CTC detection, surgical procedure
(minimally invasive esophagectomy or open surgery), differentiation
degree (poor or middle-high), tumor cutting area (≥5 or <5 cm),
infiltration depth (T1, T2, T3) and the number of positive lymph
nodes. A log-rank test was first used to filter the prognostic
factor (Table VI). As a result, the
factors of preoperative CTC detection, postoperative CTC detection,
differentiation degree (poor or middle-high), tumor cutting area,
and the number of positive lymph nodes were subjected to Cox
proportional hazards regression analysis. The results (including
hazard ratio) are displayed in Table
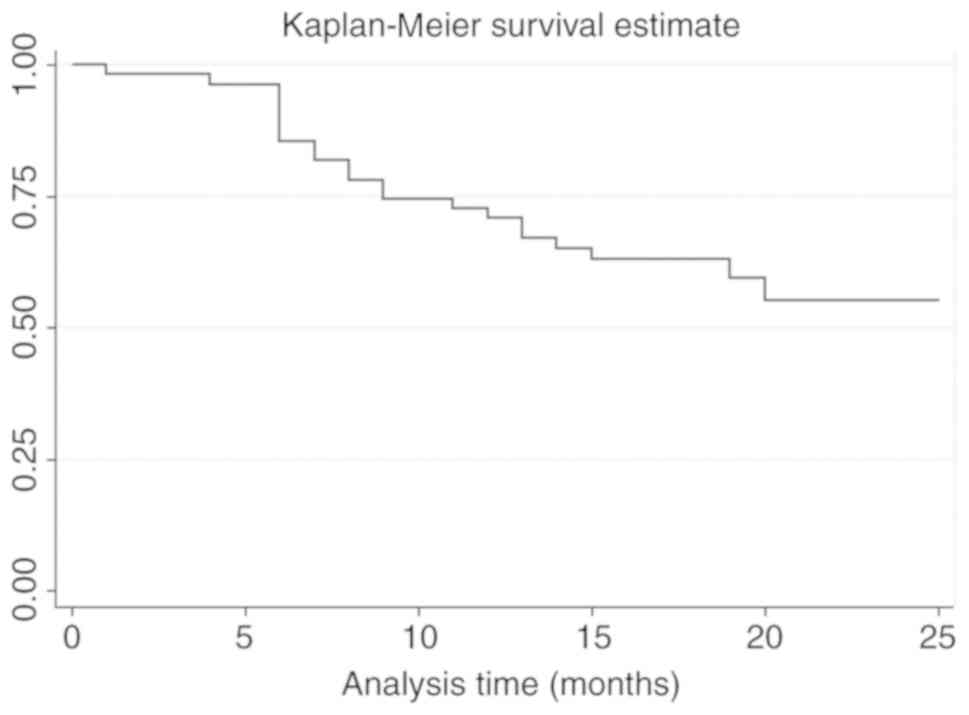
VII. The Kaplan-Meier survival curve is illustrated in Fig. 4.
 | Table VI.Log-rank test for filtering the
prognostic factors. |
Table VI.
Log-rank test for filtering the
prognostic factors.
| Prognostic
factors | χ2 | P-value |
|---|
| Sex | 0.67 | 0.4122 |
| Age | 0.30 | 0.5864 |
| CTCpre | 6.41 | 0.0113 |
| CTCpost | 4.20 | 0.0403 |
| Therapy | 0.18 | 0.6694 |
|
Differentiation | 9.49 | 0.0021 |
| Size | 6.72 | 0.0095 |
| Depth | 3.84 | 0.2788 |
| Node | 36.10 | 0.0002 |
 | Table VII.Cox proportional hazards regression
analysis. |
Table VII.
