Cancer remains a principal cause of human
mortalities worldwide. The incidence of cancer and the consequent
mortalities are a principal cause for concern. It is estimated that
by 2030, there may be 22 million novel cases of cancer and 13
million mortalities worldwide each year due to different types of
cancer (1). In China, the incidence
of cancer has increased between 215.8 cases per 100,000 people in
2003 and 250.3 cases per 100,000 people in 2011 (2). The onset of gastrointestinal malignancy
is frequently clinically silent (3),
with the early occurrence of tumor invasion and metastasis leading
to high mortality rates (4). For
example, at present, the 5-year survival rate for pancreatic cancer
is 8%, whereas, the 5-year survival rate for patients with distant
metastases is 3% (5). Due to early
vessel metastasis of pancreatic cancer, it may be difficult to
conduct radical surgical resections for advanced gastrointestinal
malignancies in certain situations (6). Therefore, effective targeted therapy
may be indispensable in reducing the severity of gastrointestinal
malignancies and the degree of radical surgery required.
F-box/WD-40 repeat-containing protein 7 (Fbw7) is a
member of the F-box protein family. Fbw7 is an essential component
of the E3 ubiquitin ligase, S-phase kinase-associated protein 1
(Skp1)-cullin-1 (Cul1)-F-box (SCF)Fbw7, which serves as
a binding site for substrates, and mediates their ubiquitination
and degradation (7). The majority of
Fbw7 substrates regulate a number of cell behaviors, including
progression through the cell cycle, differentiation and apoptosis
(7). In solid tumors, Fbw7
substrates are typically identified to be oncoproteins. Therefore,
FBW7, which encodes Fbw7, is frequently considered a tumor
suppressor gene (7). In tumor cells,
FBW7 has been demonstrated to possess mutations and
deletions, to be methylated or post-transcriptionally modified
(8). Fbw7 expression is additionally
regulated by numerous proteins, resulting in its dysfunctional
expression and subsequent tumor progression (8). The present review aimed to provide a
comprehensive overview of the mechanisms involved in dysfunctional
Fbw7 expression caused by mutations in the FBW7 gene.
Additionally, it may provide information on the shared molecular
regulatory mechanisms involved in the progression of
gastrointestinal malignancies, including colorectal, liver, gastric
and pancreatic cancer, in addition to oral and esophageal squamous
cell carcinomas.
Fbw7 (additionally termed Fbxw7, hAgo, hCdc4 and
SEL-10) is a protein encoded by the FBW7 gene and is located
on the chromosomal band 4q32 (9).
Fbw7 is present as one of three subtypes: Fbw7α exists in the
nucleoplasm; Fbw7β exists in the cytoplasm; and Fbw7γ exists in the
nucleoli (9). Each of the three
subtypes are transcribed by a different promoter, and thus, are
considered as three different proteins (9). At present, the regulatory mechanisms of
the three proteins remain largely unknown. Of the three subtypes,
Fbw7α serves a leading ubiquitylation role (9). All of the three isoforms contain a
dimerization-domain that mediates protein dimerization and
regulates substrate binding and ubiquitination, an F-box connecting
Skp1 and a WD40 repeat region that forms a β propeller, binding to
phosphorylated substrates (9). Fbw7
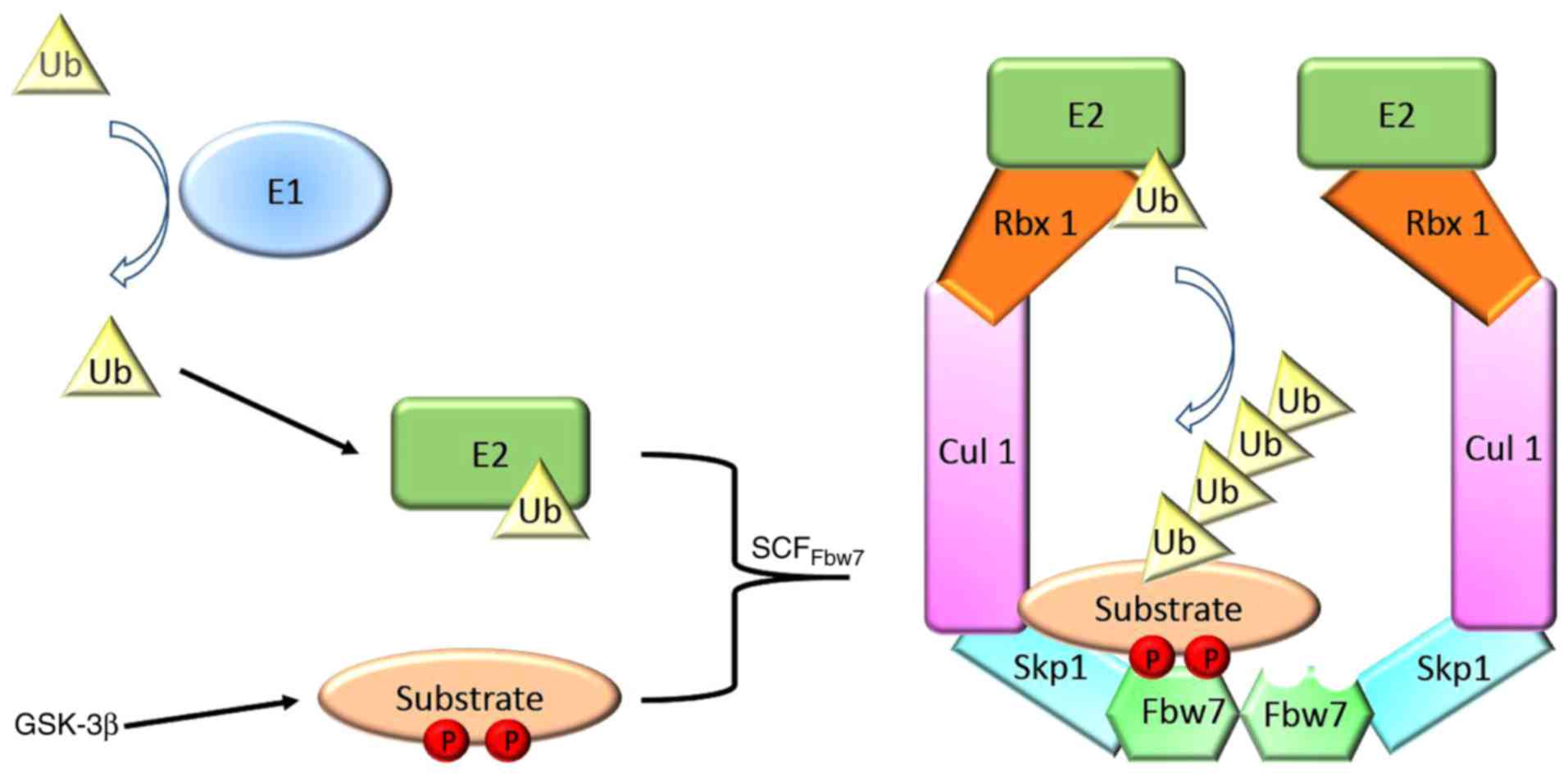
binds to Skp1, Cul1 and E3 ubiquitin-protein ligase RBX1 to form an
SCF E3 ubiquitin ligase, which allows the ubiquitination of
substrates, together with E1 ubiquitin-activating and E2
ubiquitin-conjugating enzymes (Fig.
