Introduction
Lung cancer is the most frequently diagnosed type of
cancer and the leading cause of cancer-associated mortality
worldwide. An estimated 1.8 million new lung cancer cases were
diagnosed in 2012, which accounted for ~13% of all cancer diagnoses
(1). Chemotherapy is the most common
treatment for cancer; however, it has severe side effects and
limited efficacy. Current research focuses on developing highly
efficient drugs with low toxicity for cancer treatment.
Natural products and their analogues are important
sources of anti-cancer drugs that are widely used to treat patients
with cancer (2). These agents
include vincristine and paclitaxel (PTX). