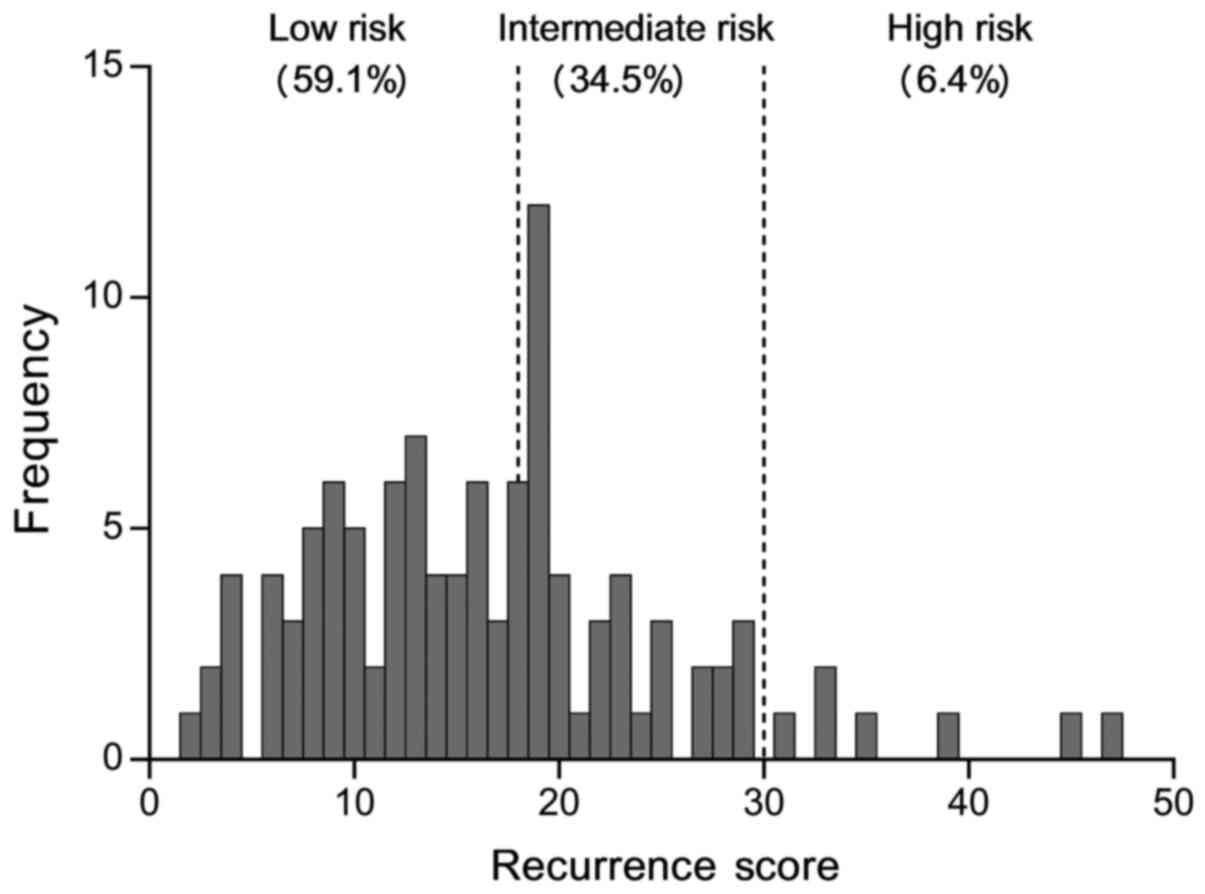
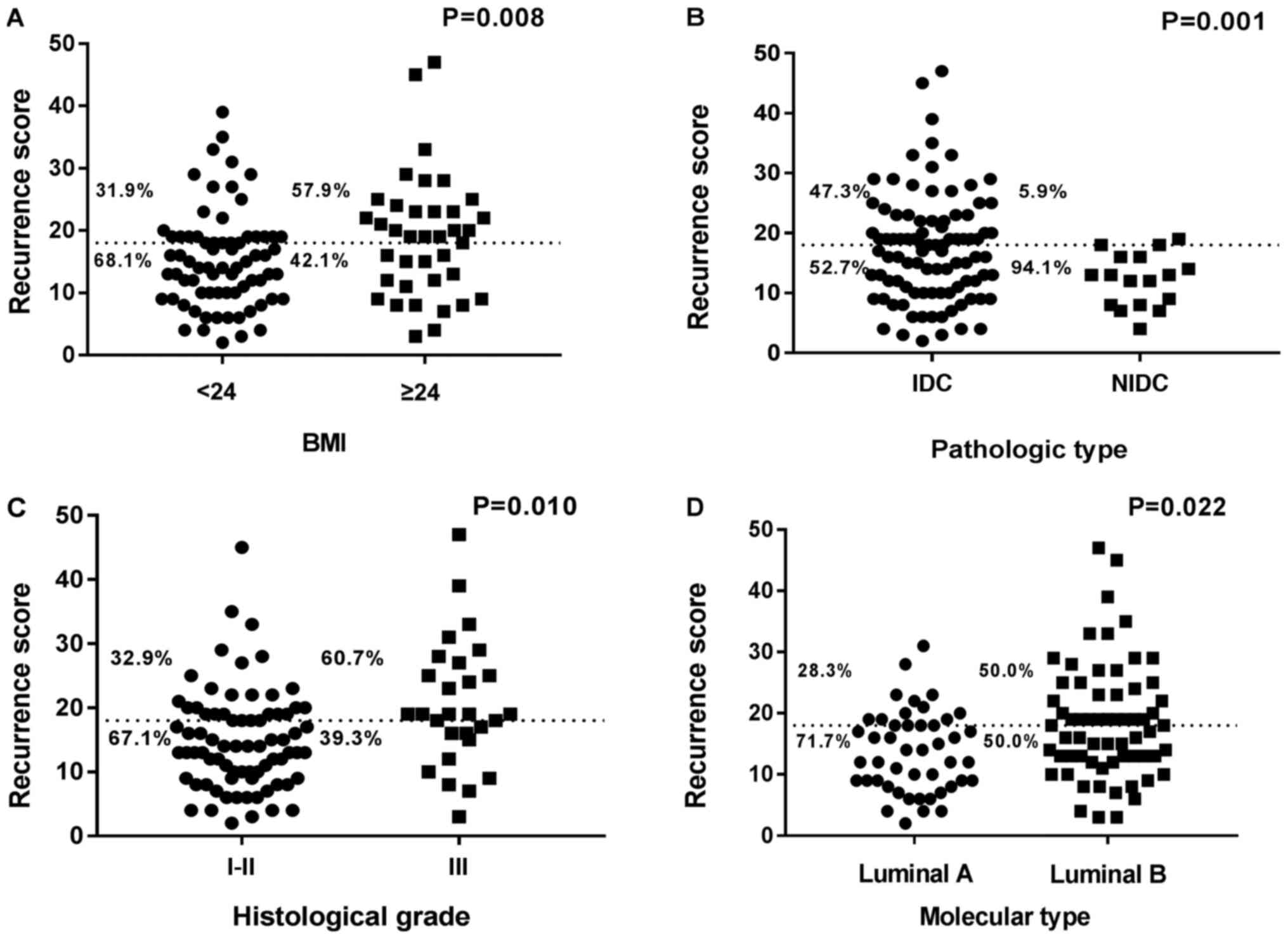
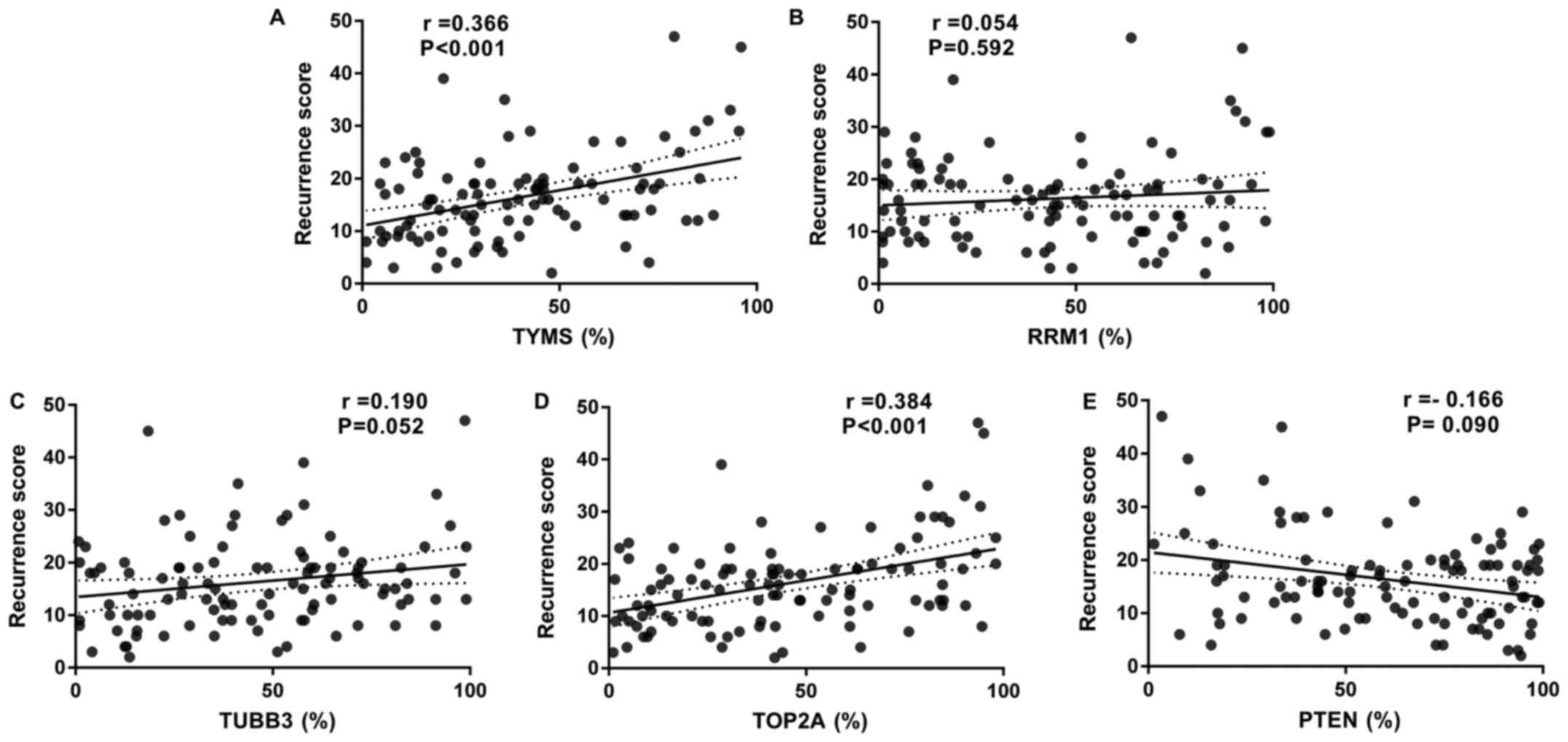
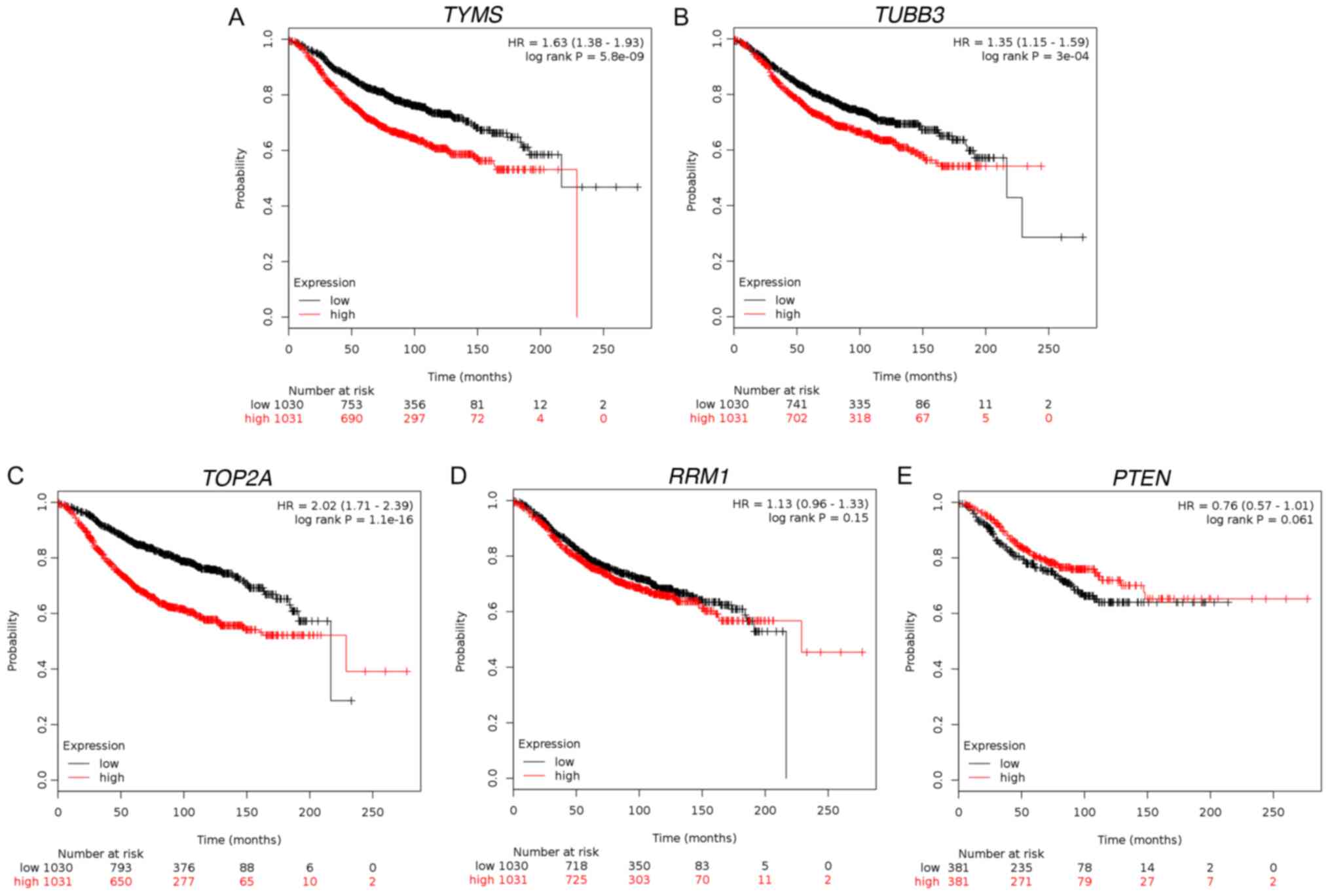
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson BO and Jakesz R: Breast cancer
issues in developing countries: An overview of the Breast Health
Global Initiative. World J Surg. 32:2578–2585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
4
|
National Bureau of Statistics of China, .