Cox proportional hazards regression
analysis.
| Prognostic
factor | Hazard ratio | Standard error | z | P>|z| | 95% confidence
interval |
|---|
| Preoperative CTC
detection | 1.84 | 1.05 | 1.06 | 0.29 | 0.60–5.66 |
| Postoperative CTC
detection | 1.19 | 0.70 | 0.30 | 0.77 | 0.38–3.75 |
| Differentiation
degree | 7.39 | 7.80 | 1.89 | 0.06 | 0.93–58.53 |
| Tumor cutting
area | 2.11 | 1.10 | 1.42 | 0.16 | 0.75–5.88 |
| Positive lymph
nodes | 1.12 | 0.06 | 1.94 | 0.052 | 0.99–1.25 |
Discussion
Various methods have been used to detect CTCs,
including those depending on tumor cell size, tumor-associated
markers, or reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)-based assays. The CellSearch® system
depends on tumor-associated markers and has been demonstrated to
have an extremely low detection rate in ESCC (17). Although RT-qPCR has been widely used
in the past few years (25), the
cell integrity is destroyed by RNA extraction, and benign cells are
present in the peripheral circulation (26). Isolation by size of epithelial tumor
cells is considered to be a suitable method for application in ESCC
(17). The present study was the
first to use isolation by size of epithelial tumor cells to detect
CTCs in patients with ESCC prior to and following surgery, and to
use immunofluorescence staining to observe the expression of P40
and CD45 by CTCs or CTM detected in patients with ESCC.
The 7th edition of the American Joint Committee on
Cancer (AJCC) Cancer Staging Manual describes stage 0 (Tis) and
stages I–IV. In the present study, the CTC detection rate prior to
surgery revealed a significant difference between stages I–II and
III–IV. This finding indicated that the CTC detection rate was
associated with the prognosis of ESCC. Other studies have also used
the CellSearch® system to isolate and enumerate CTCs
(19). Kaganoi et al
(18) used RT-qPCR to detect cancer
cells using specific mRNAs; patients who were positive for the mRNA
encoding squamous cell carcinoma antigen (SCCA mRNA) had a higher
recurrence rate compared with those who were negative for the
antigen. Also, SCCA mRNA was associated with the depth of the tumor
and venous invasion. However, in the present study, there was no
significant difference in CTC detection performed prior to and
following surgery.
Measuring the systemic inflammatory response is
another method for assessing the outcome of malignancies; this
method is simple and reliable and may be incorporated into current
staging procedures as Roxburgh and McMillan demonstrated in a
review that preoperative measures of the systemic inflammatory
response predict cancer survival (27,28).
Tumors interact with inflammatory cells, and the tumor-associated
inflammatory response may promote metastasis by upregulating
inflammatory mediators, inhibiting apoptosis, damaging DNA and
enhancing angiogenesis (29). An
elevated NLR was associated with significantly decreased
disease-free survival and overall survival (adenocarcinomas
accounted for 68% of these tumors) (30). However, another study has reported
that neither NLR nor PLR is an independent prognostic factor in
ESCC (31). Similarly, the present
study did not indicate a significant correlation between CTC
detection with NLR, PLR or platelet count, but these three
parameters displayed a positive trend for association with CTC
positivity compared with CTC negativity.
Notably, a single patient who accepted the left
thoracotomy surgery approach, was CTC positive, with postoperative
pathological staging of T1bN0M0, IB, while another patient, who
accepted the thoracoscopic and laparoscopic approach with thoracic
anastomosis (Ivor-Lewis), was CTM-positive with the same
postoperative pathological stage. This may reflect the current
staging system, or be due to a correlation with the platelet count.
The 7th Union for International Cancer Control/AJCC TNM edition
acknowledges the LNM as the current standard for N staging
(32), and the minimum suggested
number of lymph nodes harvested ranges from 12 to 18 (33,34). The
former patient had 21 lymph nodes sampled, while the latter patient
had 22. Theoretically, these numbers are sufficient for accurate
staging; however, as a result of the surgical approach the superior
mediastinal lymph nodes were not adequately sampled. In general,
two- or three-field lymph node dissection may increase the staging
accuracy. Additionally, there was a significant difference in the
CTC detection rate between stages I and II and stages III and IV,
while the platelet count did not reveal a significant difference.