1). The cell division control protein 4 phosphorylation domain
(CPD) of substrates is recognized by Fbw7 and leads to their
ubiquitylation subsequent to being phosphorylated by glycogen
synthase kinase 3 (GSK3). The majority of substrates that are
recognized for degradation by Fbw7 contain at least one CPD
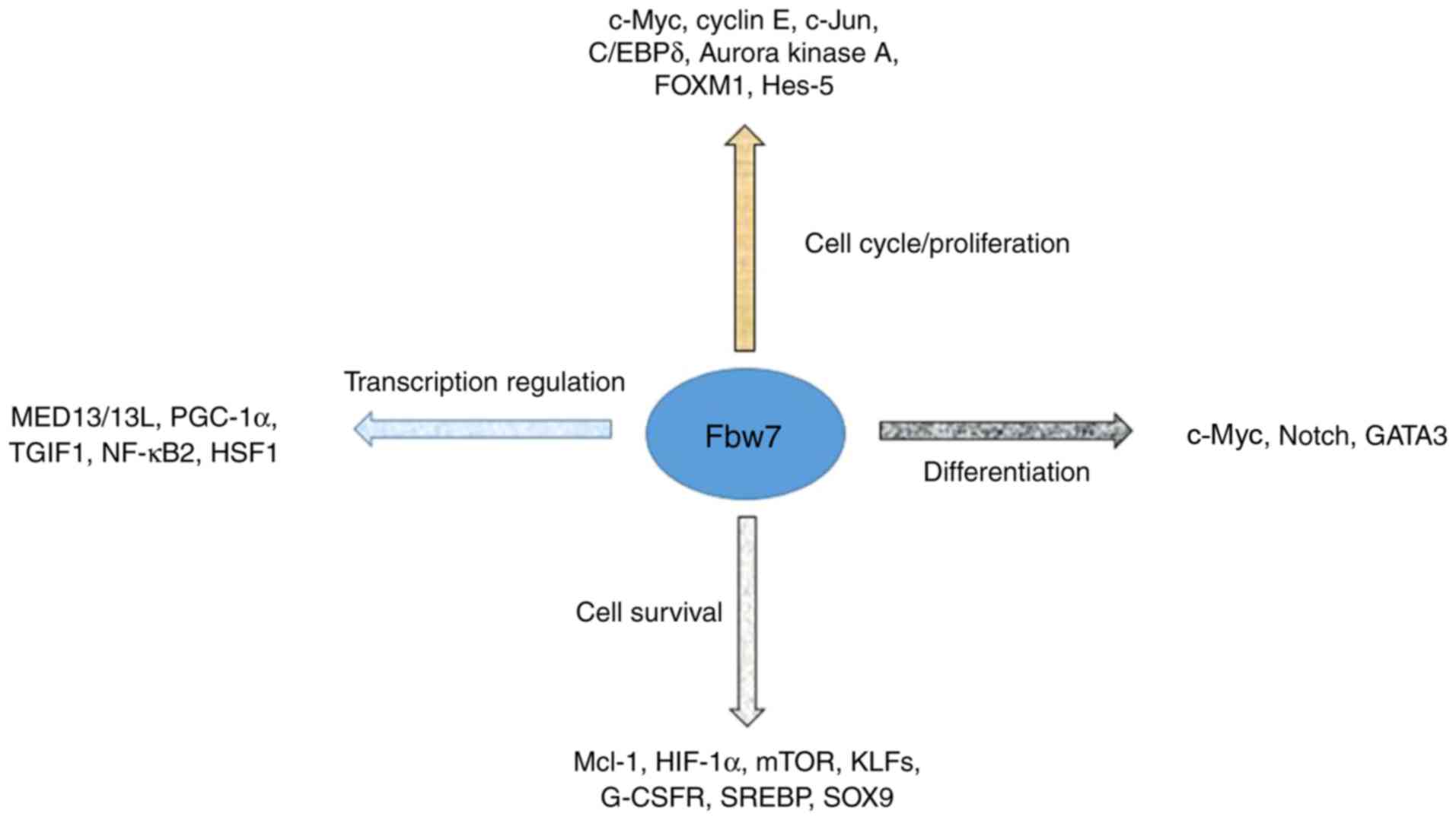
(10). Numerous substrates of Fbw7,
including Myc proto-oncogene protein (c-Myc), Neurogenic locus
notch homolog protein (Notch), cyclin E and c-Jun, are vital due to
their important regulatory role in tumor progression; Fbw7 serves
as a scavenger by degrading such substrates (9). Additionally, previous studies
demonstrated that substrates of Fbw7, including myeloid cell
leukemia 1, mediator complex subunit 13/13 ligand, Krüppel-like
factor 5, thymine guanine-interacting factor and numerous other
proteins, may additionally exert their influence on tumor
progression (Fig. 2) (11–14).
In normal tissues, Fbw7 is stably expressed,
maintaining a balance between tumor suppressor proteins and
proto-oncoproteins in vivo, thus inhibiting tumor
progression (15). In tumor tissues,
FBW7 is typically mutated, resulting in the downregulation or
dysfunction of Fbw7 (15). During
transcription, multiple transcriptional factors bind to Fbw7 mRNA
to decrease the expression level of Fbw7, including a number of
miRNAs (16,17) and p53 (18). Proteins may bind to the functional
region of Fbw7, resulting in an incomplete or non-functional E3
ubiquitin ligase and other proteins may promote the
self-ubiquitination of Fbw7, targeting itself for degradation,
including the role of Parkin (19).
Upregulation of proteins that result in the downregulation,
dysfunction or degradation of Fbw7 expression serve key roles in
tumorigenesis (Fig. 3).
