Within recent years, dermatologic imaging technology
focused on the development of optical, noninvasive tools to improve
diagnostic accuracy and to overcome the disadvantages of
histopathological examination. Of all these promising in
vivo tools, only confocal laser scanning microscopy (CLSM)
allows the visualization of cutaneous structures with a resolution
that is very close to that of light microscopy, thus performing a
skin ‘optical biopsy’ (1). It
enables the noninvasive, virtual sectioning of the skin at
different depths, with grey-scale images obtained in horizontal
planes (en face), parallel to the skin surface and it does
not require tissue processing or coloring (2,3). As it
allows repeated imaging of the same skin area in real-time, at
different time intervals, it is an excellent method for monitoring
disease progression and treatment efficacy and studying skin's
dynamic behaviour (1,4–10).
Based on the source of image contrast, CLSM can be
performed in either fluorescence or reflectance mode. Fluorescence
confocal microscopy (FCM) requires the administration of a
fluorescent agent to generate contrast (11) and has been used predominantly in
experimental studies with promising results in lesional and
nonlesional skin (12,13). Reflectance confocal microscopy (RCM)
relies on differences in the refractive indices of cellular
structures (14) and has been
extensively applied in the noninvasive assessment of melanocytic
(15–18) and non-melanocytic skin tumors
(16,19), with features demonstrating a good
correlation with dermoscopic and histologic findings. Furthermore,
this novel imaging technique proved to be useful for the diagnosis
of various inflammatory skin diseases (20), conditions with dermatologic
manifestations (21), as well as for
the study of dynamic processes like wound healing (22,23), in
real-time assessment of blood flow in response to various topical
stimuli (10,24) or leucocyte migration (5,6).
In this study, we present the RCM characteristic
features of the most common melanocytic and non-melanocytic skin
tumors (Table I), as well as future
possible CLSM applications in the study of experimental skin
tumorigenesis on animal models.
Basal cell carcinoma (BCC) is the most common of all
cancers in light-skinned individuals (27) and its incidence is still rising with
~10% each year worldwide (28). Very
often, a skin biopsy is needed to confirm the diagnosis, despite
its associated invasiveness and costs. Early diagnosis and
treatment are of paramount importance because it is locally
destructive and can lead to disfigurement (29,30). RCM
diagnostic features for various clinical types of BCC have been
described (31–36), demonstrating a good correlation with
certain dermoscopic and histopathologic findings (37,39). BCC
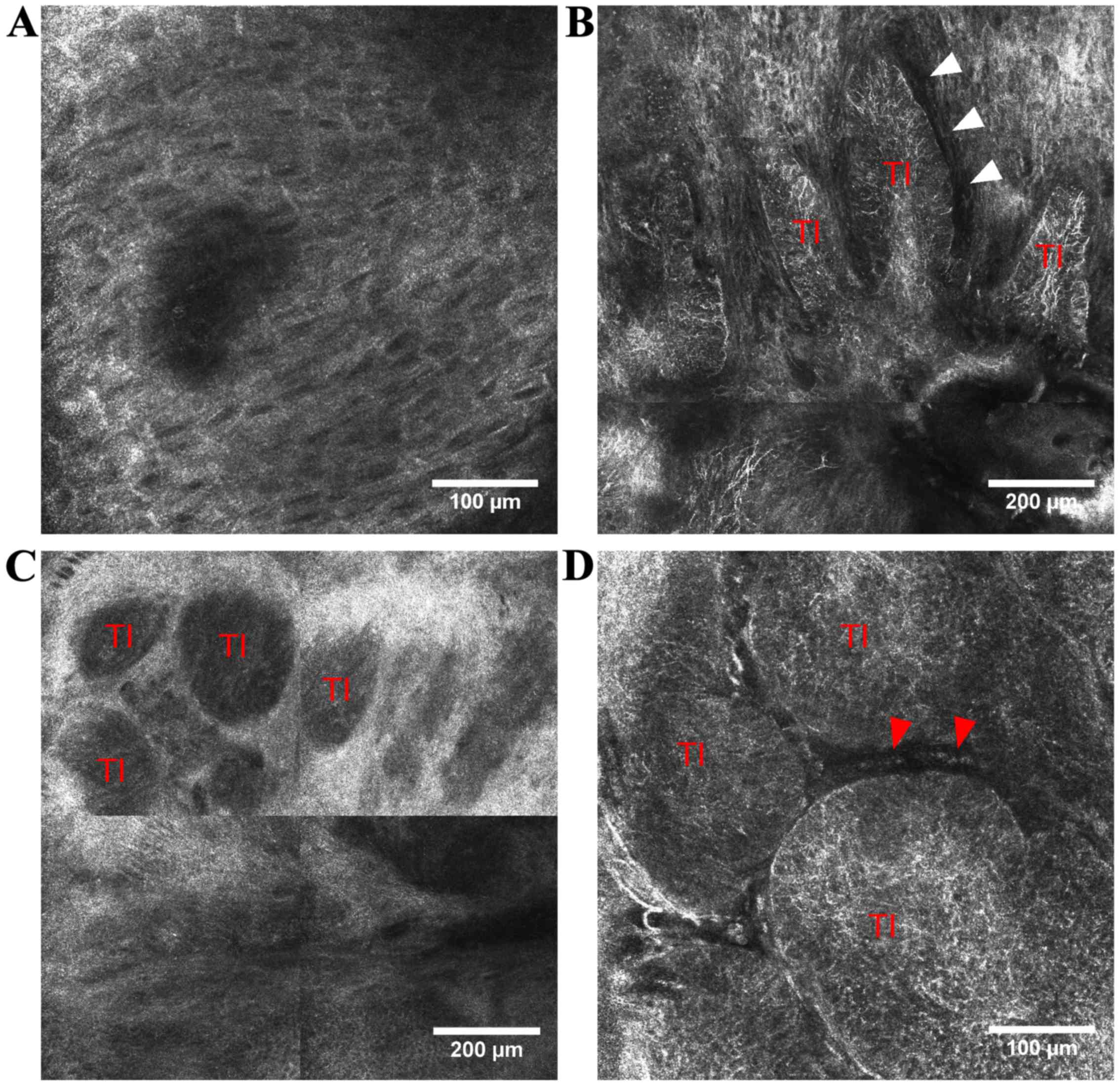
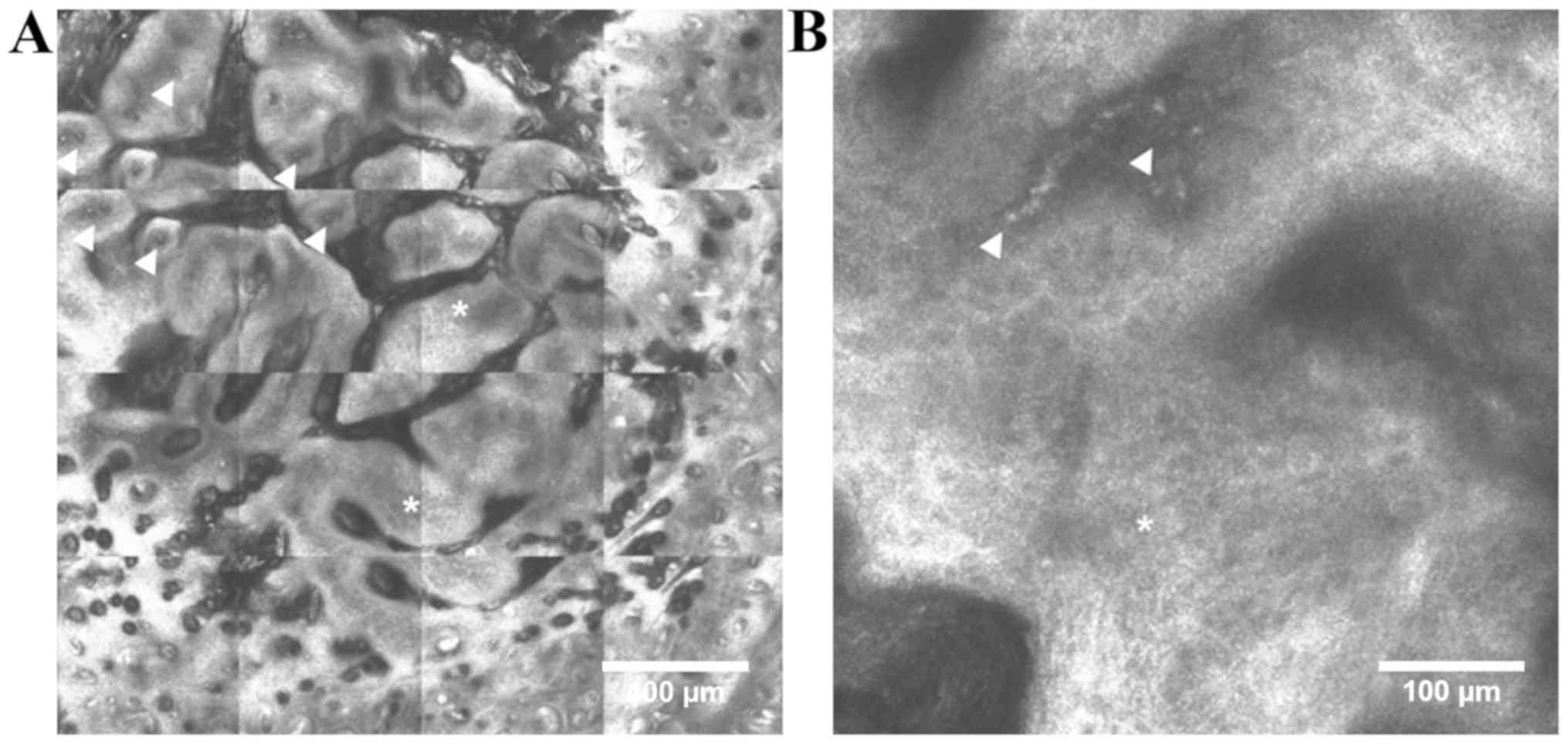
consists of aggregates of basaloid cells at the dermo-epidermal
junction or papillary dermis that appear in RCM images either as
‘bright tumor islands’, cord-like structures surrounded by
cleft-like dark spaces, either as ‘dark silhouettes’,
hyporeflective dark areas outlined by bright stromal tissue
(36,38–40).