Nevertheless, there was no significant difference in the CTC
detection rate for stages IIIB, IIIC and IV compared with stages I,
II and IIIA; though the platelet count did highlight a significant
difference. These findings suggest that an elevated platelet count
may promote cancer cell extravasation and increase the number of
CTCs in peripheral blood. Schumacher et al (35) demonstrated that platelets promote
cancer cell transendothelial migration via the P2Y2
receptor to facilitate tumor cell survival and dissemination. The
two previously mentioned patients had relatively high platelet
counts of 2.91×1011/l and 3.38×1011/l,
respectively, yet were not independently statistically analyzed due
to the small sample size. Platelet counts still revealed an obvious
trend when the presence of CTM was observed, using two-sample
Wilcoxon rank-sum tests due to the small sample size), although
there was no significant difference. From another point of view,
these results corroborate past research suggesting that a high
platelet count is associated with tumor progression and poor
survival in patients with ESCC.
Regarding the lymph node staging system, LODDS has
gained attention as a novel indicator and is defined as the log of
the ratio of the number of positive lymph nodes to the number of
negative lymph nodes. LODDS has been suggested as a powerful system
for predicting survival in gastric (22) and esophageal cancer (23). One study (23) of esophageal cancer illustrated that
LODDS predicts survival more accurately compared with the present
system of LNMs, and may serve as another indicator of the LNR.
Furthermore, LODDS is a factor that does not depend on the number
of lymph nodes sampled. Indeed, there are situations in which
surgeons cannot perform adequate lymph node resection due to
extensive pleural adhesions or advanced patient age. The present
study revealed that CTC positivity was associated with LODDS group
prior to surgery. While the connection between the presence of CTCs
and the LODDS group requires further research, the parameters hold
value for evaluating prognosis in esophageal cancer and reflecting
the tendency toward tumor cell metastasis.
An earlier study (24) demonstrated that surgery for
esophageal cancer results in cancer cell dissemination, which is
associated with metastasis. In the current study, CTCs were used to
investigate the effect of different surgical approaches which may
enhance CTC dissemination to different extents. No significant
difference was illustrated between the thoracoscopic and
laparoscopic approach and the open approach. Such research is not
easy to perform due to the uncertainty of the backflow of the vein
from the ESCC tumor body. In lung cancer, it has been demonstrated
that the pulmonary vein CTC count significantly increases at the
time of lobectomy completion. Additionally, the number of CTCs in
preoperative peripheral blood or intraoperative pulmonary venous
blood is an independent risk factor for tumor-free survival and
overall survival in patients with resected non-small-cell lung
cancer (36,37). The backflow of the
esophagus may pass through the azygos vein, inferior phrenic vein
or left gastric vein, and these veins could not be sampled at the
same time. As a result, direct evidence is difficult to obtain;
therefore, the increase in CTCs in peripheral blood may be
attributable to narcotism or stress.
Although the phenotype of CTCs and CTM harvested
from ESCC patients has been previously investigated (17), cytokeratin and vimentin levels were
used as indices to explain why CTM could not be detected by the
CellSearch® system. SCCA mRNA is detectable by RT-qPCR
(18) but with a confirmed lower
sensitivity compared with isolation by size of epithelial tumor
cells (10). In the present study,
immunofluorescence staining demonstrated for the first time that
the CTCs or CTM harvested by isolation by size of epithelial tumor
cells were indeed cells of squamous epithelial origin, and this
method was faster and less costly compared with RT-qPCR. The
procedure has great clinical significance in that abnormal cells
are definitively classified as CTCs and CTM, and different cells of
epithelial origin may be distinguished in the future. Furthermore,
chemotherapy protocols benefit from this procedure, especially for
synchronous cancers. It also revealed that identifying suspected
cells by morphology was not completely reliable. Clear
morphological standards or abnormal cell aggregates could not be
used as definite diagnostic criteria for blood samples in ECSS.
In Cox proportional hazards regression analysis,
preoperative CTC detection, postoperative CTC detection,
differentiation degree (poor or middle-high), tumor cutting area,
and the number of positive lymph nodes had a significant impact on
postoperative survival during factors screening. There was no
significant correlation between the factors and postoperative
mortality risk. Kaplan-Meier survival curves were separate
particularly when pre- and postoperative CTC detection were
positive. Long-term follow up is suggested to determine the impact
of CTC detection on postoperative survival. Circulating DNA
evaluated by next generation sequencing may be combined with the
aforementioned methods to predict tumor response, or to provide
more information on metastasis or survival.