China Statistical Yearbook, 2010. China Statistics Press; Beijing:
2010
|
|
5
|
Salek R, Shahidsales S and Mozafari V:
Changing pattern in the clinical presentation of breast cancer in
the absence of a screening program over a period of thirty-three
years in Iran. Breast. 28:95–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Somashekhar SP, Sepúlveda MJ, Puglielli S,
Norden AD, Shortliffe EH, Rohit Kumar C, Rauthan A, Arun Kumar N,
Patil P, Rhee K, et al: Watson for Oncology and breast cancer
treatment recommendations: Agreement with an expert
multidisciplinary tumor board. Ann Oncol. 29:418–423. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gray J and Druker B: Genomics: The breast
cancer landscape. Nature. 486:328–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo M, Hoshi SL, Ishiguro H,
Yoshibayashi H and Toi M: Economic evaluation of 21-gene reverse
transcriptase-polymerase chain reaction assay in
lymph-node-negative, estrogen-receptor-positive, early-stage breast
cancer in Japan. Breast Cancer Res Treat. 112:175–187. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cronin M, Sangli C, Liu ML, Pho M, Dutta
D, Nguyen A, Jeong J, Wu J, Langone KC and Watson D: Analytical
validation of the Oncotype DX genomic diagnostic test for
recurrence prognosis and therapeutic response prediction in
node-negative, estrogen receptor-positive breast cancer. Clin Chem.