These aggregates of basaloid cells often have nuclei that are
oriented along the same axis, displaying a ‘peripheral palisading’
at the periphery of tumor islands (35,36). In
the above stratum spinosum, the elongated nuclei of keratinocytes
that are polarized along the same axis form the typical ‘streaming
of the epidermis’ (35).
Additionally, prominent and tortuous blood vessels with intense
leukocyte traffic are present in the dermis and numerous
inflammatory cells with various shape and sizes (lymphocytes,
melanophages) surround the tumor nests (36). In pigmented BCC, bright dendritic
structures that correspond to dendritic melanocytes can be
identified inside aggregates of basaloid cells (41) (Fig.
1).
A retrospective, multicenter study evaluated the
sensitivity and specificity of five RCM criteria for BCC, including
architectural alteration and cellular pleomorphism of the overlying
epidermis, areas of refractile tumor cells with elongated,
monomorphic nuclei, nuclear polarization, increased dermal
vasculature and prominent inflammatory infiltrate (35). Identification of two or more of these
five criteria in a sample showed a sensitivity of 100% for BCC
diagnosis, whereas four or more of these had a specificity of 95.7%
and a sensitivity of 82.9% (35). Of
these criteria, elongated, monomorphic nuclei proved to be the most
sensitive (100%) and nuclear polarization the most sensitive
(91.6%) and specific (97.1%) (35).
Furthermore, this novel technology may be a
diagnostic guide in defining the margins of the lesion before
surgical excision (42) or laser
ablation (29). During Mohs
micrographic surgery, FCM proved to be far superior than RCM for
tumor margin assessment in BCC (36). Moreover, it offers the advantage of
monitoring noninvasive treatment in superficial-type BCC, thus
avoiding the discomfort associated with skin biopsy (43,44).
Squamous cell carcinoma (SCC) is the 2nd most
frequent non-melanoma skin cancer after BCC and appears dominantly
in sun exposed areas. Besides UV exposure, various risk factors,
including immunosuppression, viral infections, exposure to chemical
agents, neuro-endocrine factors or chronic inflammation, have been
proposed to be involved in SCC pathogenesis (45–51). It
has various clinical presentations including in situ lesions
(Bowen's disease), invasive superficial lesions or highly
infiltrative lesions (52). Actinic
keratosis (AK) is the most common precancerous skin lesion with a
risk of progression to a full-thickness SCC estimated at 5–10%
(53), but some authors consider it
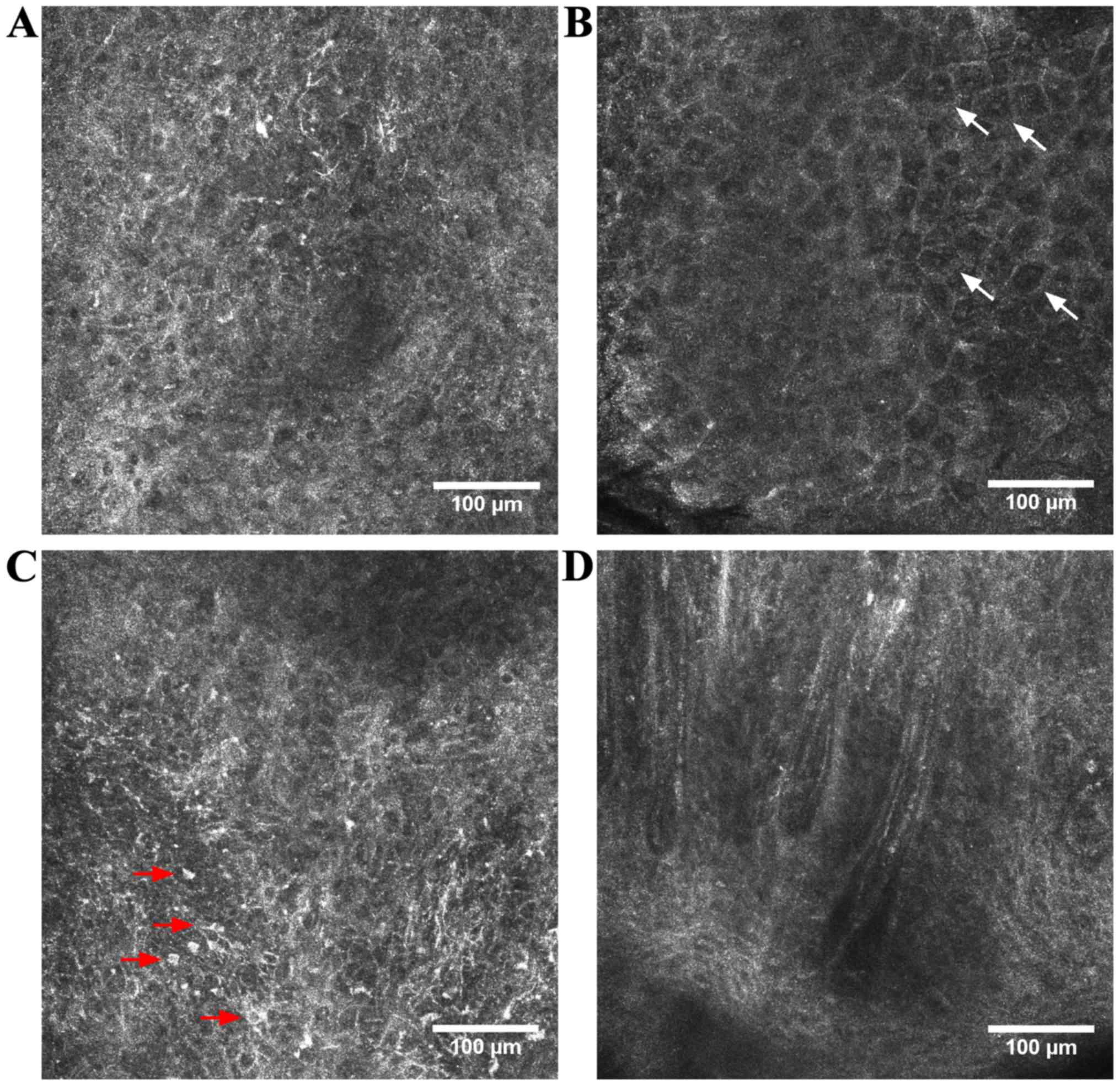
as an early form of SCC as it appears (54). Under RCM evaluation, SCC and
hypertrophic AK often present a pronounced hyperkeratosis that
limits the depth of penetration considerably (55) and provides whitewashed images because
of the strong back-reflectance at the keratin-rich surface of the
tumor (39). A more pronounced
disarranged honeycomb pattern in the spinous-granular layers and
the presence of neoplastic aggregates in the dermis can distinguish
SCC from AK (56). Moreover, nuclei
are enlarged and pleomorphic (55)
and roundish, nucleated bright cells with a pagetoid arrangement