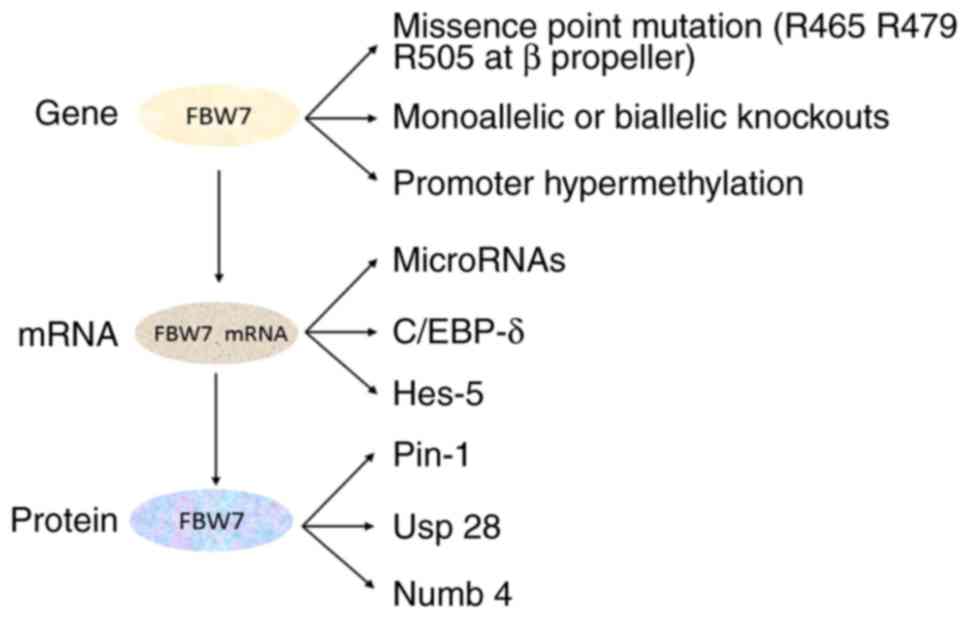
Missense point mutations on three arginine residues
(R465, R479 and R505) occurring at the β propeller
phosphorylation-binding site, impair the substrate recognition
function of Fbw7 (9). Additionally,
monoallelic or biallelic knockouts or the hypermethylation of the
promoter of FBW7 may occur in tumors, in humans and in mice
(20–22). Numerous previous studies demonstrated
that microRNAs (miRs), including miR-223 and miR-27a, bind to Fbw7
mRNA in tumors, leading to the inhibition of Fbw7 expression
(23,24). Dimerization and post-transcriptional
modifications stabilize the structure of Fbw7. Specifically,
dimerization of Fbw7 improves its affinity for substrates, whereas,
post-transcriptional modifications allow Fbw7 to alter between an
autocatalytic mode and a substrate degradation mode (7). A mutation of the CPD at the binding
site of Fbw7 decreases the affinity of Fbw7 for a substrate,
resulting in downstream substrate accumulation (25). In addition to mutations of
FBW7 and modifications of product structure subsequent to
transcription and translation, the expression of Fbw7 is regulated
by a variety of other factors. Ubiquitin specific peptidase (Usp)
28 regulates the stability of Fbw7 and the degradation of
substrates through a series of complex mechanisms (26). Loss of the deubiquitinase Usp28
monoallele stabilizes and facilitates Fbw7-mediated substrate
degradation, whereas, complete knockout of Usp28 promotes the
degradation of Fbw7, leading to the accumulation of Fbw7 substrates
(26). Cellular tumor antigen p53
(p53) directly binds to the first exon of FBW7 and promotes
Fbw7 expression. Therefore, targeted activation of the p53
signaling pathway exerts antitumor effects by restoring Fbw7
expression (18). In addition,
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1),
CCAAT/enhancer-binding protein-δ (C/EBP-δ), hairy and enhancer of
split (Hes)-5 and Numb additionally regulate the expression of Fbw7
(17). Of the aforementioned
proteins, C/EBP-δ and Hes-5 are additionally substrates of Fbw7
(13,27).
Fbw7 serves a crucial role in chemoresistance.
Chemotherapy is an effective adjuvant therapy to radical surgery.
However, the chemoresistance that cancer cells develop to various
chemotherapy drugs is one of the principal obstacles of
successfully treating cancer. The mutation in FBW7,
including FBW7 missense mutation in three arginine residues
(R465, R479 and R505), which leads to dysfunctional Fbw7, can cause
the accumulation of numerous substrates, which may be important in
chemoresistance (28). Gong et
al (29) conducted a
comprehensive review of the role of F-box proteins in
chemoresistance, including Fbw7. Numerous previous studies
investigated the involvement of Fbw7 in the chemoresistance of
numerous types of cancer (11,30–32). A
number of downstream targets of Fbw7, including c-Myc, nuclear
factor erythroid 2-related factor 2, myeloid leukemia cell
differentiation protein Mcl-1 (Mcl-1) and transcription factor
SOX-9, are involved in chemoresistance of various types of cancer,
including colorectal cancer, gastric cancer and pancreatic cancer
(11,30–32). In
comparison, a number of upstream proteins, including Epstein-Barr
virus nuclear antigen-binding protein 2 (33), C/EBP-δ (13) and miR-27a (34), may regulate FBW7 to increase
drug sensitivity. Therefore, the downregulation of such proteins
may lead to chemoresistance, and increasing the expression of these
proteins may be a novel method for overcoming chemoresistance.
Additionally, the miR-223/Fbw7 signaling pathway has been
demonstrated to serve an important role in chemoresistance of
numerous gastrointestinal malignancies, including oral and
esophageal squamous (35), gastric
(36), pancreatic (37) cancer. Therefore, targeting the
miR-223/Fbw7 pathway may be an effective way to overcome
chemoresistance in gastrointestinal cancer.
Fbw7 is able to promote ubiquitination and degrade a
number of key proteins that regulate the cell cycle, proliferation
and apoptosis and thus, serves as a tumor suppressor. In mitosis,
the deletion of Fbw7 causes hyperphosphorylation of a serine
residue at position 18 of the centromere recognition protein,
histone H3-like centromeric protein-A, by cyclin
E1/cyclin-dependent kinase 2 (CDK2) resulting in the inability of
the centromere to become localized, in addition to increased
chromosomal instability and the promotion of tumorigenesis
(38). Fbw7 additionally mediates
γ-catenin ubiquitination, resulting in an inhibition of the G2/M
cell cycle transition and cell proliferation (39). Furthermore, Fbw7 is closely
associated with epithelial to mesenchymal transition (EMT),
invasion and metastasis (40).
Numerous previous studies investigated the association between
FBW7 mutations and clinicopathological features of
gastrointestinal malignancies (Table
I). A number of the previous studies in Table I suggested that a poor prognosis was
closely associated with FBW7 mutations. In a number of
previous studies, it was demonstrated that aberrant Fbw7 expression
additionally serves a pivotal role in the resistance of numerous
gastrointestinal tumors to chemotherapeutic drugs (Table II). Therefore, examining novel
methods to restore the physiological expression levels of Fbw7 may
increase the sensitivity of tumors to chemotherapy and thus
facilitate the treatment of cancer.
Next-generation sequencing of 648 colorectal cancer
specimens demonstrated that the mutational rates of FBW7 in
the Chinese population was 5.32% (41). Disorders in Fbw7 expression caused by
FBW7 mutation, including mutation in Arg465
significantly increased the depth of invasion of colorectal cancer
and shortened the 5-year overall survival rate of patients
(41). The accumulation of cyclin E
and c-Myc caused by dysfunctional Fbw7 expression increases the
malignancy of colorectal cancer (42). A recent study demonstrated that Fbw7
restricted the metastasis of colorectal cancer cells by inhibiting
the hypoxia-inducible factor-α (HIF-α)/carcino-embryonic antigen
cell adhesion molecule 5 (CEACAM5) axis, which lead to a loss of
CEACAM5, the gene encoding a common tumor marker, CEA
(43). Polo-like kinase 2 expression
was increased in colorectal cancer tissues, and directly bound to
and phosphorylated Fbw7 on serine 176, forming a complex that
reduced the stability of Fbw7 and lead to retention of cyclin E,
which was beneficial to the proliferation of tumors (44,45).