53:1084–1091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cuzick J, Dowsett M, Pineda S, Wale C,
Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, et al:
Prognostic value of a combined estrogen receptor, progesterone
receptor, Ki-67, and human epidermal growth factor receptor 2
immunohistochemical score and comparison with the Genomic Health
recurrence score in early breast cancer. J Clin Oncol.
29:4273–4278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Győrffy B, Hatzis C, Sanft T, Hofstatter
E, Aktas B and Pusztai L: Multigene prognostic tests in breast
cancer: Past, present, future. Breast Cancer Res. 17:112015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paik S, Tang G, Shak S, Kim C, Baker J,
Kim W, Cronin M, Baehner FL, Watson D, Bryant J, et al: Gene
expression and benefit of chemotherapy in women with node-negative,
estrogen receptor-positive breast cancer. J Clin Oncol.
24:3726–3734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paik S: Development and clinical utility
of a 21-gene recurrence score prognostic assay in patients with
early breast cancer treated with tamoxifen. Oncologist. 12:631–635.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolf I, Ben-Baruch N, Shapira-Frommer R,
Rizel S, Goldberg H, Yaal-Hahoshen N, Klein B, Geffen DB and
Kaufman B: Association between standard clinical and pathologic
characteristics and the 21-gene recurrence score in breast cancer
patients: A population-based study. Cancer. 112:731–736. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Albain KS, Barlow WE, Shak S, Hortobagyi
GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL,
Davidson NE, et al Breast Cancer Intergroup of North America, :
Prognostic and predictive value of the 21-gene recurrence score
assay in postmenopausal women with node-positive,
oestrogen-receptor-positive breast cancer on chemotherapy: A
retrospective analysis of a randomised trial. Lancet Oncol.
11:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, André F,
Bergh J, et al Panel members, : Personalizing the treatment of
women with early breast cancer: Highlights of the St Gallen
International Expert Consensus on the Primary Therapy of Early
Breast Cancer 2013. Ann Oncol. 24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsunoda Y, Suzuki K, Tsunoda A, Takimoto M
and Kusano M: Evaluation of 5-fluorouracil related genes in breast
cancer to predict the effect of adjuvant therapy with oral
fluorouracil derivatives. Oncol Rep. 23:771–777. 2010.PubMed/NCBI
|
|
21
|
Yu Z, Sun J, Zhen J, Zhang Q and Yang Q:
Thymidylate synthase predicts for clinical outcome in invasive
breast cancer. Histol Histopathol. 20:871–878. 2005.PubMed/NCBI
|
|
22
|
Metro G, Zheng Z, Fabi A, Schell M,
Antoniani B, Mottolese M, Monteiro AN, Vici P, Lara Rivera S,
Boulware D, et al: In situ protein expression of RRM1, ERCC1, and
BRCA1 in metastatic breast cancer patients treated with
gemcitabine-based chemotherapy. Cancer Invest. 28:172–180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galmarini CM, Treilleux I, Cardoso F,
Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M,
Larsimont D, Piccart MJ, et al: Class III beta-tubulin isotype
predicts response in advanced breast cancer patients randomly
treated either with single-agent doxorubicin or docetaxel. Clin
Cancer Res. 14:4511–4516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nitiss JL: Targeting DNA topoisomerase II
in cancer chemotherapy. Nat Rev Cancer. 9:338–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braybrooke JP, Levitt NC, Joel S, Davis T,
Madhusudan S, Turley H, Wilner S, Harris AL and Talbot DC:
Pharmacokinetic study of cisplatin and infusional etoposide
phosphate in advanced breast cancer with correlation of response to
topoisomerase IIalpha expression. Clin Cancer Res. 9:4682–4688.
