Introduction
Lung cancer is the leading cause of tumor-related
mortality worldwide (1). Small cell
lung cancer (SCLC) accounted for 14% of lung cancer cases in 2014
(2) and progresses quickly with
distant metastasis. Overall, 60–70% of the patients have
extensive-stage SCLC (ES-SCLC) when a definite diagnosis is
established (2). Chemotherapy is the
primary treatment strategy for ES-SCLC, but the standard first-line
therapeutic regimens for SCLC, typically platinum
(cisplatin/carboplatin) combined with topoisomerase inhibitors,
achieve a median survival time of 8–13 months only (2), with high toxicity including
myelosuppression, gastrointestinal toxicity, cardiac toxicity and
nephrotoxicity (3,4).
Lobaplatin is a third-generation platinum drug,
discovered during studies of platinum compounds for
cisplatin-resistant tumors. Pre-clinical studies showed that
compared with cisplatin, lobaplatin has equivalent activity against
tumors (5–7), but with improved tolerability and
stability, and lower toxicity (8,9). A phase
II trial further suggested that lobaplatin had strong antitumor
activity in patients with SCLC who were naïve or previously treated
with one line of therapy (10). The
majority of previous studies were limited in sample size and
restricted to phase II trials, limiting the reliable assessment of
the regimen (11,12).
Therefore, the aim of the present study was to
verify the non-inferiority (in terms of efficacy) of lobaplatin
plus etoposide (EL) vs. cisplatin plus etoposide (EP) in patients
with previously untreated ES-SCLC.
Materials and methods
Study design
The present study was a non-inferiority, open label,
randomized clinical trial (Chinese Clinical Trial Registry
ChiCTR-TRC-10001047) that was performed at 17 sites in China
between September 2010 and May 2013. The study was approved by the
ethical committees of all participating hospitals, and followed the
Good Clinical Practice (GCP) principle and Declaration of Helsinki.
No substantial protocol revision was made during study
implementation. All patients provided written informed consent
prior to enrollment.
Patients
The inclusion criteria were as follows: i)
Definitive histological diagnosis of ES-SCLC (defined as a tumor
that spreads beyond the hemithorax, hilar, mediastinal or
supraclavicular nodes); ii) an age of 18–70 years; iii) no
chemotherapy history; iv) palliative radiotherapy or surgery
received for metastatic lesions, completed for >14 days; v)
measurable lesions (non-irradiated sites); according to the
Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1)
(13), patients with lesions with a
diameter ≥20 mm by plain computed tomography (CT) or ≥10 mm by
helical CT, and the maximum length of >2 times the section
thickness; vi) an Eastern Cooperative Oncology Group performance
status (ECOG-PS) (14) of 0–1; and
vii) an estimated survival time of >3 months.
The exclusion criteria were as follows: i) A
previous history of allergy to platinum compounds; ii) an active
ulcer; iii) surgery or radiotherapy for the primary lesion; iv)
interstitial pneumonia or pulmonary fibrosis; v) active brain
metastasis and a stable status for <4 weeks and/or with
symptoms, and/or requiring anticonvulsant drugs or steroids and/or
treatment for leptomeningeal disease; vi) severe main bronchial or
lobe bronchial stenosis and obstruction caused by tumor invasion or
oppression, or presenting with superior vena cava syndrome (SVCS),
or with uncontrolled malignant hydrothorax, ascites or pericardial
effusion at an above medium degree; vii) severe infection, severe
anti-diuretic hormone abnormal secretion syndrome, poorly
controlled diabetes mellitus (requirement of continuous daily
insulin of >40 units, or fasting plasma glucose remaining
>7.8 mmol/l and hemoglobin A1c >9.0% with or without
continuous daily insulin of <40 units), or with severe
complications of treatment-required SVCS; viii) severe
cardiovascular diseases, including high blood pressure
uncontrollable by medications, unstable angina, myocardial
infarction attack in the past 6 months, congestive heart failure of
New York Heart Association (15)
grade >3, or severe arrhythmias; ix) requirement for long-term
anticoagulants or vitamin K antagonists, including warfarin,
heparin or its analogues, except for prophylactic low-dose warfarin
(≤1 mg/day) or aspirin (100 mg/day); x) participated in another
clinical trial within 4 weeks of allocation, or had quit this study
following allocation; xi) active cancer relapse, except for
intraepithelial carcinoma, or no recurrence of relapsed cancer for
>5 years; or xii) pregnancy, possible pregnancy or an intention
for pregnancy, a lack of effective contraceptive measures or
currently lactating.
Randomization
All enrolled patients were assigned at a 1:1 ratio
to receive EL or EP. Randomization was implemented using a central
computerized randomization system managed by an independent
statistician. Randomization was stratified according to ECOG PS,
sex, age, and presence or absence of liver or brain metastasis.
Treatment protocol
According to phase I studies, the recommended dose
of lobaplatin is 50 mg/m2 as single-agent chemotherapy
(16,17). In a phase I study (Clinical Trial
Approval Number: 2008L09400), the maximum tolerated dose for the
lobaplatin combination regimen as first-line therapy in patients
with non-small cell lung cancer (NSCLC) was 30 mg/m2
lobaplatin on day 2 and 175 mg/m2 paclitaxel on day 1 of
a 21-day treatment cycle. Therefore, 30 mg/m2 lobaplatin
was selected in the present study.
The EL regimen was composed of six cycles of 30
mg/m2 lobaplatin (10 mg/vial; National Medical
Authorization No. H20080359; Hainan Changan International
Pharmaceutical Co., Ltd, Haikou, Hainan, China) on day 1 and 100
mg/m2 etoposide (100 mg/vial; National Medical
Authorization No. H37023183; Qilu Pharmaceutical Co., Ltd, Jinan,
Shandong, China) on days 1–3. The EP regimen consisted of six
cycles of 80 mg/m2 cisplatin (10 mg/vial; National
Medical Authorization No. H37021358; Qilu Pharmaceutical Co., Ltd,
Jinan, Shandong, China) on day 1 and 100 mg/m2 etoposide
on days 1–3. The cycle length was 21 days for each arm.