are often observed in the suprabasal epidermis. When the thickness
of the lesion allows the dermo-epidermal junction imaging, dermal
papillae may appear elongated with looping, round vessels inside
them (39,57) (Fig.
2). However, in case of infiltrative lesions, the level of
invasion is usually inaccessible in CLSM (58). Even with ex vivo CLSM during
Mohs surgery, the detection of residual SCC is rarely possible also
because of the non-reflecting features of keratinization (58,59).
When it comes to RCM evaluation of SCC localized on
the lips, distinctive features were described (60). Moreover, RCM evaluation has the
potential to distinguish between features of normal mucosa,
dysplasia and lip SCC in real-time and therefore may be useful for
the preoperative assessment of tumor resection margins (61).
Cutaneous melanoma is one of the most aggressive
human malignancies, associated with high mortality rates, despite
latest advances in therapy (62–65). An
important genetic background and several environmental factors are
key players in melanoma development and progression (66–70). Two
crucial points have to be taken into account in this form of
aggressive skin cancer. One is particularly in high-risk patients,
where melanomas may be complicated to distinguish from nevi
(71) and the fact that numerous
biopsy specimens for screening are associated with patient
morbidity. Therefore, if a dermatologist is confronted with a
lesion obeying the ABCDE rule of melanoma (72) or if the atypical lesion is
solitary/is the ‘ugly duckling’ (73) there are no particular issues for a
dermatologist. Conversely, in patients with numerous and clinically
atypical nevi, visually identifying the lesion with the greatest
atypical features that may represent a new or developing melanoma
is almost impossible. Removing high numbers of nevi in such
patients for finding one melanoma can be a screening method, but
although there are extended publications on how many nevi should be
removed in high-risk patients to identify one melanoma (74–77),
there are still issues such as removing too few nevi can be
associated with overlooked melanomas and/or significant medical
system costs (78).
In the case of melanoma, the melanocytic lesions
have in the upper parts of the tumor pagetoid roundish or dendritic
cells in the superficial epidermis, atypical nests at the
dermo-epidermal junction, non-edged papillae and atypical nucleated
cells in the papillary dermis. The benefit of RCM in vivo
examination in real-time is important also in particular cases of
melanoma like lentigo maligna melanoma and amelanotic melanoma.
Moreover, this technology can add information on management of
subclinical margins, recurrences, or monitoring noninvasive
treatment of tumors (80).
Studies that focused on the application of this
technology in melanoma diagnosis have shown that melanocyte-derived
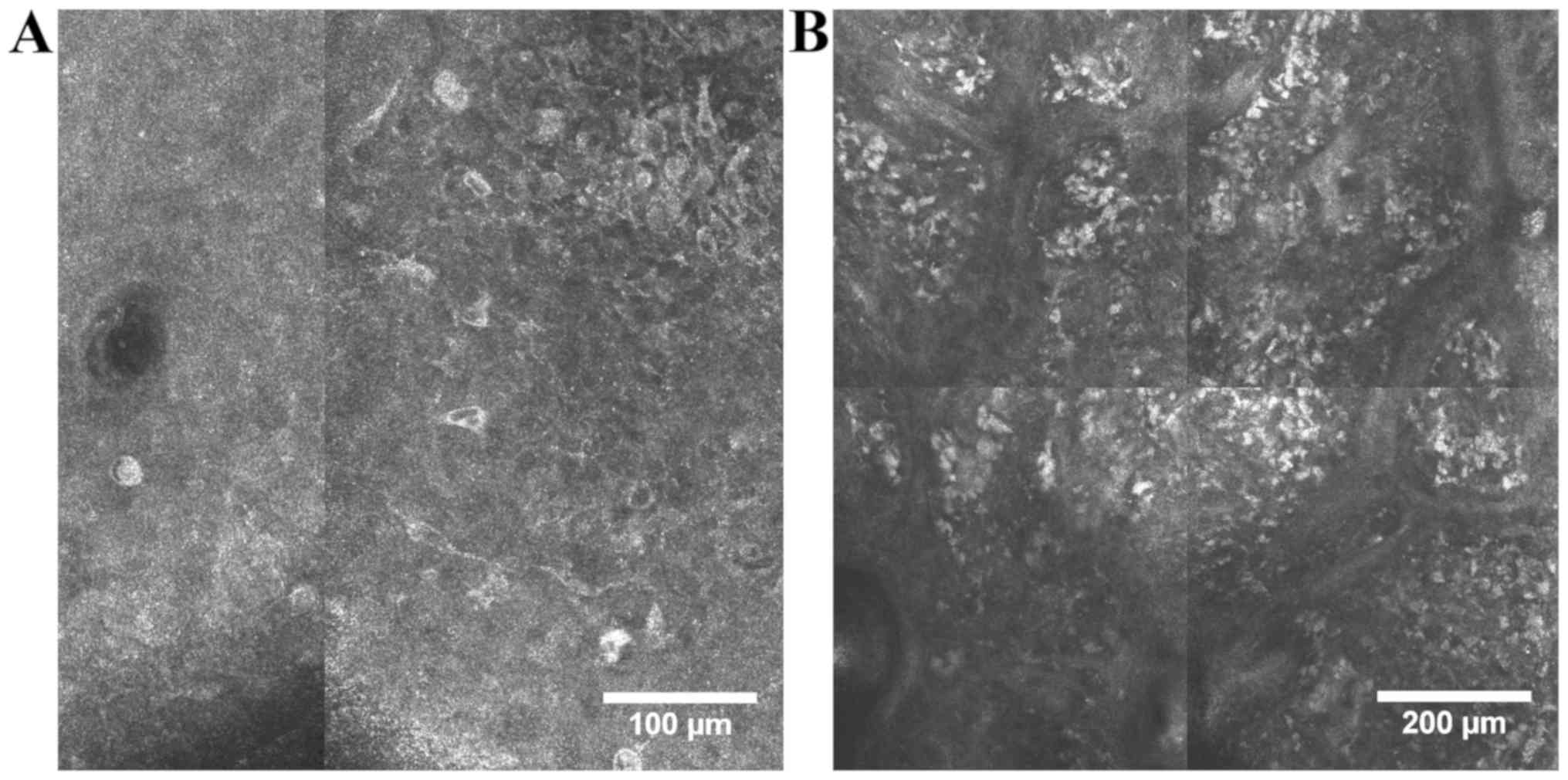
tumor cells can be demarcated from non-melanocytic ones. Thus, our
experience has shown that, while benign nevi have monomorphic
cells, round to oval in shape, with bright appearance, in melanomas
cells are bright, polymorphic and irregular, roundish or with
branching dendrites (Fig. 3). In