In conclusion, the present study illustrated the
value of CTC detection in patients with ESCC, and provides a
specific approach for confirming other CTCs of epithelial origin.
Testing for the P40+/CD45− phenotype is
strongly advocated to ensure accurate identification.
Acknowledgements
The authors would like to thank Dr Chengke Zhang
(Second Hospital of Shandong University (Shandong, China) and Dr
Shili Han (Wuhan YZY Medical Science and Technology Co., Ltd.,
Wuhan, China) for their technical support.
Funding
The present study was funded by the National Key
Research and Development Program of China (grant no.
2016YFC0106005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ analyzed the patient data and wrote the
manuscript. SZ performed the experiments. YC made substantial
contributions to quality control of the experiments, and analyzed
and described the figures. XD, CP and QS acquired the data and were
involved in drafting the manuscript. LS and ZW interpreted the
data. XZ conceived and designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Hospital of Shandong University. All
procedures performed in studies involving human participants were
in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allum WH, Stenning SP, Bancewicz J, Clark
PI and Langley RE: Long-term results of a randomized trial of
surgery with or without preoperative chemotherapy in esophageal
cancer. J Clin Oncol. 27:5062–5067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographicregions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cells (CTC) detection: Clinical impact and future
directions. Cancer Lett. 253:180–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cristofanilli M, Hayes DF, Budd GT, Ellis
MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC,
et al: Circulating tumor cells: A novel prognostic factor for newly
diagnosed metastatic breast cancer. J Clin Oncol. 23:1420–1430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayes DF, Cristofanilli M, Budd GT, Ellis
MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV and
Terstappen LW: Circulating tumor cells at each follow-up time point
during therapy of metastatic breast cancer patients predict
progression-free and overall survival. Clin Cancer Res.
12:4218–4224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sabile A, Louha M, Bonte E, Poussin K,
Vona G, Mejean A, Chretien Y, Bougas L, Lacour B, Capron F, et al:
Efficiency of Ber-EP4 antibody in isolating circulating epithelial
tumor cells before RT-PCR detection. Am J Clin Pathol. 112:171–178.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulating tumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anker P, Mulcahy H and Stroun M:
Circulating nucleic acids in plasma and serum as a noninvasive
investigation for cancer: Time for large-scale clinical studies?
Int J Cancer. 103:149–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clarke LE, Leitzel K, Smith J, Ali SM and
Lipton A: Epidermal growth factor receptor mRNA in peripheral blood
of patients with pancreatic, lung, and colon carcinomas detected by
RT-PCR. Int J Oncol. 22:425–430. 2003.PubMed/NCBI
|
|
13
|
Chen TF, Jiang GL, Fu XL, Wang LJ, Qian H,
Wu KL and Zhao S: CK19 mRNA expression measured by
reverse-transcription polymerase chain reaction (RT-PCR) in the
peripheral blood of patients with non-small cell lung cancer
treated by chemo-radiation: An independent prognostic factor. Lung
Cancer. 56:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo J, Xiao B, Jin Z, Qin L, Chen J, Chen
H, Zhang X and Liu Z: Detection of cytokeratin 20 mRNA in the
peripheral blood of patients with colorectal cancer by
immunomagnetic bead enrichment and real-time reverse
transcriptase-polymerase chain reaction. J Gastroenterol Hepatol.
20:1279–1284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayes DC, Secrist H, Bangur CS, Wang T,
Zhang X, Harlan D, Goodman GE, Houghton RL, Persing DH and
Zehentner BK: Multigene real-time PCR detection of circulating
tumor cells in peripheral blood of lung cancer patients. Anticancer
Res. 26:1567–1575. 2006.PubMed/NCBI
|
|
16
|
Gervasoni A, Monasterio Muñoz RM, Wengler
GS, Rizzi A, Zaniboni A and Parolini O: Molecular signature
detection of circulating tumor cells using a panel of selected
genes. Cancer Lett. 263:267–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Song P, Zou B, Liu M, Cui K, Zhou P,
Li S and Zhang B: Circulating tumor cell analyses in patients with
esophageal squamous cell carcinoma using epithelial
marker-dependent and -independent approaches. Medicine (Baltimore).