If a patient presented with hematological toxicities
[platelets <25×109/l or absolute neutrophil count
(ANC) <0.5×109/l for 4 consecutive days, or ANC
<1.0×109/l combined with a fever of >38.5°C during
the prior course], the doses of lobaplatin and etoposide were
reduced by 10 and 20 mg/m2, respectively, in the
subsequent cycles. A 25% dose reduction of cisplatin was required
when grade >2 neurotoxicity or nephrotoxicity occurred. Once the
dose was reduced, the new dose was kept for the subsequent cycles
and there was no return to the original dose. The dose of etoposide
could be reduced a maximum of two times (i.e. reduced to 60
mg/m2). If grade 3/4 toxicity still occurred following
two dose adjustments, the treatment was terminated and the patient
was withdrawn from the study.
Any radiotherapy or other antitumor therapy was
forbidden until disease progression. Chinese medicine and
immunomodulatory agents with explicit lung cancer treatment
indications that were approved by the State Food and Drug
Administration were not permitted for concomitant administration.
Patients were administered the antiemetic prophylaxis and
treatments according to the routine medical practice at each
participating center. The recommended prophylactic antiemetic
regimen was a 5-hydroxytryptamine receptor 3 antagonist in
combination with glucocorticoids and, if necessary, in combination
with other antiemetics or sedatives. When patients presented with
grade 3/4 thrombocytopenia, thrombopoietin (TPO) or interleukin
(IL)-11 could be used. If the patients developed bone metastases,
bisphosphonates could be used. Hospitals were allowed to provide
any relevant supportive and symptomatic measures according to their
routine medical care practice.
Treatment assessments
During treatment, physical examination,
electrocardiogram, blood coagulation tests, tumor marker tests and
urinalysis were performed within 1 week of each cycle. Blood count
and blood biochemistry analyses were performed at least once a
week. Spiral CT scan or magnetic resonance imaging was used for
tumor assessment according to RECIST (version 1.1) at baseline
(within 2 weeks of treatment) and once every two cycles following
treatment (the 21st day of the second cycle, the 21st day of the
4th cycle and the 21st day of the 6th cycle, ±3 day window), until
progressive disease (PD) was diagnosed or the patient withdrew from
the study. Toxicity was evaluated according to the Common
Terminology Criteria for Adverse Events (18) (CTC AE, version 3.0). Quality of life
(QoL) was assessed using EuroQol (EQ)-5D, EQ-visual analogue scales
(VAS), QoL questionnaire (QLQ)-C30 (version 3.0) (19) and QLQ-lung cancer 13 (QLQ-LC13)
(20), all of which were collected
on day 1 of each cycle. Questionnaires were filled in by the
patients under the instruction of nurses from each study center who
received specific and GCP training.
Endpoints
Progression-free survival (PFS) referred to the time
interval from randomization to disease progression or mortality
from any cause. In cases where the information on mortality or
disease progression was not available, PFS referred to the time
interval between randomization and the last follow-up.
Overall survival (OS) referred to the time interval
from randomization to mortality from any cause. In cases where the
information on mortality was not available, OS referred to the time
interval between randomization and the last follow-up.
The overall response rate (ORR) referred to the
ratio of cases with optimal efficacy [complete remission (CR) +
partial remission (PR)] vs. the total cases. The disease control
rate (DCR) referred to the ratio of cases with CR, PR and stable
disease (SD) [namely CR + PR + SD (≥8 weeks)], vs. the total
cases.
The QoL variables originated from the QLQ-C30,
QLQ-LC13, EQ-5D and EQ-VAS. QLQ-C30 comprises 30 items organized
into five functional scales, three symptom scales, one overall
health/quality-of-life scale, and six single items. QLQ-LC13 is a
supplementary questionnaire for patients with lung cancer and
includes questions assessing cough, hemoptysis, dyspnea,
site-specific pain, treatment-associated side effects and the
efficacy of pain medications. The EQ-5D records the level of
self-reported problems according to five dimensions. EQ-VAS is a
VAS used by respondents to describe their own health from 0 (the
worst imaginable health) to 100 (the best imaginable health). The
raw score (RS) for one area was calculated by the sum of scores
from every item under the corresponding area divided by the number
of items. The standard score (SS) was calculated using the
following formula: SS=[1-(RS-1)/R] ×100.
The primary endpoint was PFS. The secondary
endpoints included OS, ORR, DCR, toxicity and QoL. Adverse events
(AEs) taking place during the trial were documented and reviewed
for all patients.
Statistical analysis
The primary analysis of this study aimed to
demonstrate the non-inferiority of EL to EP with regard to PFS. The
clinical non-inferiority margin for the hazard ratio (HR) was set
at 1.4 based on the primary objective of this study, current
treatment, prognosis of the disease and historical data. According
to the literature (21,22) and the experience of the
investigators, the median PFS (mPFS) time in the EL group and the
EP group was considered to be 5.0 months. Taking two-sided α=0.05
and 1-β=80%, and considering a drop-out rate of 10% in each group
(patient enrollment was planned as 24 months, followed by 18 months
of follow-up), this clinical trial required a total of 234 patients
(23,24).
Interim analysis was not preset, and the termination
was 80% PD events. Normally distributed continuous variables are
presented as the mean ± standard deviation. Non-normally
distributed continuous variables are presented as the median
(range). Categorical variables are presented as frequencies and
percentages. Comparisons of baseline demographics and disease
characteristics between groups were made using Student's t-test for
continuous variables and the χ2 test for categorical
variables. The survival curve of PFS was analyzed by log-rank test,
and the HR and confidence interval (CI) of PFS were calculated by
Cox proportional hazards regression model. The Cox proportional
hazards regression model incorporating covariates, including sex
(males vs. females), age (≥65 vs. <65 years), stage (stage IV
vs. IIIB) and ECOG PS (1 vs. 0), was used to estimate HR and its
95% CI. The Kaplan-Meier method was used to estimate the mPFS and
mOS times, and survival curves were plotted. Fisher's exact test
was used for the comparison of the AEs. The Wilcoxon rank sum test
was used for comparison of changes in the QoL scores prior to and
after treatment between the two groups. Statistical analyses were
conducted using SAS 9.2 (SAS Institute, Cary, NY, USA). Two-sided
P-values of <0.05 were considered to indicate a statistically
significant difference.