benign nevi, junctional and dermal nevus cell nests can be found,
while in melanomas there is a disarray of the melanocytic cell
architecture. Keratinocyte cell borders can be detected readily but
are poorly defined or absent in melanomas. The horizontal optical
sections in RCM offer a better visualization of malignant
melanocyte morphology than classical hematoxylin and eosin stained
histologic sections (15,81). In addition, based on their cell
morphology in RCM, four types of melanomas have been identified,
namely dendritic cell melanomas, melanomas with roundish
melanocytes, melanomas with predominant dermal nesting
proliferation and combined type melanomas, each with different
tumor and patient characteristics (15).
More elaborated studies seeking to evaluate
specificity and sensitivity of this technology have reported that
there is a good differentiation between benign versus malignant
tumors. Thus, depending on the observers, the sensitivity ranged
from 90.42 to 97.62% and the specificity from 96.67 to 100%. These
values generated good performance of the investigation:
sensitivity, 94.65%; specificity, 96.67%; positive predictive value
97.50%; and negative predictive value 92.99% (82).
Cutaneous lymphomas are a heterogeneous group of
lymphoproliferative disorders involving the skin that are
characterized by clonal proliferation of mature T-lymphocytes
(>60% of all cases), B-lymphocytes or NK cells (83,84).
Histopathological examination combined with immunohistochemistry of
the skin biopsy specimen is the mainstay of the diagnosis, although
sequential biopsies are often needed, especially in case of early
stage lesions.
Mycosis fungoides, early patch lesions in
particular, can imitate a wide variety of erythematosquamous skin
diseases and its clinical and histopathological diagnosis is often
a challenge. Most commonly reported RCM features of mycosis
fungoides correlate with histopathologic findings and include
weakly refractile cells (lymphocytes) and vesicle-like spaces
(Pautrier collections) within the epidermis, hypo-refractile
papillary rings and dilated capillaries with thick walls at the
dermo-epidermal junction (90).
Detection of Pautrier collections with RCM is associated with
improved histopathologic diagnosis and presence of TCR gene
clonality (86).
The rest of the RCM findings are non-specific and
reflect the heterogeneous clinical and histopathologic presentation
of the lesions (86). However, in
vivo RCM seems to be reliable in guiding skin biopsy
collection, therefore reducing the number of unsuccessful
histopathological examinations for mycosis fungoides lesions
(85,87).
In contrast to cutaneous T-cell lymphomas, to date
no RCM features have been described for the diagnosis of B-cell
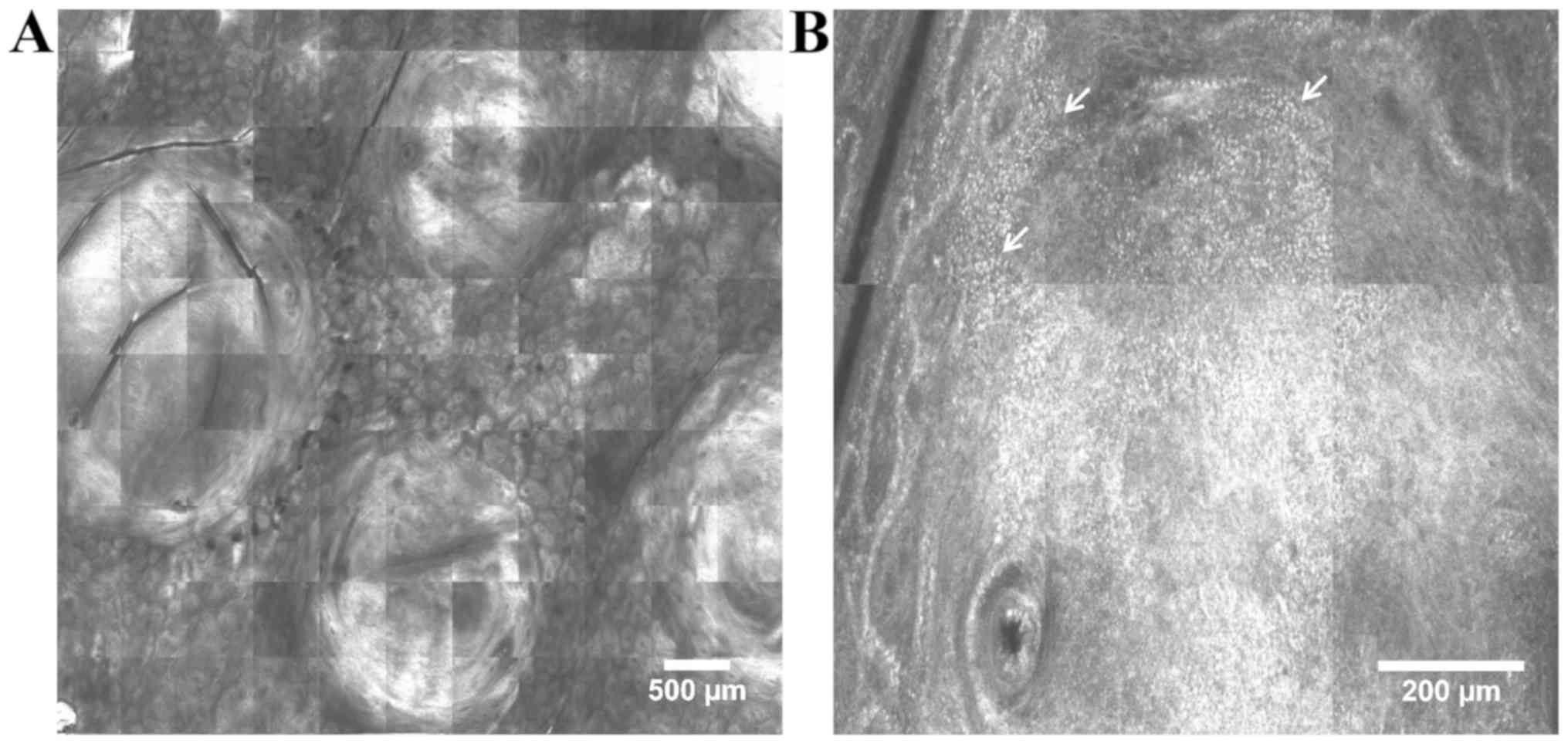
lymphomas. Our research team recently described the in vivo
RCM features observed in primary cutaneous folliculocentric
lymphoma lesions (unpublished results). These correlate with
histopathology and include round-shaped, highly-refractive tumor
masses in the dermis, bright cells of various sizes dispersed
throughout the dermis and aggregates of bright small cells
(lymphocytes) at the periphery of tumor masses (Fig. 4).