Zinc finger protein 746 additionally inhibited c-Myc
phosphorylation by GSK3 and decreased the degradation of c-Myc by
Fbw7 (46). Furthermore, the
sequential upregulation of miR-182 and miR-503, may transform
colorectal adenomas into colorectal cancer by inhibiting Fbw7
expression (47). In contrast, the
deubiquitinase, ubiquitin specific peptidase 9, X-linked, inhibited
Fbw7 self-ubiquitination and proteolysis, stabilized and restored
Fbw7 function, and suppressed the progression of colorectal cancer
(48). The sensitivity of colorectal
cancer to chemotherapeutics is, to a certain extent, associated
with the expression of Fbw7. Downregulation of Fbw7 resulted in the
accumulation of cryptochrome 2, which increased the resistance of
colorectal cancer to oxaliplatin (49). Additionally, an increase in the Fbw7
substrate Mcl-1, mediated resistance of colorectal cancer to
regorafenib (28).
A previous Japanese study, which examined liver
cancer tissue samples from 66 cases and followed up the survival
rates of the patients, demonstrated that tumor-free survival of
patients with high Fbw7 expression was considerably longer compared
with patients with low Fbw7 expression (50). Multivariate analysis identified that
the decreased expression of Fbw7 was the strongest independent risk
factor for the recurrence of hepatocellular carcinoma (50). The positive correlation between Fbw7
and the survival rate of patients with liver cancer may be
associated with the downregulation of Notch-1 and the expression of
downstream matrix metalloproteinase (MMP)-2, MMP-9 and urokinase
plasminogen activator, which obstruct the invasion and metastasis
of liver cancer (51). Expression of
Fbw7 in liver cancer is additionally regulated by a wide-range of
proteins. Signal transduction and activator of transcription
(STAT)-1 expression downregulates the expression level of Fbw7 and
p53, increases the expression of downstream cyclin E, CDK2, Hes-1
and nuclear factor-κB (NF-κB) transcription factor p65, accelerates
cell growth and promotes G0/G1 cell cycle transition and apoptosis
(52). Long non-coding RNA cancer
susceptibility candidate 2 can bind and sequester miR-367,
restoring Fbw7 expression (53).
Fbw7 is associated with chemoresistance of liver cancer.
Hepatocellular carcinoma cell lines with increased expression of
Fbw7 were more sensitive to doxorubicin and demonstrated decreased
EMT (54). In addition, the increase
in expression of Mcl-1 by the Pin1-mediated downregulation of Fbw7
expression may enhance the resistance of liver cancer to sorafenib
(55).
To date, four missense mutations, a frame shift
mutation and a nonsense mutation of FBW7 have been
identified in gastric cancer tissues; and an FBW7 mutation
has been identified in early gastric cancer (56). An FBW7 monoallele deletion
increased the carcinogenic risk of healthy cells to
methylnitrosourea (57). A recent
Chinese study demonstrated that dysfunctional Fbw7 expression
caused by FBW7 mutations were associated with poor tissue
differentiation, survival and adjuvant chemotherapy resistance
(58). FBW7 mutations were
additionally associated with lymph node metastasis, tumor size and
p53 mutations in gastric cancer, leading to a poor prognosis
(59). The tumorigenic effects of
FBW7 mutations are manifested as a result of its
interactions with numerous substrates. Therefore, screening for
FBW7 mutations may improve the diagnosis and detection of
early gastric cancer. As a substrate of Fbw7, transforming protein
RhoA interacts with downstream effectors to damage the
cytoskeleton, restrict cell migration, disrupt the cell cycle of
tumors, and is closely associated with tumorigenesis and tumor
invasion (60). The expression of
Fbw7 in gastric cancer was inhibited by miR-223, which promoted
tumor apoptosis and proliferation. A previous in-depth study
demonstrated that the upregulation of miR-223 additionally
increased the resistance of gastric cancer cells to cisplatin by
downregulating the expression of Fbw7 and thus affecting G1/S cell
cycle transition (35). In receptor
tyrosine-protein kinase erbB-2 positive gastric cancer cell lines,
the miR-223/Fbw7 axis is a pivotal pathway for mediating
trastuzumab resistance (61,62).
A number of miRs regulate the expression of Fbw7 in
oral and esophageal cancer. miR-223 serves a role in the
progression of gastric cancer and esophageal cancer. Additionally,
miR-223 was upregulated and inhibited the expression of Fbw7,
resulting in a poor prognosis (34).
Furthermore, miR-25 was involved in the poor prognosis of patients
with esophageal cancer, by promoting invasion and metastasis of
esophageal cancer by downregulating the expression of Fbw7
(67). Although previous studies
identified an intrinsic association between FBW7 mutations
and the pathological features, and prognosis of oral and esophageal
squamous cell carcinomas (65–68),
There are a limited number of studies, to the best of our
knowledge, on the regulatory mechanisms of upstream and downstream
molecules.
A previous study on pancreatic cancer cell lines
overexpressing cyclin E demonstrated that a number of the cell
lines possessed an FBW7 allele loss, which promoted the
progression of pancreatic cancer (68). Fbw7 may inhibit cancer by degrading
numerous oncoproteins in pancreatic cancer. The expression of
exportin-1 (Xpo1) facilitates the transport of Fbw7, which prevents
the degradation of Notch-1 in the nucleus, leading to the
accumulation of c-Myc, cyclin D, Hes-1 and vascular endothelial
growth factor in the Notch signaling pathway (69). The Xpo1-specific inhibitor, KPT-185,
and Xpo1 inhibition by an inhibitor of nuclear export interferes
with this process (69). Fbw7
additionally degrades β-catenin to block an abnormally regulated
Wnt signaling pathway in pancreatic cancer (70). In the diagnosis of pancreatic cancer,
the Fbw7 expression level in pancreatic cancer was inversely
correlated with the maximum standardized uptake value in positron
emission tomography and coaxial tomography, and inhibited glucose
metabolism via an Fbw7/c-Myc/thioredoxin-interacting protein axis
(71). Additionally, Fbw7 expedited
the accumulation of equilibrative nucleoside transporter 1 by
inhibiting the degradation function of lysosomes, and enhanced the
sensitivity of pancreatic cancer to gemcitabine (72). The function of Fbw7 is additionally
regulated by a variety of mechanisms in pancreatic cancer. A
KRAS proto-oncogene, GTPase mutation activated extracellular
signal-regulated kinase (ERK) to directly bind to a threonine at
position 205 of Fbw7, resulting in ERK degradation. However, a lack
of threonine at this site in Fbw7 resulted in ERK dysfunction and
lead to an inability of Fbw7 degradation (73). Genistein inhibits cell growth,
invasion and metastasis, and induces apoptosis due to the increased
expression of miR-223 and decreased expression of Fbw7, promoting
EMT to enhance pancreatic cancer resistance to gemcitabine
(36,39). Notably, Fbw7 degrades the GSK3
phosphorylated miR-223 agonist, heterogenous nuclear
ribonucleoprotein K, resulting in miR-223 dysfunction (74).