Results
Enrollment
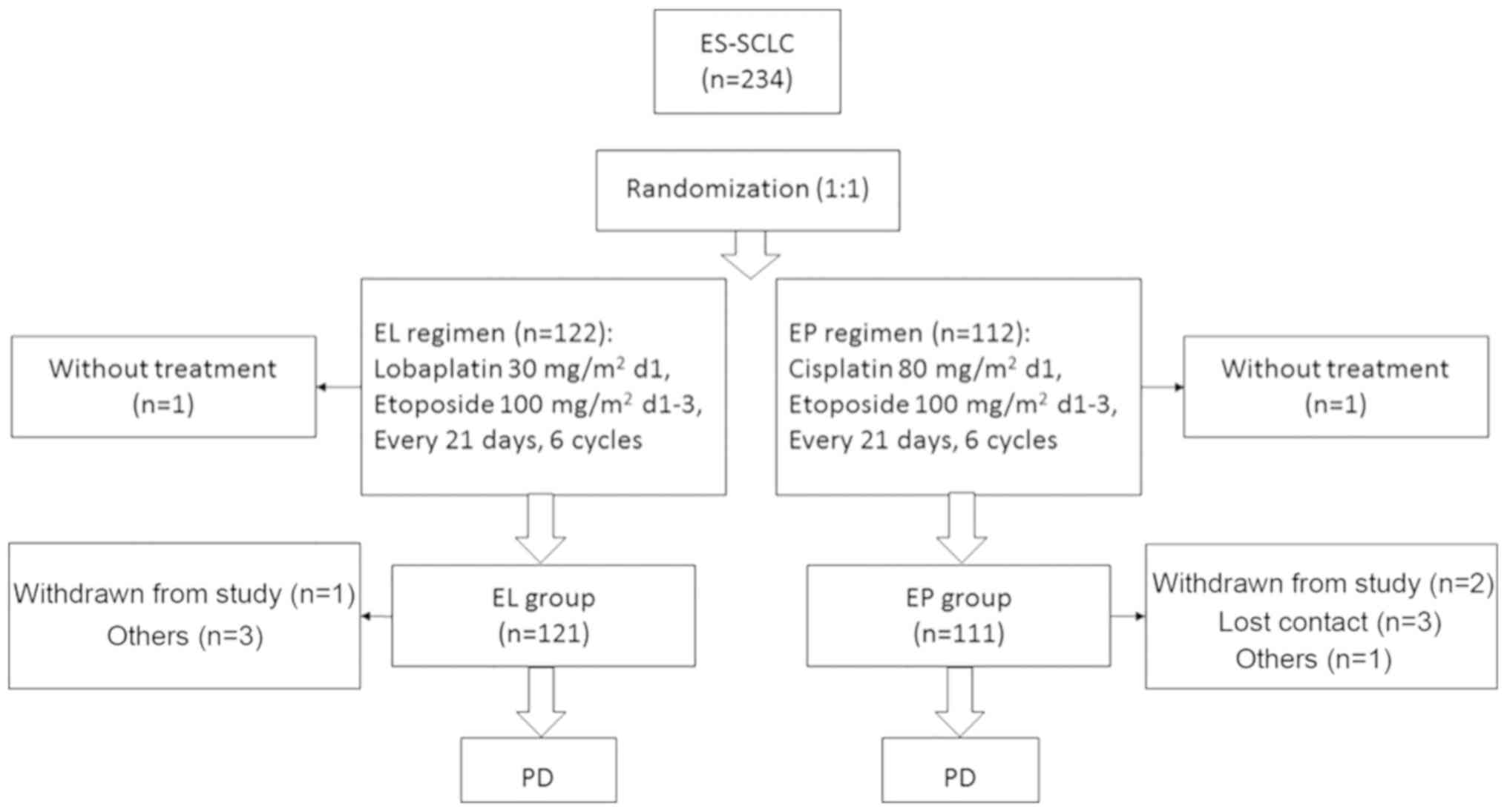
A total of 234 were enrolled: 122 in the EL group
and 112 in the EP group. In the EL group, 1 patient was finally
pathologically diagnosed with NSCLC, and 1 patient in the EP group
withdrew consent prior to treatment, but following randomization.
Overall, 232 patients were included in the intention-to-treat
analysis. In the EL group, 3 patients violated the protocol and 1
withdrew consent during treatment. In the EP group, 1 patient
violated the protocol, 2 withdrew consent and 3 were lost to
follow-up (Fig. 1). Finally, 222
patients strictly adhered to the protocol (117 in the EL group and
105 in the EP group) and were included in the per-protocol
analysis. Baseline characteristics were similar between the two
groups (Table I).