Skin carcinogenesis is a complex, multifactorial
process and the topic of intensive research given the continuously
increasing incidence of skin cancer. In addition to the recognized
genetic and environmental factors (93), prolonged exposure to pro-inflammatory
cytokines and chemokines within chronic inflammation is
experimentally sustained to favor initiation and progression of
skin cancer (94).
Mouse models of chemically induced skin
carcinogenesis are one of the most available and cost-effective
models to analyze early alterations and pathways involved in skin
tumorigenesis (95). The two-stage
skin carcinogenesis model has been used to study mechanisms of
epithelial cancers (96) and it
refers to the two-step topical administration of chemicals to mouse
skin for the initiation and promotion phases of skin tumorigenesis.
This delimitation of phases allows the observation of premalignant
lesions (96) and it offers more
reliable results when testing the effects of environmental factors
and drugs on skin tumors (95).
Fluorescence mode CLSM studies have been done on
transgenic mice using green fluorescent protein marker to visualize
cellular details of the skin (11).
As it allows sequential noninvasive examination of the same skin
area, CLSM technology seems to be ideal for monitoring tumor
progression (98) and therapeutic
effects of anticancer agents in mouse models of skin carcinogenesis
(95). Recently, a dual mode in
vivo reflectance and fluorescence CLSM has been developed and
holds significant promise for imaging tumor progression in murine
skin (98). This system combines the
fluorescence contrast of targeted tumor cells with the acquired
reflectance contrast of examined cells and tissues and place them
within a histologically meaningful framework (98). In addition to the in situ
visualization of tumor cell proliferation and vascular structures,
it has been shown that combined reflectance/fluorescence in
vivo CLSM has the ability to image dendritic immune cell
trafficking to inoculated tumors and to monitor tumor induced
immune response in the skin (98).
Despite the great advantages CLSM adds to
dermatological practice, it also has some limitations, the most
recognized being the restricted depth of penetration to 200–300 µm,
that allows imaging only of the epidermis and upper dermis
(3,4). Therefore, the deeper part of the dermis
and the hypodermis cannot be visualized using the currently
commercially available confocal microscopes. Examination of deeper
skin structures could be achieved using higher laser power, but at
the expense of damaging the skin area under evaluation (52). There are attempts to develop new
devices that improve light collection from the examined plane in
order to increase depth of penetration (99). Moreover, examination of skin lesions
by means of CLSM is more time consuming than clinical evaluation or
dermoscopy and it needs training and experience for the
interpretation of CLSM images. Recent technological breakthroughs
have led to the development of new, smaller and more practical
hand-held devices that offer faster image acquisition and allow the
examination of lesions located in less accessible body areas
(52). Unlike vertical sections
obtained in conventional histology, CLSM enables virtual sectioning
of the skin at different depths, in horizontal planes (en
face), parallel to the skin surface (3). For a better correlation with histology
sections, current efforts are aimed at developing devices that
could also perform optical sections of vertical planes and then
compile 3-D reconstructions of the lesions (100). In addition, CLSM does not require
tissue processing or coloring and images are obtained in greyscale,
similar to X-rays or ultrasonography (2,3). To
improve contrast of epidermal and dermal structures and toward
color-enhanced in vivo CSLM imaging, fluorescent dyes like
indocyanine-green are being tested (101).
CLSM is a modern imaging technique that enables the
noninvasive characterization of skin lesions located in the
epidermis and superficial dermis with a high resolution. Currently,
it is considered to be the most promising imaging technique for the
quasi-microscopic morphological and dynamic characterization of
superficial skin tumors. The in vivo mode adds the advantage
of noninvasive, dynamic, in real-time assessment of the tumor
associated vasculature and inflammation. It allows sequential
noninvasive examination of the same skin area without causing any
damage and to monitor disease progression and treatment outcome.
Furthermore, CLSM technology seems to be ideal for monitoring tumor
progression and therapeutic effects of anticancer agents in mouse
models of experimental skin carcinogenesis.
Not applicable.
This study was partially supported by a grant of the
Romanian Ministry of Research and Innovation (CCCDI-UEFISCDI;
project no. 61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341) within
PNCDI–III.
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
MAI, CC, ML, DL, MT, SGR, AB, CC, MN, SAZ and DB
were responsible for acquisition of references, analysis and
systematization of data, and contributed to writing the manuscript
and revising it critically for important intellectual content. All
authors read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Diaconeasa A, Boda D, Neagu M, Constantin
C, Căruntu C, Vlădău L and Guţu D: The role of confocal microscopy
in the dermato-oncology practice. J Med Life. 4:63–74.
2011.PubMed/NCBI
|
|
2
|
Aghassi D, González E, Anderson RR,
Rajadhyaksha M and González S: Elucidating the pulsed-dye laser
treatment of sebaceous hyperplasia in vivo with real-time confocal
scanning laser microscopy. J Am Acad Dermatol. 43:49–53. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
González S, Swindells K, Rajadhyaksha M
and Torres A: Changing paradigms in dermatology: Confocal
microscopy in clinical and surgical dermatology. Clin Dermatol.
21:359–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajadhyaksha M, González S, Zavislan JM,
Anderson RR and Webb RH: In vivo confocal scanning laser microscopy
of human skin II: Advances in instrumentation and comparison with
histology. J Invest Dermatol. 113:293–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
González S, Sackstein R, Anderson RR and
Rajadhyaksha M: Real-time evidence of in vivo leukocyte trafficking
in human skin by reflectance confocal microscopy. J Invest
Dermatol. 117:384–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peppelman M, Wolberink EA, Gerritsen MJ,
van de Kerkhof PC and van Erp PE: Application of leukotriene B4 and
reflectance confocal microscopy as a noninvasive in vivo model to
study the dynamics of skin inflammation. Skin Res Technol.
21:232–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
8
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl 3):1191–1196.