2003.PubMed/NCBI
|
|
26
|
Lánczky A, Nagy Á, Bottai G, Munkácsy G,
Szabó A, Santarpia L and Győrffy B: miRpower: A web-tool to
validate survival-associated miRNAs utilizing expression data from
2178 breast cancer patients. Breast Cancer Res Treat. 160:439–446.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orucevic A, Bell JL, McNabb AP and Heidel
RE: Oncotype DX breast cancer recurrence score can be predicted
with a novel nomogram using clinicopathologic data. Breast Cancer
Res Treat. 163:51–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stemmer SM, Klang SH, Ben-Baruch N, Geffen
DB, Steiner M, Soussan-Gutman L, Merling S, Svedman C, Rizel S and
Lieberman N: The impact of the 21-gene Recurrence Score assay on
clinical decision-making in node-positive (up to 3 positive nodes)
estrogen receptor-positive breast cancer patients. Breast Cancer
Res Treat. 140:83–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stemmer SM, Steiner M, Rizel S,
Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, Geffen DB,
Nisenbaum B, Isaacs K, Fried G, et al: Clinical outcomes in
patients with node-negative breast cancer treated based on the
recurrence score results: Evidence from a large prospectively
designed registry. NPJ Breast Cancer. 3:332017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stemmer SM, Steiner M, Rizel S, Geffen DB,
Nisenbaum B, Peretz T, Soussan-Gutman L, Bareket-Samish A, Isaacs
K, Rosengarten O, et al: Clinical outcomes in ER+ HER2
-node-positive breast cancer patients who were treated according to
the Recurrence Score results: Evidence from a large prospectively
designed registry. NPJ Breast Cancer. 3:322017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee MH, Han W, Lee JE, Kim KS, Park H, Kim
J, Bae SY, Shin HJ, Lee JW and Lee ES: The clinical impact of
21-gene recurrence score on treatment decisions for patients with
hormone receptor-positive early breast cancer in Korea. Cancer Res
Treat. 47:208–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holt S, Bertelli G, Humphreys I, Valentine
W, Durrani S, Pudney D, Rolles M, Moe M, Khawaja S, Sharaiha Y, et
al: A decision impact, decision conflict and economic assessment of
routine Oncotype DX testing of 146 women with node-negative or
pNImi, ER-positive breast cancer in the U.K. Br J Cancer.
108:2250–2258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lo SS, Mumby PB, Norton J, Rychlik K,
Smerage J, Kash J, Chew HK, Gaynor ER, Hayes DF, Epstein A, et al:
Prospective multicenter study of the impact of the 21-gene
recurrence score assay on medical oncologist and patient adjuvant
breast cancer treatment selection. J Clin Oncol. 28:1671–1676.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martínez Del Prado P, Alvarez-López I,
Domínguez-Fernández S, Plazaola A, Ibarrondo O, Galve-Calvo E,
Ancizar-Lizarraga N, Gutierrez-Toribio M, Lahuerta-Martínez A and
Mar J: Clinical and economic impact of the 21-gene recurrence score
assay in adjuvant therapy decision making in patients with
early-stage breast cancer: Pooled analysis in 4 Basque Country
university hospitals. Clinicoecon Outcomes Res. 10:189–199. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dieci MV, Guarneri V, Giarratano T, Mion
M, Tortora G, De Rossi C, Gori S, Oliani C, Merlini L, Pasini F, et
al: First prospective nulticenter Italian study on the impact of
the 21-gene recurrence score in adjuvant clinical decisions for
patients with ER positive/HER2 negative breast cancer. Oncologist.
23:297–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamauchi H, Nakagawa C, Takei H, Chao C,
Yoshizawa C, Yagata H, Yoshida A, Hayashi N, Hell S and Nakamura S:
Prospective study of the effect of the 21-gene assay on adjuvant
clinical decision-making in Japanese women with estrogen
receptor-positive, node-negative, and node-positive breast cancer.
Clin Breast Cancer. 14:191–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eiermann W, Rezai M, Kümmel S, Kühn T,
Warm M, Friedrichs K, Schneeweiss A, Markmann S, Eggemann H,
Hilfrich J, et al: The 21-gene recurrence score assay impacts
adjuvant therapy recommendations for ER-positive, node-negative and
node-positive early breast cancer resulting in a risk-adapted
change in chemotherapy use. Ann Oncol. 24:618–624. 2013. View Article : Google Scholar : PubMed/NCBI
|