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Variable | EL (n=121) | EP (n=111) | P-value |
|---|
| Age |
|
|
|
| Mean ±
standard deviation, years | 56.3±8.0 | 56.4±8.4 | 0.952 |
| ≥65
years, n (%) | 19 (15.7) | 18 (16.2) | 0.481 |
| Sex, n (%) |
|
| 0.878 |
|
Male | 93 (76.9) | 84 (75.7) |
|
|
Female | 28 (23.1) | 27 (24.3) |
|
| ECOG, n (%) |
|
| 0.251 |
| 0 | 13 (10.7) | 7 (6.3) |
|
| 1 | 108 (89.3) | 104 (93.7) |
|
| Affected lung, n
(%) |
|
| 0.429 |
|
Left | 52 (43.0) | 54 (48.6) |
|
|
Right | 68 (56.2) | 57 (51.4) |
|
|
Both | 1 (0.8) | 0 (0.0) |
|
| History of smoking,
n (%) |
|
| 0.460 |
|
Non-smokers | 35 (28.9) | 27 (24.3) |
|
|
Smokers | 86 (71.1) | 84 (75.7) |
|
| TNM stage, n
(%) |
|
| 0.318 |
|
IIIB | 3 (2.5) | 6 (5.4) |
|
| IV | 118 (97.5) | 105 (94.6) |
|
| Liver/brain
metastasis, n (%) |
|
| 0.791 |
|
With | 49 (40.5) | 47 (42.3) |
|
|
Without | 72 (59.5) | 64 (57.7) |
|
| Bone metastasis, n
(%) |
|
| 0.236 |
|
With | 27 (22.3) | 32 (28.8) |
|
|
Without | 94 (77.7) | 79 (71.2) |
|
| History of
malignant tumor, n (%) |
|
| 0.248 |
| No | 118 (97.5) | 111 (100.0) |
|
|
Yes | 3 (2.5) | 0 (0.0) |
|
| Other major health
problems, n (%) |
|
| 0.674 |
| No | 84 (69.4) | 74 (66.7) |
|
|
Yes | 37 (30.6) | 37 (33.3) |
|
Efficacy
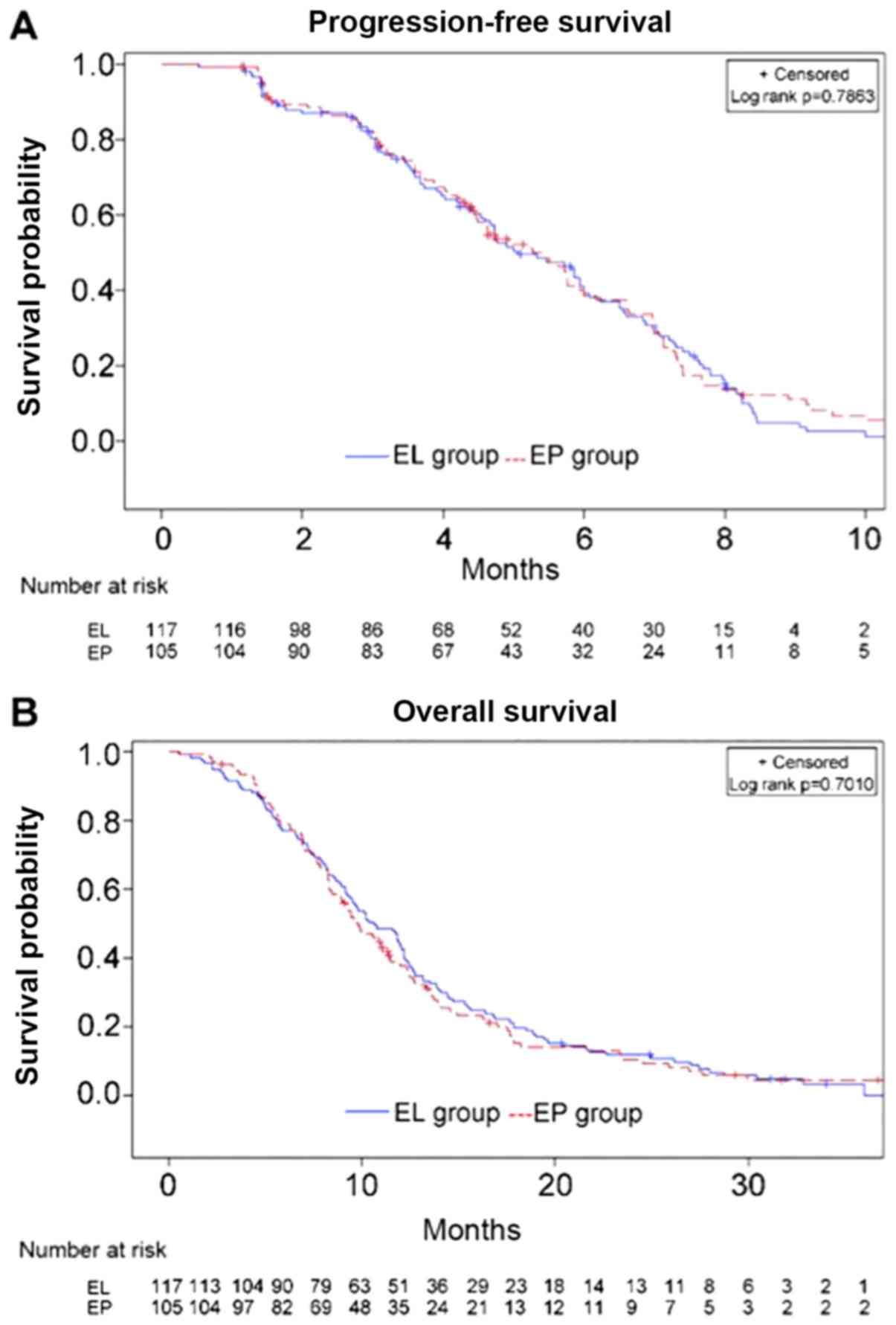
The median PFS time in the EL (n=117) and EP (n=105)
groups was 5.1 months (range, 1.3–16.3 months) and 5.3 months
(range, 1.1–13.8 months), respectively (HR, 1.041; 95%CI,
0.777–1.391; P=0.786) (Fig. 2A). The
median OS time in the EL and EP groups was 10.6 vs. 9.7 months,