2014.PubMed/NCBI
|
|
9
|
Ghiţă MA, Căruntu C, Rosca AE, Căruntu A,
Moraru L, Constantin C, Neagu M and Boda D: Real-time investigation
of skin blood flow changes induced by topical capsaicin. Acta
Dermatovenerol Croat. 25:223–227. 2017.PubMed/NCBI
|
|
10
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okabe M, Ikawa M, Kominami K, Nakanishi T
and Nishimune Y: ‘Green mice’ as a source of ubiquitous green
cells. FEBS Lett. 407:313–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meyer LE, Otberg N, Sterry W and Lademann
J: In vivo confocal scanning laser microscopy: Comparison of the
reflectance and fluorescence mode by imaging human skin. J Biomed
Opt. 11:0440122006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skvara H, Plut U, Schmid JA and Jonak C:
Combining in vivo reflectance with fluorescence confocal microscopy
provides additive information on skin morphology. Dermatol Pract
Concept. 2:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajadhyaksha M, Grossman M, Esterowitz D,
Webb RH and Anderson RR: In vivo confocal scanning laser microscopy
of human skin: Melanin provides strong contrast. J Invest Dermatol.
104:946–952. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pellacani G, Guitera P, Longo C, Avramidis
M, Seidenari S and Menzies S: The impact of in vivo reflectance
confocal microscopy for the diagnostic accuracy of melanoma and
equivocal melanocytic lesions. J Invest Dermatol. 127:2759–2765.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guida S, Longo C, Casari A, Ciardo S,
Manfredini M, Reggiani C, Pellacani G and Farnetani F: Update on
the use of confocal microscopy in melanoma and non-melanoma skin
cancer. G Ital Dermatol Venereol. 150:547–563. 2015.PubMed/NCBI
|
|
17
|
Pellacani G, De Pace B, Reggiani C,
Cesinaro AM, Argenziano G, Zalaudek I, Soyer HP and Longo C:
Distinct melanoma types based on reflectance confocal microscopy.
Exp Dermatol. 23:414–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Serban ED, Farnetani F, Pellacani G and
Constantin MM: Role of in vivo reflectance confocal microscopy in
the analysis of melanocytic lesions. Acta Dermatovenerol Croat.
26:64–67. 2018.PubMed/NCBI
|
|
19
|
González S, Sánchez V, González-Rodríguez
A, Parrado C and Ullrich M: Confocal microscopy patterns in
nonmelanoma skin cancer and clinical applications. Actas
Dermosifiliogr. 105:446–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Białek-Galas K, Wielowieyska-Szybińska D,
Dyduch G and Wojas-Pelc A: The use of reflectance confocal
microscopy in selected inflammatory skin diseases. Pol J Pathol.
66:103–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Constantin MM, Bucur S, Serban DE, Caruntu
C, Orzan OA and Constantin T: Dermoscopic and laser confocal
features of an exogenous ochronosis case. G Ital Dermatol Venereol.
Jun 29–2018.(Epub ahead of print). PubMed/NCBI
|
|
22
|
Lange-Asschenfeldt S, Bob A, Terhorst D,
Ulrich M, Fluhr J, Mendez G, Roewert-Huber HJ, Stockfleth E and
Lange-Asschenfeldt B: Applicability of confocal laser scanning
microscopy for evaluation and monitoring of cutaneous wound
healing. J Biomed Opt. 17:0760162012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altintas AA, Altintas MA, Ipaktchi K,
Guggenheim M, Theodorou P, Amini P and Spilker G: Assessment of
microcirculatory influence on cellular morphology in human burn
wound healing using reflectance-mode-confocal microscopy. Wound
Repair Regen. 17:498–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altintas AA, Guggenheim M, Oezcelik A,
Gehl B, Aust MC and Altintas MA: Local burn versus local cold
induced acute effects on in vivo microcirculation and
histomorphology of the human skin. Microsc Res Tech. 74:963–969.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Longo C, Ragazzi M, Rajadhyaksha M, Nehal
K, Bennassar A, Pellacani G and Malvehy Guilera J: In vivo and ex
vivo confocal microscopy for dermatologic and Mohs surgeons.
Dermatol Clin. 34:497–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennàssar A, Vilata A, Puig S and Malvehy
J: Ex vivo fluorescence confocal microscopy for fast evaluation of
tumour margins during Mohs surgery. Br J Dermatol. 170:360–365.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller DL and Weinstock MA: Nonmelanoma
skin cancer in the United States: Incidence. J Am Acad Dermatol.
30:774–778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lomas A, Leonardi-Bee J and Bath-Hextall
F: A systematic review of worldwide incidence of nonmelanoma skin
cancer. Br J Dermatol. 166:1069–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CS, Sierra H, Cordova M and
Rajadhyaksha M: Confocal microscopy-guided laser ablation for
superficial and early nodular Basal cell carcinoma: A promising
surgical alternative for superficial skin cancers. JAMA Dermatol.
150:994–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lupu M, Caruntu C, Ghita MA, Voiculescu V,
Voiculescu S, Rosca AE, Caruntu A, Moraru L, Popa IM, Calenic B, et
al: Gene expression and proteome analysis as sources of biomarkers
in basal cell carcinoma. Dis Markers. 2016:98312372016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghita MA, Caruntu C, Rosca AE, Kaleshi H,
Caruntu A, Moraru L, Docea AO, Zurac S, Boda D, Neagu M, et al:
Reflectance confocal microscopy and dermoscopy for in vivo,
non-invasive skin imaging of superficial basal cell carcinoma.
Oncol Lett. 11:3019–3024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Longo C, Lallas A, Kyrgidis A, Rabinovitz
H, Moscarella E, Ciardo S, Zalaudek I, Oliviero M, Losi A, Gonzalez
S, et al: Classifying distinct basal cell carcinoma subtype by
means of dermatoscopy and reflectance confocal microscopy. J Am
Acad Dermatol. 71:716–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Căruntu C, Boda D, Guţu DE and Căruntu A:
In vivo reflectance confocal microscopy of basal cell carcinoma
with cystic degeneration. Rom J Morphol Embryol. 55:1437–1441.
2014.PubMed/NCBI
|
|
34
|
Peppelman M, Wolberink EA, Blokx WA, van
de Kerkhof PC, van Erp PE and Gerritsen MJ: In vivo diagnosis of
basal cell carcinoma subtype by reflectance confocal microscopy.
Dermatology. 227:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nori S, Rius-Díaz F, Cuevas J, Goldgeier
M, Jaen P, Torres A and González S: Sensitivity and specificity of
reflectance-mode confocal microscopy for in vivo diagnosis of basal
cell carcinoma: A multicenter study. J Am Acad Dermatol.