Additionally, Fbw7 is involved in the progression of
other tumors. In a previous study by Yu et al (54), transfection of Fbw7 into a non-small
cell lung cancer cell line, NCI-H1299, which exhibits low
endogenous expression levels of Fbw7, significantly increased its
chemosensitivity to cisplatin, which was closely associated with
the effect of Fbw7 on EMT. Therefore, Fbw7 may be regarded as a
potential target for lung cancer resistance (75). An additional previous study on drug
resistance in non-small cell lung cancer demonstrated that ERK,
combined with GSK3β, phosphorylated the serine 159 residue of
Mcl-1, which promoted the degradation of Mcl-1 in the nucleus by
binding to Fbw7. In tumor tissues, the lack of Fbw7 disrupted this
process and induced drug resistance in non-small cell lung cancer
(76). Lin et al (77) demonstrated that Fbw7 overexpression
inhibited proliferation, invasion and metastasis of the glioma cell
lines, U251 and U373, by downregulating Aurora B, Mcl-1 and Notch-1
expression levels, and enhanced the cytotoxicity of temozolomide.
Fbw7 additionally serves a role in the development of
triple-negative breast cancer. Takada et al (78) demonstrated that EgIN2 prolyl
hydroxylase promoted tumorigenesis in breast cancer and was
regulated by Fbw7. Following phosphorylation of GSK3β at the
C-terminus of EgIN2, the latter became ubiquitylated and degraded
by Fbw7 (78). In addition, Fbw7
overexpression significantly decreased the viability of the
activated B cell-like diffuse large B lymphoma cell lines, SU-DHL-2
and OCI-LY-3, and increased their apoptotic rate (79). This process is caused by Fbw7
ubiquitination and degradation of STAT3 and phosphorylated
STAT3Tyr705, resulting in the dysfunction of downstream
anti-apoptotic proteins, Myc, survivin, Mcl-1,
serine/threonine-protein kinase pim-1, B-cell lymphoma-2 (Bcl-2)
and Bcl2-associated agonist of cell death (79).
Dysfunctional Fbw7 is closely associated with the
progression, invasion, metastasis and chemoresistance of tumors as
a result of deficiencies in the ubiquitylation and degradation of
substrates that serve as oncoproteins in tumor progression. In
gastrointestinal tumors, the Fbw7 expression level is typically
aberrantly downregulated, and the detailed mechanisms involved are
diverse. At the genetic level, FBW7 mutations may lead to
increased dysfunctional expression. At the transcriptional level,
miRs bind to Fbw7 mRNA, thus the proteins are not translated. At
the post-transcriptional level, the stability of Fbw7 is regulated
by numerous various upstream regulatory proteins, whereas,
physiological ubiquitination function is abrogated by
deubiquitinases. In addition, mutations in the CPD region of
substrates result in a loss of affinity to Fbw7, resulting in
dysfunctional ubiquitination by Fbw7. The frequent presence of
mutations in FBW7 in tumors may constitute FBW7 as an
early tumor screening gene. Fbw7 may additionally be used as an
essential protein target for targeted therapy to reverse
chemoresistance. Approaches against Fbw7 in anti-tumor therapy may
include knocking out FBW7 variant genes, targeting upstream
proteins of Fbw7 and substrates of Fbw7 that have cancer-promoting
effects, and exogenously upregulating the expression of Fbw7 to
reverse tumorigenesis.
Bromodomain containing protein-7 (BRD7), is a
subunit of switch/sucrose non-fermenting (SWI/SNF), which is an
evolutionarily conserved, large (~2 MDa) multi-subunit,
ATP-dependent chromatin remodeling complex that regulates
epigenetic architecture and cellular identity (80). Similar to Fbw7, mutations of BRD7 may
lead to SWI/SNF dysfunction, which causes aberrant gene expression
leading to various diseases, including prostate cancer, breast
cancer and nasopharyngeal cancer (80–82). The
mechanisms include regulating the cell cycle, serving as a
co-activator of p53, c-Myc, HIF-α and NF-κB, and downregulating the
expression of BRD7 in cancer (83).
Therefore, it was hypothesized that Fbw7 and BRD7 may be tumor
suppressors, and they may be synergistic in tumor inhibition.
However, the specific association between Fbw7 and BRD7 remains
unknown, and requires further study.
Compared with other gastrointestinal malignancies,
the survival rate for pancreatic cancer is decreased, suggesting
the urgency in identifying specific targets to reduce the degree of
radical surgery undertaken. Although a variety of adjuvant and
neoadjuvant chemotherapeutic agents exist, the overall survival
rate of pancreatic cancer has remained unaltered over the past few
decades. Therefore, studies on Fbw7 may provide novel insight for
targeted therapy for advanced pancreatic cancer. However, studies
of the role of Fbw7 in pancreatic cancer have only gradually
emerged in recent years (71,72,84).
A previous study demonstrated that Fbw7 regulates the expression of
NF-κB and vice versa (85). NF-κB is
closely correlated with angiogenesis, invasion and metastasis of
pancreatic cancer (86). However,
limited studies exist on the association between Fbw7 and NF-κB in
pancreatic cancer. Therefore, the regulation of Fbw7 and the NF-κB
signaling pathway may become the focus of future studies in
targeted therapy for pancreatic cancer.
In conclusion, the present review discusses the
mechanisms of Fbw7 in the progression of gastrointestinal
malignancies. Fbw7, in addition to upstream and downstream
regulatory proteins, were suggested to be potential tumor
therapeutic targets. Previous studies provided insight for the
regulatory mechanisms of Fbw7 in gastrointestinal malignancies.
However, specific issues require investigation. Upstream and
downstream signaling molecules and signaling pathways that
specifically affect Fbw7 expression have not yet been examined.
Additionally, the diagnostic specificity and sensitivity of
FBW7 as a biomarker for cancer and the veracity of prognosis
prediction requires further improvement. Furthermore, large-scale
clinical and multicenter trials as opposed to cell culture or
animal studies require gradual implementation to examine
chemotherapeutics targeting Fbw7.
Not applicable.
The present study was supported by The China Academy
of Medical Sciences Innovation Fund for Medical Sciences (grant no.
2016-I2M-3-019) and the non-profit Central Research Institute Fund
of Chinese of Academy of Medical Sciences (grant no.
2018PT32014).
Not applicable.
In this review, study concept and design was
conducted by YW and JG. YW and YA drafted the manuscript. Analysis
of data was performed by YA and YM. Critical revision of the
manuscript for important intellectual content was conducted by YW,
YA and JG. All authors agreed the final version.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsoi KK, Hirai HW, Chan FC, Griffiths S
and Sung JJ: Cancer burden with ageing population in urban regions
in China: Projection on cancer registry data from World Health
Organization. Br Med Bull. 121:83–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lieberman D, Ladabaum U, Cruz-Correa M,
Ginsburg C, Inadomi JM, Kim LS, Giardiello FM and Wender RC:
Screening for colorectal cancer and evolving issues for physicians
and patients: A review. JAMA. 316:2135–2145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arkan MC: Cancer: Fat and the fate of
pancreatic tumours. Nature. 536:157–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao
HL, Li H, Zhang SR, Xu JZ, Qi ZH, et al: Angiogenesis in pancreatic
cancer: Current research status and clinical implications.
Angiogenesis. Aug 24–2018.(Epub ahead of print).
|
|
7
|
Xu W, Taranets L and Popov N: Regulating
Fbw7 on the road to cancer. Semin Cancer Biol. 36:62–70. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao J, Ge MH and Ling ZQ: Fbxw7 tumor
suppressor: A vital regulator contributes to human tumorigenesis.