respectively (HR, 0.947; 95%CI, 0.719–1.248; P=0.701) (Fig. 2B). There were no differences when
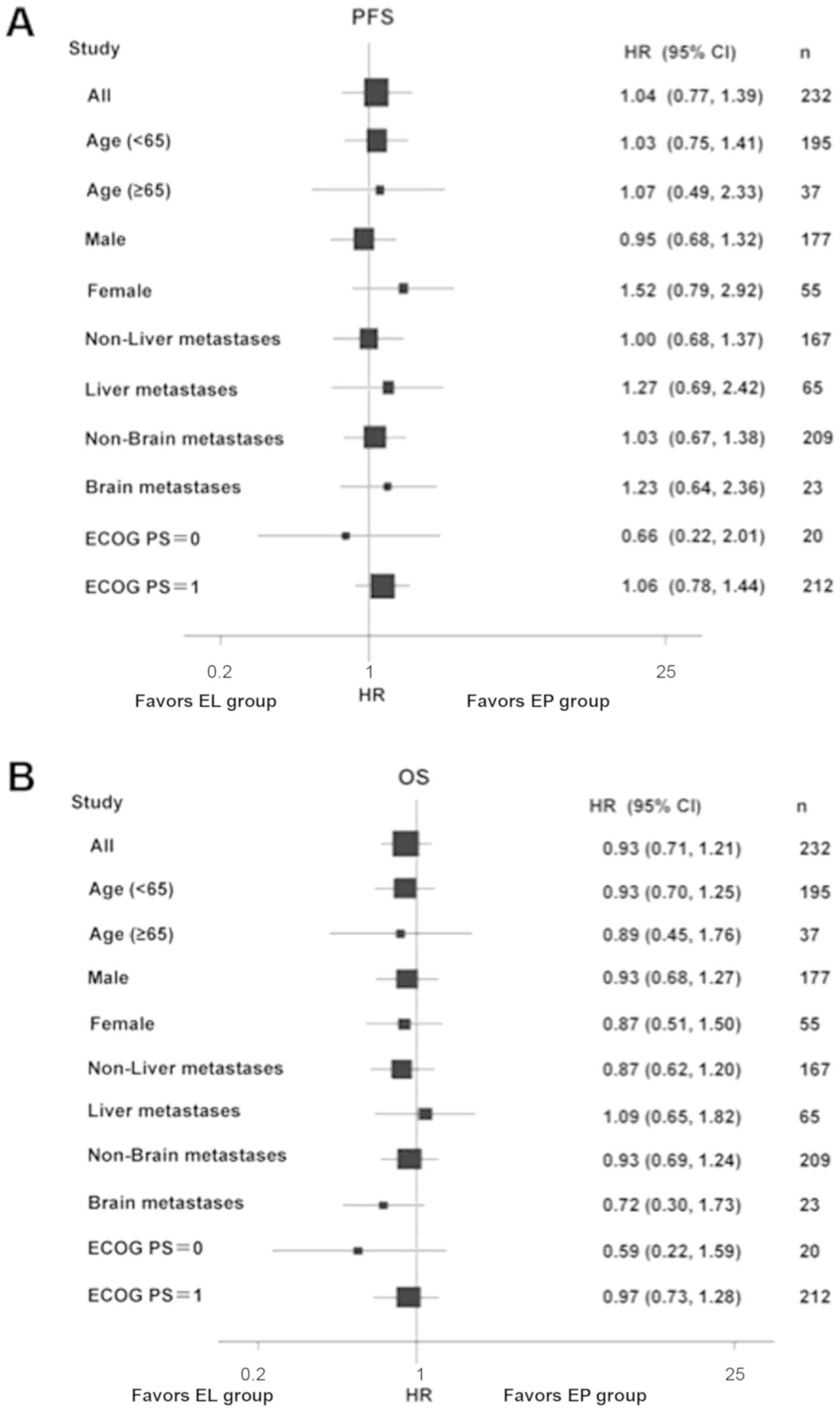
stratifying by age, sex, ECOG PS and presence/absence liver/brain
metastases (Fig. 3). The two groups
did not exhibit any significant difference in ORR (67.6 vs. 53.9%;
P=0.051) or DCR (86.7 vs. 85.5%; P=0.850).
Toxicity
Toxicity analysis was conducted among 232 patients
who received treatment and the common treatment-emergent AEs are
summarized in Table II. There were
54 (44.6%) patients in the EL group and 77 (69.4%) patients in the
EP group who experienced AEs (P<0.001). In terms of Grade 3/4
AEs, the EL group had a significantly lower frequency than the EP
group (5.8 vs. 15.3%; P=0.019). Renal toxicity, nausea, vomiting,
loss of appetite, hiccup, fatigue and particularly Grade 3/4 (11.7
vs. 0.8%; P<0.001) were more common in the EP group. The two
groups did not have significant differences in the frequency and
severity of anemia, leucopenia or neutropenia, although the EL
group had a significantly higher frequency of Grade 3/4
thrombocytopenia than the EP group (28.9 vs. 10.8%; P=0.001). Due
to thrombocytopenia, 5 patients in the EL group and 1 patient in
the EP group required a 6- to 10-day delay prior to the following
cycle. A total of 18 patients (14.9%) in the EL group and 8 (7.2%)
in the EP group received IL-11 or a platelet transfusion (P=0.090).
No bleeding was observed in either group. The two groups had a
similar frequency of serious AEs (6.6 vs. 6.3%; P=1.000).
 | Table II.Adverse events in the EL and EP
groups. |
Table II.
Adverse events in the EL and EP
groups.