51:923–930. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
González S and Tannous Z: Real-time, in
vivo confocal reflectance microscopy of basal cell carcinoma. J Am
Acad Dermatol. 47:869–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stephens A, Fraga-Braghiroli N, Oliviero
M, Rabinovitz H and Scope A: Spoke wheel-like structures in
superficial basal cell carcinoma: A correlation between dermoscopy,
histopathology, and reflective confocal microscopy. J Am Acad
Dermatol. 69:e219–e221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ulrich M, Roewert-Huber J, González S,
Rius-Diaz F, Stockfleth E and Kanitakis J: Peritumoral clefting in
basal cell carcinoma: Correlation of in vivo reflectance confocal
microscopy and routine histology. J Cutan Pathol. 38:190–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Que SK, Fraga-Braghiroli N, Grant-Kels JM,
Rabinovitz HS, Oliviero M and Scope A: Through the looking glass:
Basics and principles of reflectance confocal microscopy. J Am Acad
Dermatol. 73:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lupu M, Caruntu C, Solomon I, Popa A,
Lisievici C, Draghici C, Papagheorghe L, Voiculescu VM and
Giurcaneanu C: The use of in vivo reflectance confocal microscopy
and dermoscopy in the preoperative determination of basal cell
carcinoma histopathological subtypes. DermatoVenerol. 62:7–13.
2017.
|
|
41
|
Segura S, Puig S, Carrera C, Palou J and
Malvehy J: Dendritic cells in pigmented basal cell carcinoma: A
relevant finding by reflectance-mode confocal microscopy. Arch
Dermatol. 143:883–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan ZY, Lin JR, Cheng TT, Wu JQ and Wu WY:
In vivo reflectance confocal microscopy of basal cell carcinoma:
Feasibility of preoperative mapping of cancer margins. Dermatol
Surg. 38:1945–1950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Webber SA, Wurm EMT, Douglas NC, Lambie D,
Longo C, Pellacani G and Soyer HP: Effectiveness and limitations of
reflectance confocal microscopy in detecting persistence of basal
cell carcinomas: A preliminary study. Australas J Dermatol.
52:179–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ahlgrimm-Siess V, Horn M, Koller S, Ludwig
R, Gerger A and Hofmann-Wellenhof R: Monitoring efficacy of
cryotherapy for superficial basal cell carcinomas with in vivo
reflectance confocal microscopy: A preliminary study. J Dermatol
Sci. 53:60–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Voiculescu V, Calenic B, Ghita M, Lupu M,
Caruntu A, Moraru L, Voiculescu S, Ion A, Greabu M, Ishkitiev N, et
al: From normal skin to squamous cell carcinoma: A quest for novel
biomarkers. Dis Markers. 2016:45174922016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boda D, Neagu M, Constantin C, Voinescu
RN, Caruntu C, Zurac S, Spandidos DA, Drakoulis N, Tsoukalas D and
Tsatsakis AM: HPV strain distribution in patients with genital
warts in a female population sample. Oncol Lett. 12:1779–1782.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boda D, Docea AO, Calina D, Ilie MA,
Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE,
Voiculescu V, et al: Human papilloma virus: Apprehending the link
with carcinogenesis and unveiling new research avenues (Review).
Int J Oncol. 52:637–655. 2018.PubMed/NCBI
|
|
48
|
Georgescu SR, Sârbu MI, Matei C, Ilie MA,
Caruntu C, Constantin C, Neagu M and Tampa M: Capsaicin: Friend or
foe in skin cancer and other related malignancies? Nutrients.
9:13652017. View Article : Google Scholar
|
|
49
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers. 2018:97878312018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tampa M, Caruntu C, Mitran M, Mitran C,
Sarbu I, Rusu LC, Matei C, Constantin C, Neagu M and Georgescu SR:
Markers of oral lichen planus malignant transformation. Dis
Markers. 2018:19595062018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
González S and Ahlgrimm-Siess V:
Reflectance confocal microscopy in dermatology: Fundamentals and
clinical applications. Aula Médica. p1112012.
|
|
53
|
Rossi R, Mori M and Lotti T: Actinic
keratosis. Int J Dermatol. 46:895–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wolff K and Johnson RA: Fitzpatrick's
Color Atlas and Synopsis of Clinical Dermatology. 6th. McGraw-Hill;
New York, NY: pp. 267–270. 2009
|
|
55
|
Aghassi D, Anderson RR and González S:
Confocal laser microscopic imaging of actinic keratoses in vivo: A
preliminary report. J Am Acad Dermatol. 43:42–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Peppelman M, Nguyen KP, Hoogedoorn L, van
Erp PE and Gerritsen MJ: Reflectance confocal microscopy:
Non-invasive distinction between actinic keratosis and squamous
cell carcinoma. J Eur Acad Dermatol Venereol. 29:1302–1309. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rishpon A, Kim N, Scope A, Porges L,
Oliviero MC, Braun RP, Marghoob AA, Fox CA and Rabinovitz HS:
Reflectance confocal microscopy criteria for squamous cell
carcinomas and actinic keratoses. Arch Dermatol. 145:766–772. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Branzan AL, Landthaler M and Szeimies RM:
In vivo confocal scanning laser microscopy in dermatology. Lasers
Med Sci. 22:73–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chung VQ, Dwyer PJ, Nehal KS, Rajadhyaksha
M, Menaker GM, Charles C and Jiang SB: Use of ex vivo confocal
scanning laser microscopy during Mohs surgery for nonmelanoma skin
cancers. Dermatol Surg. 30:1470–1478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lupu M, Caruntu A, Caruntu C, Boda D,
Moraru L, Voiculescu V and Bastian A: Non-invasive imaging of
actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin
Oncol. 8:640–646. 2018.PubMed/NCBI
|
|
61
|
Clark AL, Gillenwater AM, Collier TG,
Alizadeh-Naderi R, El-Naggar AK and Richards-Kortum RR: Confocal
microscopy for real-time detection of oral cavity neoplasia. Clin
Cancer Res. 9:4714–4721. 2003.PubMed/NCBI
|
|
62
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Neagu M, Constantin C and Zurac S: Immune
parameters in the prognosis and therapy monitoring of cutaneous
melanoma patients: Experience, role, and limitations. BioMed Res
Int. 2013:1079402013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Neagu M, Constantin C, Manda G and
Margaritescu I: Biomarkers of metastatic melanoma. Biomarkers Med.
3:71–89. 2009. View Article : Google Scholar
|
|
65
|
Neagu M, Constantin C and Tanase C:
Immune-related biomarkers for diagnosis/prognosis and therapy
monitoring of cutaneous melanoma. Expert Rev Mol Diagn. 10:897–919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Surcel M, Constantin C, Caruntu C, Zurac S
and Neagu M: Inflammatory cytokine pattern is sex-dependent in
mouse cutaneous melanoma experimental model. J Immunol Res.
2017:92121342017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Caruntu C, Mirica A, Rosca A, Mirica R,
Caruntu A, Tampa M, Matei C, Constantin C, Neagu M, Badarau A, et
al: The role of estrogens and estrogen receptors in melanoma
development and progression. Acta Endo Buc. 12:234–241. 2016.
View Article : Google Scholar
|
|
68
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endo Buc. 10:545–558. 2014.
View Article : Google Scholar
|
|
69
|
Neagu M, Constantin C, Dumitrascu GR, Lupu
AR, Caruntu C, Boda D and Zurac S: Inflammation markers in
cutaneous melanoma - edgy biomarkers for prognosis. Discoveries
(Craiova). 3:e382015. View Article : Google Scholar
|
|
70
|
Ene CD, Anghel AE, Neagu M and Nicolae I:
25-OH vitamin D and interleukin-8: Emerging biomarkers in cutaneous
melanoma development and progression. Mediators Inflamm.