94:e15652015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaganoi J, Shimada Y, Kano M, Okumura T,
Watanabe G and Imamura M: Detection of circulating oesophageal
squamous cancer cells in peripheral blood and its impact on
prognosis. Br J Surg. 91:1055–1060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita D, Uenosono Y, Arigami T,
Yanagita S, Nishizono Y, Hagihara T, Hirata M, Haraguchi N, Arima
H, Kijima Y, et al: Clinical significance of circulating tumor
cells in peripheral blood of patients with esophageal squamous cell
carcinoma. Ann Surg Oncol. 22:3674–3680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McKeown KC: Total three-stage
oesophagectomy for cancer of the oesophagus. Br J Surg. 63:259–262.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis I: The surgical treatment of
carcinoma of the oesophagus; with special reference to a new
operation for growths of the middle third. Br J Surg. 34:18–31.
1946. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jian-Hui C, Shi-Rong C, Hui W, Si-le C,
Jian-Bo X, Er-Tao Z, Chuang-Qi C and Yu-Long H: Prognostic value of
three different lymph node staging systems in the survival of
patients with gastric cancer following D2 lymphadenectomy. Tumor
Biol. 37:11105–11113. 2016. View Article : Google Scholar
|
|
23
|
Cao J, Yuan P, Ma H, Ye P, Wang Y, Yuan X,
Bao F, Lv W and Hu J: Log odds of positive lymph nodes predicts
survival in patients after resection for esophageal cancer. Ann
Thorac Surg. 102:424–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Jiang M, Zhao J and Ju H:
Circulating tumor cells in perioperative esophageal cancer
patients: Quantitative assay system and potential clinical utility.
Clin Cancer Res. 13:2992–2997. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pelkey TJ, Frierson HF Jr and Bruns DE:
Molecular and immunological detection of circulating tumor cells
and micrometastases from solid tumors. Clin Chem. 42:1369–1381.
1996.PubMed/NCBI
|
|
26
|
Hofman VJ, Ilie MI, Bonnetaud C, Selva E,
Long E, Molina T, Vignaud JM, Fléjou JF, Lantuejoul S, Piaton E, et
al: Cytopathologic detection of circulating tumor cells using the
isolation by size of epithelial tumor cell method. Am J Clin
Pathol. 135:146–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roxburgh CS and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeNardo DG, Johansson M and Coussens LM:
Immune cells as mediators of solid tumor metastasis. Cancer
Metastasis Rev. 27:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharaiha RZ, Halazun KJ, Mirza F, Port JL,
Lee PC, Neugut AI, Altorki NK and Abrams JA: Elevated preoperative
neutrophil: Lymphocyte ratio as a predictor of postoperative
disease recurrence in esophageal cancer. Ann Surg Oncol.
18:3362–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiaolei W: Clinical study on the
prognostic value of pre_operative neutrophil lymphocyte ratio and
platelet lymphocyte ratio in patients of esophageal squamous cell
carcinomaJinan, Shandong University: 2013
|
|
32
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: 2010
|
|
33
|
Dutkowski P, Hommel G, Böttger T, Schlick
T and Junginger T: How many lymph nodes are needed for an accurate
pN classification in esophageal cancer? Evidence for a new
threshold value. Hepatogastroenterology. 49:176–180.
2002.PubMed/NCBI
|
|
34
|
Rizk NP, Ishwaran H, Rice TW, Chen LQ,
Schipper PH, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, et al:
Optimum lymphadenectomy for esophageal cancer. Ann Surg. 251:46–50.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schumacher D, Strilic B, Sivaraj KK,
Wettschureck N and Offermanns S: Platelet-derived nucleotides
promote tumor-cell transendothelial migration and metastasis via
P2Y2 receptor. Cancer Cell. 24:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Cheng X, Chen Z, Liu Y, Liu Z and Xu
S: Circulating tumor cells in peripheral and pulmonary venous blood
predict poor long-term survival in resected non-small cell lung
cancer patients. Sci Rep. 7:49712017. View Article : Google Scholar : PubMed/NCBI
|