|
| EL | EP | P-value |
|---|
|
|
|
|
|
|---|
| Variable, n
(%) | Any grade | Grade III/IV | Any grade | Grade III/IV | Any grade | Grade III/IV |
|---|
| All events | 54 (44.6) | 7 (5.8) | 77 (69.4) | 17 (15.3) | <0.001 | 0.019 |
| Renal toxicity | 3 (2.5) | – | 13 (11.7) | – | 0.006 | – |
| Elevated
bilirubin | 30 (24.8) | 11 (9.1) | 41 (36.9) | 13 (11.7) | 0.048 | 0.527 |
| Nausea | 27 (22.3) | 1 (0.8) | 45 (40.5) | 3 (2.7) | 0.003 | 0.351 |
| Vomiting | 17 (14.0) | 1 (0.8) | 39 (35.1) | 13 (11.7) | <0.001 | <0.001 |
| Loss of
appetite | 8 (6.6) | – | 18 (16.2) | – | 0.003 | – |
| Hiccup | 0 (0.0) | – | 7 (6.3) | – | 0.005 | – |
| Fatigue | 13 (10.7) | 1 (0.8) | 25 (22.5) | 1 (0.9) | 0.020 | 1.000 |
| Leucopenia | 108 (89.3) | 41 (33.9) | 99 (89.2) | 37 (33.3) | 1.000 | 1.000 |
| Neutropenia | 95 (78.5) | 78 (64.5) | 95 (85.6) | 82 (73.9) | 0.176 | 0.155 |
| Anemia | 96 (79.3) | 28 (23.1) | 87 (78.4) | 17 (15.3) | 0.873 | 0.139 |
|
Thrombocytopenia | 63 (52.1) | 35 (28.9) | 46 (41.4) | 12 (10.8) | 0.115 | 0.001 |
QoL
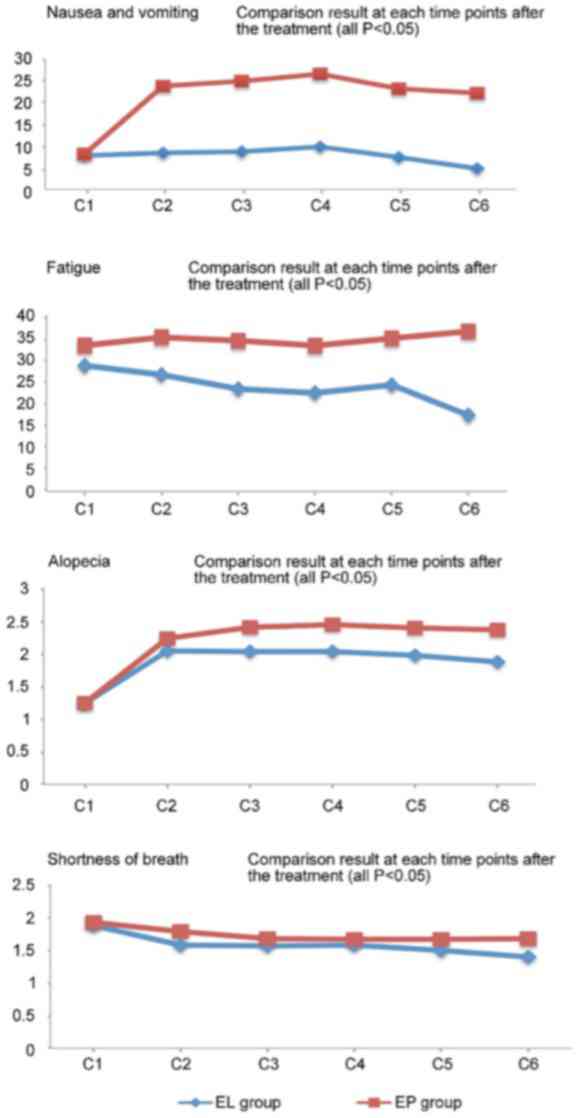
According to the QLQ-C30, the EL group had
significantly better outcomes in terms of nausea/vomiting and
fatigue at various time points following chemotherapy compared with
the EP group (P<0.05). With regard to the QLQ-LC13, the EL group
had significantly better outcomes for alopecia and shortness of
breath at various time points following the second treatment cycle
compared with the EP group (P<0.05) (Fig. 4). The two groups did not show
significant differences in patient-reported outcomes and other QoL
indicators (P>0.05). Prior to treatment, patients in the two
groups achieved varying degrees of improvement in their QoL scores,
particularly with regard tonausea/vomiting (P<0.001), loss of
appetite (P<0.001; data not show) and fatigue (P<0.001).
Concomitant medications
The proportion of patients in the EL group who
received concomitant medications was significant lower than that in
the EP group in terms of diuretic agents (36.4 vs. 100%;
P<0.001), renal protective agents (8.3 vs. 24.3%; P=0.001),
gastric mucosa protective agents (57.0 vs. 71.1%; P=0.029),
electrolytes (28.1 vs. 42.2%; P=0.028) and vitamins (22.3 vs.
35.1%; P=0.041). There were no significant differences between the
two groups in other concomitant medications.
Subsequent antitumor therapy
Once they presented with PD, 51 (42.1%) patients in
the EL group received second-line chemotherapy, of which 14 (11.6%)
patients received both second-line chemotherapy and local
palliative radiotherapy, with 8 (6.6%) patients only receiving
local palliative radiotherapy, 8 (6.6%) patients receiving other
therapies, 12 (9.9%) patients without information on subsequent
treatment and 42 (34.7%) patients without any antitumor therapy. In
the EP group, the corresponding figures were 38 (34.2%), where 7
patients received both second-line chemotherapy and local
palliative radiotherapy, and 9 (8.1%), 2 (1.8%), 5 (4.5%) and 57
(51.4%) with the respective aforementioned conditions; the
differences in numbers were significant (P=0.028).
Discussion
This phase III non-inferiority randomized clinical
trial aimed to verify the non-inferiority (in terms of efficacy) of
EL vs. EP in patients with previously untreated ES-SCLC. The
results showed that EL is not inferior to EP in terms of PFS and
OS. The tolerance and QoL of the EL regimen were greater than that
for the EP regimen. Therefore, EL is an alternative choice for the
first-line treatment of ES-SCLC in China.
A significant finding of the present study is that
the EL regimen, as first-line therapy for ES-SCLC, reduced the
frequency of non-hematological AEs. Renal toxicity of cisplatin,
which is dose-limiting, has a frequency of up to 20–30% (25). Although hydration and application of
diuretics aid in reducing the occurrence, the frequency increases
with dosage accumulation. In the present study, the frequency of
renal toxicity associated with EL was only 2.5% and mostly grade
1/2, suggesting that EL has less renal toxicity, which was also
confirmed by a study using lobaplatin plus paclitaxel to treat
esophageal squamous carcinoma (26).
Given the median onset age of SCLC, the decreased renal reserve
function with age and renal impairment resulting from possible
complications, lobaplatin may be a more appropriate treatment
option for elderly SCLC patients with renal dysfunction, but this
will have to be validated due to the small number of patients
>65 years old in the present study and as the patients were not
analyzed in terms of renal function. Gastrointestinal AEs,
including nausea and vomiting, are additional considerations that
restrict the clinical use of cisplatin. In the present study, the
EL group had a significantly lower frequency of grade 3/4 nausea
and vomiting than the EP group. Similar results were also found in
other studies of lobaplatin-based chemotherapy regimens (26–28),
although the types of tumors were different. Therefore, the EL
regimen is possibly an ideal option for SCLC patients who are
clinically intolerant to the gastrointestinal AEs of cisplatin.