Medicine (Baltimore). 95:e24962016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis RJ, Welcker M and Clurman BE: Tumor
suppression by the Fbw7 ubiquitin ligase: Mechanisms and
opportunities. Cancer Cell. 26:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu K, Nihira NT, Inuzuka H and Wei W:
Physiological functions of FBW7 in cancer and metabolism. Cell
Signal. 46:15–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis MA, Larimore EA, Fissel BM, Swanger
J, Taatjes DJ and Clurman BE: The SCF-Fbw7 ubiquitin ligase
degrades MED13 and MED13L and regulates CDK8 module association
with Mediator. Genes Dev. 27:151–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balamurugan K, Sharan S, Klarmann KD,
Zhang Y, Coppola V, Summers GH, Roger T, Morrison DK, Keller JR and
Sterneck E: FBXW7α attenuates inflammatory signalling by
downregulating C/EBPδ and its target gene Tlr4. Nat Commun.
4:16622013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bengoechea-Alonso MT and Ericsson J: Tumor
suppressor Fbxw7 regulates TGFβ signaling by targeting TGIF1 for
degradation. Oncogene. 29:5322–5328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao B, Oehlmann S, Sowa ME, Harper JW and
Pavletich NP: Structure of a Fbw7-Skp1-cyclin E complex:
Multisite-phosphorylated substrate recognition by SCF ubiquitin
ligases. Mol Cell. 26:131–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olive V, Sabio E, Bennett MJ, De Jong CS,
Biton A, McGann JC, Greaney SK, Sodir NM, Zhou AY, Balakrishnan A,
et al: A component of the mir-17-92 polycistronic oncomir promotes
oncogene-dependent apoptosis. Elife. 2:e008222013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Ye X, Liu Y, Wei W and Wang Z:
Aberrant regulation of FBW7 in cancer. Oncotarget. 5:2000–2015.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura T, Gotoh M, Nakamura Y and Arakawa
H: hCDC4b, a regulator of cyclin E, as a direct transcriptional
target of p53. Cancer Sci. 94:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ekholm-Reed S, Goldberg MS, Schlossmacher
MG and Reed SI: Parkin-dependent degradation of the F-box protein
Fbw7b promotes neuronal survival in response to oxidative stress by
stabilizing Mcl-1. Mol Cell Biol. 33:3627–3643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhoondi S, Lindström L, Widschwendter M,
Corcoran M, Bergh J, Spruck C, Grandér D and Sangfelt O:
Inactivation of FBXW7/hCDC4-β expression by promoter
hypermethylation is associated with favorable prognosis in primary
breast cancer. Breast Cancer Res. 12:R1052010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Inuzuka H, Zhong J, Wan L,
Fukushima H, Sarkar FH and Wei W: Tumor suppressor functions of
FBW7 in cancer development and progression. FEBS Lett.
586:1409–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Sengupta T, Kukreja L and Minella
AC: MicroRNA-223 regulates cyclin E activity by modulating
expression of F-box and WD-40 domain protein 7. J Biol Chem.
285:34439–34446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng DD, Zhang H, Zhang P, Zheng YS, Zhang
XJ, Han BW, Luo XQ, Xu L, Zhou H, Qu LH, et al: Down-regulated
miR-331-5p and miR-27a are associated with chemotherapy resistance
and relapse in leukemia. J Cell Mol Med. 15:2164–2175. 2010.
View Article : Google Scholar
|
|
25
|
Cheng X, Hao Y, Shu W, Zhao M, Zhao C, Wu
Y, Peng X, Yao P, Xiao D, Qing G, et al: Cell cycle-dependent
degradation of the methyltransferase SETD3 attenuates cell
proliferation and liver tumorigenesis. J Biol Chem. 292:9022–9033.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schülein-Völk C, Wolf E, Zhu J, Xu W,
Taranets L, Hellmann A, Jänicke LA, Diefenbacher ME, Behrens A,
Eilers M, et al: Dual regulation of Fbw7 function and oncogenic
transformation by Usp28. Cell Rep. 9:1099–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sancho R, Blake SM, Tendeng C, Clurman BE,
Lewis J and Behrens A: Fbw7 repression by hes5 creates a feedback
loop that modulates Notch-mediated intestinal and neural stem cell
fate decisions. PLoS Biol. 11:e10015862013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong J, Tan S, Zou F, Yu J and Zhang L:
FBW7 mutations mediate resistance of colorectal cancer to targeted
therapies by blocking Mcl-1 degradation. Oncogene. 36:787–796.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong J, Zhou Y, Liu D and Huo J: F-box
proteins involved in cancer-associated drug resistance. Oncol Lett.
15:8891–8900. 2018.PubMed/NCBI
|
|
30
|
Suryo Rahmanto A, Swartling FJ and
Sangfelt O: Targeting SOX9 for degradation to inhibit
chemoresistance, metastatic spread, and recurrence. Mol Cell Oncol.
4:e12528712016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yada M, Hatakeyama S, Kamura T, Nishiyama
M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K and
Nakayama KI: Phosphorylation-dependent degradation of c-Myc is
mediated by the F-box protein Fbw7. EMBO J. 23:2116–2125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biswas M, Phan D, Watanabe M and Chan JY:
The Fbw7 tumor suppressor regulates nuclear factor E2-related
factor 1 transcription factor turnover through proteasome-mediated
proteolysis. J Biol Chem. 286:39282–39289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welcker M, Larimore EA, Frappier L and
Clurman BE: Nucleolar targeting of the fbw7 ubiquitin ligase by a
pseudosubstrate and glycogen synthase kinase 3. Mol Cell Biol.
31:1214–1224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lerner M, Lundgren J, Akhoondi S, Jahn A,
Ng HF, Akbari Moqadam F, Oude Vrielink JA, Agami R, Den Boer ML,
Grandér D and Sangfelt O: MiRNA-27a controls FBW7/hCDC4-dependent
cyclin E degradation and cell cycle progression. Cell Cycle.
10:2172–2183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurashige J, Watanabe M, Iwatsuki M,
Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K and
Baba H: Overexpression of microRNA-223 regulates the ubiquitin
ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer.
106:182–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Guo Y, Liang X, Sun M, Wang G, De W
and Wu W: MicroRNA-223 functions as an oncogene in human gastric
cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol.
138:763–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng
F, Miele L, Sarkar FH, Xia J and Wang Z: Genistein down-regulates
miR-223 expression in pancreatic cancer cells. Curr Drug Targets.
14:1150–1156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takada M, Zhang W, Suzuki A, Kuroda TS, Yu
Z, Inuzuka H, Gao D, Wan L, Zhuang M, Hu L, et al: FBW7 loss
promotes chromosomal instability and tumorigenesis via Cyclin
E1/CDK2-mediated phosphorylation of CENP-A. Cancer Res.
77:4881–4893. 2017.PubMed/NCBI
|
|
39
|
Li Y, Hu K, Xiao X, Wu W, Yan H, Chen H,
Chen Z and Yin D: FBW7 suppresses cell proliferation and G2/M cell
cycle transition via promoting γ-catenin K63-linked ubiquitylation.