2015:9048762015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Solovastru LG, Vâta D, Statescu L,
Constantin MM and Andrese E: Skin cancer between myth and reality,
yet ethically constrained. Rev Rom Bioet. 12:47–52. 2014.
|
|
72
|
Rigel DS, Friedman RJ, Kopf AW and Polsky
D: ABCDE - an evolving concept in the early detection of melanoma.
Arch Dermatol. 141:1032–1034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Grob JJ and Bonerandi JJ: The ‘ugly
duckling’ sign: Identification of the common characteristics of
nevi in an individual as a basis for melanoma screening. Arch
Dermatol. 134:103–104. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hansen C, Wilkinson D, Hansen M and
Argenziano G: How good are skin cancer clinics at melanoma
detection? Number needed to treat variability across a national
clinic group in Australia. J Am Acad Dermatol. 61:599–604. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sidhu S, Bodger O, Williams N and Roberts
DL: The number of benign moles excised for each malignant melanoma:
The number needed to treat. Clin Exp Dermatol. 37:6–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Goodson AG, Florell SR, Hyde M, Bowen GM
and Grossman D: Comparative analysis of total body and
dermatoscopic photographic monitoring of nevi in similar patient
populations at risk for cutaneous melanoma. Dermatol Surg.
36:1087–1098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Moloney FJ, Guitera P, Coates E, Haass NK,
Ho K, Khoury R, O'Connell RL, Raudonikis L, Schmid H, Mann GJ, et
al: Detection of primary melanoma in individuals at extreme high
risk: A prospective 5-year follow-up study. JAMA Dermatol.
150:819–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
March J, Hand M and Grossman D: Practical
application of new technologies for melanoma diagnosis: Part I.
Noninvasive approaches. J Am Acad Dermatol. 72:929–942. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ulrich M and Lange-Asschenfeldt S: In vivo
confocal microscopy in dermatology: From research to clinical
application. J Biomed Opt. 18:0612122013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Carrera C, Puig S and Malvehy J: In vivo
confocal reflectance microscopy in melanoma. Dermatol Ther
(Heidelb). 25:410–422. 2012. View Article : Google Scholar
|
|
81
|
Longo C, Farnetani F, Ciardo S, Cesinaro
AM, Moscarella E, Ponti G, Zalaudek I, Argenziano G and Pellacani
G: Is confocal microscopy a valuable tool in diagnosing nodular
lesions? A study of 140 cases. Br J Dermatol. 169:58–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gerger A, Koller S, Weger W, Richtig E,
Kerl H, Samonigg H, Krippl P and Smolle J: Sensitivity and
specificity of confocal laser-scanning microscopy for in vivo
diagnosis of malignant skin tumors. Cancer. 107:193–200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sokołowska-Wojdyło M, Olek-Hrab K and
Ruckemann-Dziurdzińska K: Primary cutaneous lymphomas: Diagnosis
and treatment. Postepy Dermatol Alergol. 32:368–383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ion A, Popa IM, Papagheorghe LML,
Lisievici C, Lupu M, Voiculescu V, Caruntu C and Boda D: Proteomic
approaches to biomarker discovery in cutaneous T-cell lymphoma. Dis
Markers. 2016:96024722016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Fabbrocini G, Mazzella C, Cantelli M,
Baldo A, Russo D, De Rosa G and Monfrecola G: Reflectance confocal
microscopy as new diagnostic tool in folliculotropic mycosis
fungoides. Skin Appendage Disord. 4:118–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mancebo SE, Cordova M, Myskowski PL,
Flores ES, Busam K, Jawed SI, Skripnik A, Rajadhyaksha M and
Querfeld C: Reflectance confocal microscopy features of mycosis
fungoides and Sézary syndrome: Correlation with histopathologic and
T-cell receptor rearrangement studies. J Cutan Pathol. 43:505–515.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li W, Dai H, Li Z and Xu AE: Reflectance
confocal microscopy for the characterization of mycosis fungoides
and correlation with histology: A pilot study. Skin Res Technol.
19:352–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lange-Asschenfeldt S, Babilli J, Beyer M,
Ríus-Diaz F, González S, Stockfleth E and Ulrich M: Consistency and
distribution of reflectance confocal microscopy features for
diagnosis of cutaneous T cell lymphoma. J Biomed Opt.
17:0160012012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Koller S, Gerger A, Ahlgrimm-Siess V,
Weger W, Smolle J and Hofmann-Wellenhof R: In vivo reflectance
confocal microscopy of erythematosquamous skin diseases. Exp
Dermatol. 18:536–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Agero AL, Gill M, Ardigo M, Myskowski P,
Halpern AC and González S: In vivo reflectance confocal microscopy
of mycosis fungoides: A preliminary study. J Am Acad Dermatol.
57:435–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ardigò M, Donadio C, Vega H, Cota C,
Moscarella E and Agozzino M: Concordance between in vivo
reflectance confocal microscopy and optical histology of
lymphomatoid papulosis. Skin Res Technol. 19:308–313. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ardigò M, El Shabrawi-Caelen L and Tosti
A: In vivo reflectance confocal microscopy assessment of the
therapeutic follow-up of cutaneous T-cell lymphomas causing scalp
alopecia. Dermatol Ther (Heidelb). 27:248–251. 2014. View Article : Google Scholar
|
|
93
|
de Vries E, Trakatelli M, Kalabalikis D,
Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D,
Ioannides D, Aquilina S, Apap C, et al EPIDERM Group, : Known and
potential new risk factors for skin cancer in European populations:
A multicentre case-control study. Br J Dermatol. 167 (Suppl
2):1–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Maru GB, Gandhi K, Ramchandani A and Kumar
G: The role of inflammation in skin cancer. Adv Exp Med Biol.
816:437–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Abel EL, Angel JM, Kiguchi K and
DiGiovanni J: Multi-stage chemical carcinogenesis in mouse skin:
Fundamentals and applications. Nat Protoc. 4:1350–1362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hashemi P, Pulitzer MP, Scope A,
Kovalyshyn I, Halpern AC and Marghoob AA: Langerhans cells and
melanocytes share similar morphologic features under in vivo
reflectance confocal microscopy: A challenge for melanoma
diagnosis. J Am Acad Dermatol. 66:452–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li Y, Gonzalez S, Terwey TH, Wolchok J, Li
Y, Aranda I, Toledo-Crow R and Halpern AC: Dual mode reflectance
and fluorescence confocal laser scanning microscopy for in vivo
imaging melanoma progression in murine skin. J Invest Dermatol.
125:798–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Izatt JA, Kulkarni MD, Hsing-Wen W,
Kobayashi K and Sivak MV: Optical coherence tomography and
microscopy in gastrointestinal tissues. IEEE J Sel Top Quantum
Electron. 2:1017–1028. 1996. View Article : Google Scholar
|
|
100
|
Ono I, Sakemoto A, Ogino J, Kamiya T,
Yamashita T and Jimbow K: The real-time, three-dimensional analyses
of benign and malignant skin tumors by confocal laser scanning
microscopy. J Dermatol Sci. 43:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Skvara H, Kittler H, Schmid JA, Plut U and
Jonak C: In vivo fluorescence confocal microscopy: Indocyanine
green enhances the contrast of epidermal and dermal structures. J
Biomed Opt. 16:0960102011. View Article : Google Scholar : PubMed/NCBI
|