In the present study, the EL group had a higher
frequency of thrombocytopenia than the EP group, but the majority
of the patients recovered within 2 weeks. The EL group, in
comparison with the EP group, did not show statistical differences
in the frequency and severity of leucopenia, neutropenia or anemia.
Thrombocytopenia is a dose-limiting toxicity of lobaplatin, and its
severity is associated with the dose of lobaplatin and level of
creatinine clearance (Ccr) (16). A
study showed that the maximum tolerated dose of lobaplatin was 40
mg/m2 in a population with a Ccr of 60–80 ml/min, 70
mg/m2 in a population with a Ccr of 81–100 ml/min and 85
mg/m2 in a population with a Ccr>100 ml/min; the
median time to occurrence of thrombocytopenia was 10 days (range,
7–14 days), and the median time to recovery was 7 days (range, 2–18
days) (17). Therefore, determining
the individual dosage of lobaplatin according to the serum Ccr of
the patient is an effective measure to prevent the occurrence of
severe thrombocytopenia. Furthermore, coagulation routine
examination should be performed prior to receiving the
lobaplatin-containing regimen. Caution should be taken when using
the EL regimen in patients with SCLC and coagulation disorders or
bleeding events within 4 weeks prior to treatment.
Another important finding is that the EL regimen was
superior to the EP regimen in terms of improving QoL. As ES-SCLC is
an incurable disease, improving QoL is a common goal pursued by the
patient and the physician in addition to prolonging survival. In
the present study, EQ-5D and EQ-VAS, QLQ-C30 and QLQ-LC13 were used
to analyze and evaluate the patients' QoL. The results showed that
the EL group achieved more significant improvements in fatigue,
nausea/vomiting and loss of appetite than the EP group. These
results are supported by a trial in patients with cervical cancer
(29). Along with improvements in
survival, the patients in the EL group showed greater compliance
with treatment, possibly due a more tolerable AE profile of the EL
regimen. In addition, the better tolerability of the EL regimen
could possibly make the patients fitter to receive subsequent lines
of therapy, making improvements in survival possible. In the
present study, more patients received treatments following PD.
However, as those subsequent treatments were administered outside
the protocol, data were scarce and QoL was not evaluated. This will
require assessment to be examined in a future study.
Finally, it is noteworthy that the cost of
lobaplatin is 10–12 times higher compared with cisplatin, as it is
still in the patent protection period in China. Nevertheless, the
lobaplatin-based regimen has more advantages in reducing
concomitant medications compared with the cisplatin-based regimen.
In addition, the overall cost of lobaplatin-based regimen will be
reduced following the patent expiration of lobaplatin.
The present study is not without limitations.
Despite the fact that it was a multicenter study, the sample size
was relatively small and all patients were Chinese. Differences in
cancer genetics among populations may preclude the generalizability
of the present trial and confirmation in other populations is
required. Nevertheless, the present trial shows promising results
for the treatment of SCLC and the applicability of the EL
regimen.
In conclusion, for ES-SCLC treatment-naïve patients,
the EL regimen was not inferior to the EP regimen in terms of
efficacy in the present study. In addition, the EL regimen had a
significantly lower overall frequency and severity of AEs compared
with the EP regimen. The EL regimen may offer an alternative
first-line therapy for patients with ES-SCLC. Meanwhile, further
research is necessary to evaluate the efficacy and safety of EL in
the treatment of SCLC in larger patient populations, including
elderly patients and patients with renal dysfunction.
Acknowledgements
This abstract was presented at the American Society
of Clinical Oncology Annual Meeting, May 30-June 3, 2014 in
Chicago, IL, USA and was published as Abstract no. 7595 in Journal
of Clinical Oncology 32 (Suppl 15) 2014.
Funding
This study was funded by the China State Project for
Essential Drug Research and Development (grant no.
2013ZX09104001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, YF, XL, YL, JWL, DW, YY, SQ, WL, CH, HZ, JL, JS
and LS performed the studies, participated in collecting data and
offering suggestions for revision of the manuscript. HY performed
the statistical analysis and participated in its design. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the ethical committees of
following affiliations: Jilin Cancer Hospital, Changchun, Jilin;
Zhejiang Cancer Hospital, Hangzhou, Zhejiang; 307th Hospital of the
Academy of Military Medical Sciences, Beijing; The First Hospital
of China Medical University, Shenyang, Liaoning; The First
Affiliated Hospital of Dalian Medical University, Dalian, Liaoning;
The Daping Hospital of Third Military Medical University,
Chongqing; The Cancer Hospital of Harbin Medical University,
Harbin, Heilongjiang; People's Liberation Army Cancer Center of
Nanjing 81 Hospital, Nanjing, Jiangsu; Hebei Cancer Hospital,
Shijiazhuang, Hebei; Fujian Province Cancer Hospital, Fuzhou,
Fujian; The Tangdu Hospital of Fourth Military Medical University,
Xi'an, Shanxi; Peking University International Hospital, Peking
University, Beijing; Linyi Cancer Hospital, Linyi, Shandong;
Affiliated Hospital of Shandong Academy of Medical Sciences, Jinan,
Shandong and followed the Good Clinical Practice principle and
Declaration of Helsinki. No substantial protocol revision was made
during study implementation. All patients provided written informed
consent prior to enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Noone A and Krapcho M: SEER
cancer statistics review, 1975–2013, based on November 2015 SEER
data submission, posted to the SEER web site, April 2016. Bethesda:
National Cancer Institute; 2016
|
|
2
|
Pietanza MC, Byers LA, Minna JD and Rudin
CM: Small cell lung cancer: Will recent progress lead to improved
outcomes? Clin Cancer Res. 21:2244–2255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seiter K: Toxicity of the topoisomerase II
inhibitors. Expert Opin Drug Saf. 4:219–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayati F, Hossainzadeh M, Shayanpour S,
Abedi-Gheshlaghi Z and Beladi Mousavi SS: Prevention of cisplatin
nephrotoxicity. J Nephropharmacol. 5:57–60. 2016.PubMed/NCBI
|
|
5
|
McKeage MJ: Lobaplatin: A new antitumour
platinum drug. Expert Opin Investig Drugs. 10:119–128. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harstrick A, Bokemeyer C, Scharnofkse M,
Hapke G, Reile D and Schmoll HJ: Preclinical activity of a new
platinum analogue, lobaplatin, in cisplatin-sensitive and
-resistant human testicular, ovarian, and gastric carcinoma cell
lines. Cancer Chemother Pharmacol. 33:43–47. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang Y, Sui X and Cheng Y: Clinical
research progress of the new antitumor drug Lobaplatin in the
treatment of small cell lung cancer. Chin J N Drugs. 23:184–188.