Biochem Biophys Res Commun. 497:473–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng
L, Shi Y, Wang H, Yin B, Xia J and Wang Z: Down-regulation of
miR-223 reverses epithelial-mesenchymal transition in
gemcitabine-resistant pancreatic cancer cells. Oncotarget.
6:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Liu H, Hou Y, Zhou X, Liang L,
Zhang Z, Shi H, Xu S, Hu P, Zheng Z, et al: Performance validation
of an amplicon-based targeted next-generation sequencing assay and
mutation profiling of 648 Chinese colorectal cancer patients.
Virchows Arch. 472:959–968. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iwatsuki M, Mimori K, Ishii H, Yokobori T,
Takatsuno Y, Sato T, Toh H, Onoyama I, Nakayama KI, Baba H and Mori
M: Loss of FBXW7, a cell cycle regulating gene, in colorectal
cancer: Clinical significance. Int J Cancer. 126:1828–1837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Q, Li Y, Li J, Ma Y, Dai W, Mo S, Xu Y,
Li X and Cai S: FBW7 suppresses metastasis of colorectal cancer by
inhibiting HIF1α/CEACAM5 functional axis. Int J Biol Sci.
14:726–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ou B, Zhao J, Guan S, Wangpu X, Zhu C,
Zong Y, Ma J, Sun J, Zheng M, Feng H and Lu A: Plk2 promotes tumor
growth and inhibits apoptosis by targeting Fbxw7/Cyclin E in
colorectal cancer. Cancer Lett. 380:457–466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cizmecioglu O, Krause A, Bahtz R, Ehret L,
Malek N and Hoffmann I: Plk2 regulates centriole duplication
through phosphorylation-mediated degradation of Fbxw7 (human Cdc4).
J Cell Sci. 125:981–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jung JH, Jung DB, Kim H, Lee H, Kang SE,
Srivastava SK, Yun M and Kim SH: Zinc finger protein 746 promotes
colorectal cancer progression via c-Myc stability mediated by
glycogen synthase kinase 3β and F-box and WD repeat
domain-containing 7. Oncogene. 37:3715–3728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li L, Sarver AL, Khatri R, Hajeri PB,
Kamenev I, French AJ, Thibodeau SN, Steer CJ and Subramanian S:
Sequential expression of miR-182 and miR-503 cooperatively targets
FBXW7, contributing to the malignant transformation of colon
adenoma to adenocarcinoma. J Pathol. 234:488–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Khan OM, Carvalho J, Spencer-Dene B,
Mitter R, Frith D, Snijders AP, Wood SA and Behrens A: The
deubiquitinase USP9X regulates FBW7 stability and suppresses
colorectal cancer. J Clin Invest. 128:1326–1337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fang L, Yang Z, Zhou J, Tung JY, Hsiao CD,
Wang L, Deng Y, Wang P, Wang J and Lee MH: Circadian clock gene
CRY2 degradation is involved in chemoresistance of colorectal
cancer. Mol Cancer Ther. 14:1476–1487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Imura S, Tovuu LO, Utsunomiya T, Morine Y,
Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Takasu C, et
al: The role of Fbxw7 expression in hepatocellular carcinoma and
adjacent non-tumor liver tissue. J Gastroenterol Hepatol.
29:1822–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Zhang J, Zhou L, Sun W, Zheng ZG,
Lu P, Gao Y, Yang XS, Zhang ZC, Tao KS and Dou KF: Fbxw7 regulates
hepatocellular carcinoma migration and invasion via Notch1
signaling pathway. Int J Oncol. 47:231–243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen J, Wang H, Wang J, Huang S and Zhang
W: STAT1 inhibits human hepatocellular carcinoma cell growth
through induction of p53 and Fbxw7. Cancer Cell Int. 15:1112015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang
C, Dou C, Xu M, Liu Q and Tu K: Long non-coding RNA CASC2
suppresses epithelial-mesenchymal transition of hepatocellular
carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer.
16:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu J, Zhang W, Gao F, Liu YX, Chen ZY,
Cheng LY, Xie SF and Zheng SS: FBW7 increases chemosensitivity in
hepatocellular carcinoma cells through suppression of
epithelial-mesenchymal transition. Hepatobiliary Pancreat Dis Int.
13:184–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zheng M, Xu H, Liao XH, Chen CP, Zhang AL,
Lu W, Wang L, Yang D, Wang J, Liu H, et al: Inhibition of the
prolyl isomerase Pin1 enhances the ability of sorafenib to induce
cell death and inhibit tumor growth in hepatocellular carcinoma.
Oncotarget. 8:29771–29784. 2017.PubMed/NCBI
|
|
56
|
Lee JW, Soung YH, Kim HJ, Park WS, Nam SW,
Kim SH, Lee JY, Yoo NJ and Lee SH: Mutational analysis of the hCDC4
gene in gastric carcinomas. Eur J Cancer. 42:2369–2373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang Y, Qi X, Liu X, Zhang J, Ji J, Zhu
Z, Ren J and Yu Y: Fbxw7 haploinsufficiency loses its protection
against DNA damage and accelerates MNU-induced gastric
carcinogenesis. Oncotarget. 8:33444–33456. 2017.PubMed/NCBI
|
|
58
|
Li MR, Zhu CC, Ling TL, Zhang YQ, Xu J,
Zhao EH and Zhao G: FBXW7 expression is associated with prognosis
and chemotherapeutic outcome in Chinese patients with gastric
adenocarcinoma. BMC Gastroenterol. 17:602017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Onoyama I, Fukagawa T, Kuwano H, Nakayama KI and Mori M:
p53-Altered FBXW7 expression determines poor prognosis in gastric
cancer cases. Cancer Res. 69:3788–3794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li H, Wang Z, Zhang W, Qian K, Xu W and
Zhang S: Fbxw7 regulates tumor apoptosis, growth arrest and the
epithelial-to-mesenchymal transition in part through the RhoA
signaling pathway in gastric cancer. Cancer Lett. 370:39–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou X, Jin W, Jia H, Yan J and Zhang G:
MiR-223 promotes the cisplatin resistance of human gastric cancer
cells via regulating cell cycle by targeting FBXW7. J Exp Clin
Cancer Res. 34:282015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Eto K, Iwatsuki M, Watanabe M, Ishimoto T,
Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, et al:
The sensitivity of gastric cancer to trastuzumab is regulated by
the miR-223/FBXW7 pathway. Int J Cancer. 136:1537–1545. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Naganawa Y, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Katada T, Tanaka T, Shiozaki M, Fujii Y and Takeyama
H: Decreased expression of FBXW7 is correlated with poor prognosis
in patients with esophageal squamous cell carcinoma. Exp Ther Med.
1:841–846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Tanaka F, Sato T, Toh H, Sudo T, Iwaya T, Tanaka Y, et al: Copy
number loss of FBXW7 is related to gene expression and poor
prognosis in esophageal squamous cell carcinoma. Int J Oncol.