2014.
|
|
8
|
Jakupec MA, Galanski M and Keppler BK:
Tumour-inhibiting platinum complexes-state of the art and future
perspectives. Rev Physiol Biochem Pharmacol. 146:1–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monneret C: Platinum anticancer drugs.
From serendipity to rational design. Ann Pharm Fr. 69:286–295.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiebig HH, Henss H, von Pawel I,
Gatzemeier U, Manegold CH, Edler L and Berdel W: Phase II clinical
trial of lobaplatin (D-19466) in pretreated patients with
small-cell lung cancer. Onkologie. 19:328–332. 1996.
|
|
11
|
Yang L and Qin S: Progression of
Lobaplatin as the third generation platinum drug. Chin Clin Oncol.
14:1134–1139. 2009.
|
|
12
|
De Vore RF: Chemotherapy for small cell
lung cancer. Pass HI: Lung Cancer Principles and Practice. 2nd.
Philadelphia: Lippincott Williams & Wilkins; 2000
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satouchi M, Kotani Y, Shibata T, Ando M,
Nakagawa K, Yamamoto N, Ichinose Y, Ohe Y, Nishio M, Hida T, et al:
Phase III study comparing amrubicin plus cisplatin with irinotecan
plus cisplatin in the treatment of extensive-disease small-cell
lung cancer: JCOG 0509. J Clin Oncol. 32:1262–1268. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russell SD, Saval MA, Robbins JL, Ellestad
MH, Gottlieb SS, Handberg EM, Zhou Y and Chandler B: HF-ACTION
Investigators: New York Heart Association functional class predicts
exercise parameters in the current era. Am Heart J. 158 (4
Suppl):S24–S30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gietema JA, de Vries EG, Sleijfer DT,
Willemse PH, Guchelaar HJ, Uges DR, Aulenbacher P, Voegeli R and
Mulder NH: A phase I study of
1,2-diamminomethyl-cyclobutane-platinum (II)-lactate (D-19466;
lobaplatin) administered daily for 5 days. Br J Cancer. 67:396–401.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fiebig HH, Henss H, Mross K, Meyberg F,
Aulenbacher P, Burk K and Queißer W: Phase I clinical trial of
lobaplatin (D-19466) after intravenous bolus injection. Onkologie.
17:142–148. 1994.
|
|
18
|
Basch E, Iasonos A, McDonough T, Barz A,
Culkin A, Kris MG, Scher HI and Schrag D: Patient versus clinician
symptom reporting using the national cancer institute common
terminology criteria for adverse events: Results of a
questionnaire-based study. Lancet Oncol. 7:903–909. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osoba D, Rodrigues G, Myles J, Zee B and
Pater J: Interpreting the significance of changes in health-related
quality-of-life scores. J Clin Oncol. 16:139–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson H, Hopwood P, Stephens RJ,
Thatcher N, Cottier B, Nicholson M, Milroy R, Maughan TS, Falk SJ,
Bond MG, et al: Gemcitabine plus best supportive care (BSC) vs BSC
in inoperable non-small cell lung cancer-a randomized trial with
quality of life as the primary outcome. UK NSCLC Gemcitabine Group.
Non-small cell lung cancer. Br J Cancer. 83:447–453. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noda K, Nishiwaki Y, Kawahara M, Negoro S,
Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, et
al: Irinotecan plus cisplatin compared with etoposide plus
cisplatin for extensive small-cell lung cancer. N Engl J Med.
346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lara PN Jr, Natale R, Crowley J, Lenz HJ,
Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR,
et al: Phase III trial of irinotecan/cisplatin compared with
etoposide/cisplatin in extensive-stage small-cell lung cancer:
Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol.
27:2530–2535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung SH, Kang SJ, McCall LM and
Blumenstein B: Sample size computation for two-sample
noninferiority log-rank test. J Biopharm Stat. 15:969–979. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakatos E: Sample sizes based on the
log-rank statistic in complex clinical trials. Biometrics.
44:229–241. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller RP, Tadagavadi RK, Ramesh G and
Reeves WB: Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel).
2:2490–2518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen MQ, Chen C, Lu HJ and Xu BH: The
efficacy and toxicities of combined lobaplatin with paclitaxel as a
first-line chemotherapy for advanced esophageal squamous cell
carcinoma. J Thorac Dis. 7:1749–1755. 2015.PubMed/NCBI
|
|
27
|
Yang JS, Wang T, Qiu MQ and Li QL:
Comparison of efficacy and toxicity profiles between
paclitaxel/lobapoatin- and cisplatin/5-fluorouracil-based
concurrent chemoradiotherapy of advanced inoperable oesophageal
cancer. Intern Med J. 45:757–761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WP, Liu H, Chen L, Yao YQ and Zhao EF:
A clinical comparison of lobaplatin or cisplatin with mitomycine
and vincristine in treating patients with cervical squamous
carcinoma. Asian Pac J Cancer Prev. 16:4629–4631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang JQ, Wang T, Shi F, Yang YY, Su J,
Chai YL and Liu Z: A randomized controlled trial comparing clinical
outcomes and toxicity of lobaplatin-versus cisplatin-based
concurrent chemotherapy plus radiotherapy and high-dose-rate
brachytherapy for FIGO Stage II and III cervical cancer. Asian Pac
J Cancer Prev. 16:5957–5961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|