41:253–259. 2012.PubMed/NCBI
|
|
65
|
Er TK, Wang YY, Chen CC,
Herreros-Villanueva M, Liu TC and Yuan SS: Molecular
characterization of oral squamous cell carcinoma using targeted
next-generation sequencing. Oral Dis. 21:872–878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Arita H, Nagata M, Yoshida R, Matsuoka Y,
Hirosue A, Kawahara K, Sakata J, Nakashima H, Kojima T, Toya R, et
al: FBXW7 expression affects the response to chemoradiotherapy and
overall survival among patients with oral squamous cell carcinoma:
A single-center retrospective study. Tumour Biol. Oct 26–2017.(Epub
ahead of print). doi: 1010428317731771, 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hua Y, Zhao K, Tao G, Dai C and Su Y:
miR-25 promotes metastasis via targeting FBXW7 in esophageal
squamous cell carcinoma. Oncol Rep. 38:3030–3038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Calhoun ES, Jones JB, Ashfaq R, Adsay V,
Baker SJ, Valentine V, Hempen PM, Hilgers W, Yeo CJ, Hruban RH and
Kern SE: BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in
distinct subsets of pancreatic cancer: Potential therapeutic
targets. Am J Pathol. 163:1255–1260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gao J, Azmi AS, Aboukameel A, Kauffman M,
Shacham S, Abou-Samra AB and Mohammad RM: Nuclear retention of Fbw7
by specific inhibitors of nuclear export leads to Notch1
degradation in pancreatic cancer. Oncotarget. 5:3444–3454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang JX, Sun CY, Tian S, Yu C, Chen MY
and Zhang H: Tumor suppressor Fbxw7 antagonizes WNT signaling by
targeting β-catenin for degradation in pancreatic cancer. Tumour
Biol. 37:13893–13902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ji S, Qin Y, Liang C, Huang R, Shi S, Liu
J, Jin K, Liang D, Xu W, Zhang B, et al: FBW7 (F-box and WD Repeat
Domain-Containing 7) negatively regulates glucose metabolism by
targeting the c-Myc/TXNIP (Thioredoxin-Binding Protein) axis in
pancreatic cancer. Clin Cancer Res. 22:3950–3960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu Q, Qin Y, Zhang B, Liang C, Ji S, Shi
S, Xu W, Xiang J, Liang D, Ni Q, et al: FBW7 increases the
chemosensitivity of pancreatic cancer cells to gemcitabine through
upregulation of ENT1. Oncol Rep. 38:2069–2077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H,
Gao J, Zhang B, Xu W, Liu J, et al: ERK kinase phosphorylates and
destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell
Res. 25:561–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He D, Huang C, Zhou Q, Liu D, Xiong L,
Xiang H, Ma G and Zhang Z: HnRNPK/miR-223/FBXW7 feedback cascade
promotes pancreatic cancer cell growth and invasion. Oncotarget.
8:20165–20178. 2017.PubMed/NCBI
|
|
75
|
Yu HG, Wei W, Xia LH, Han WL, Zhao P, Wu
SJ, Li WD and Chen W: FBW7 upregulation enhances cisplatin
cytotoxicity in non-small cell lung cancer cells. Asian Pac J
Cancer Prev. 14:6321–6326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ye M, Zhang Y, Zhang X and Zhang J, Jing
P, Cao L, Li N, Li X, Yao L and Zhang J and Zhang J: Targeting FBW7
as a strategy to overcome resistance to targeted therapy in
non-small cell lung cancer. Cancer Res. 77:3527–3539. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lin J, Ji A, Qiu G, Feng H, Li J, Li S,
Zou Y, Cui Y, Song C, He H and Lu Y: FBW7 is associated with
prognosis, inhibits malignancies and enhances temozolomide
sensitivity in glioblastoma cells. Cancer Sci. 109:1001–1011. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Takada M, Zhuang M, Inuzuka H, Zhang J,
Zurlo G, Zhang J and Zhang Q: EglN2 contributes to triple negative
breast tumorigenesis by functioning as a substrate for the FBW7
tumor suppressor. Oncotarget. 8:6787–6795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yao S, Xu F, Chen Y, Ge Y, Zhang F, Huang
H, Li L, Lin D, Luo X, Xu J, et al: Fbw7 regulates apoptosis in
activated B-cell like diffuse large B-cell lymphoma by targeting
Stat3 for ubiquitylation and degradation. J Exp Clin Cancer Res.
36:102017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liang Y, Dong B, Shen J, Ma C and Ma Z:
Clinical significance of bromodomain-containing protein 7 and its
association with tumor progression in prostate cancer. Oncol Lett.
17:849–856. 2019.PubMed/NCBI
|
|
81
|
Niu W, Luo Y, Wang X, Zhou Y, Li H, Wang
H, Fu Y, Liu S, Yin S, Li J, et al: BRD7 inhibits the Warburg
effect and tumor progression through inactivation of HIF1α/LDHA
axis in breast cancer. Cell Death Dis. 9:5192018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu Y, Zhao R, Wei Y, Li M, Wang H, Niu W,
Zhou Y, Qiu Y, Fan S, Zhan Y, et al: BRD7 expression and c-Myc
activation forms a double-negative feedback loop that controls the
cell proliferation and tumor growth of nasopharyngeal carcinoma by
targeting oncogenic miR-141. J Exp Clin Cancer Res. 37:642018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yu X, Li Z and Shen J: BRD7: A novel tumor
suppressor gene in different cancers. Am J Transl Res. 8:742–748.
2016.PubMed/NCBI
|
|
84
|
Jin X, Yang C, Fan P, Xiao J, Zhang W,
Zhan S, Liu T, Wang D and Wu H: CDK5/FBW7-dependent ubiquitination
and degradation of EZH2 inhibits pancreatic cancer cell migration
and invasion. J Biol Chem. 292:6269–6280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Arabi A, Ullah K, Branca RM, Johansson J,
Bandarra D, Haneklaus M, Fu J, Ariës I, Nilsson P, Den Boer ML, et
al: Proteomic screen reveals Fbw7 as a modulator of the NF-κB
pathway. Nat Commun. 3:9762012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zeligs KP, Neuman MK and Annunziata CM:
Molecular pathways: The balance between cancer and the immune
system challenges the therapeutic specificity of targeting nuclear
Factor-κB signaling for cancer treatment. Clin Cancer Res.
22:4302–4308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chang CC, Lin HH, Lin JK, Lin CC, Lan YT,
Wang HS, Yang SH, Chen WS, Lin TC, Jiang JK and Chang SC: FBXW7
mutation analysis and its correlation with clinicopathological
features and prognosis in colorectal cancer patients. Int J Biol
Markers. 30:e88–e95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tu K, Zheng X, Zan X, Han S, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with the
expression of c-Myc, cyclin E and p53 in human hepatocellular
carcinoma. Hepatol Res. 42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Enkhbold C, Utsunomiya T, Morine Y, Imura
S, Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Ishikawa
D and Shimada M: Loss of FBXW7 expression is associated with poor
prognosis in intrahepatic cholangiocarcinoma. Hepatol Res.
44:E346–E352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ishii N, Araki K, Yokobori T, Gantumur D,
Yamanaka T, Altan B, Tsukagoshi M, Igarashi T, Watanabe A, Kubo N,
et al: Reduced FBXW7 expression in pancreatic cancer correlates
with poor prognosis and chemotherapeutic resistance via
accumulation of MCL1. Oncotarget. 8:112636–112646. 2017. View Article : Google Scholar : PubMed/NCBI
|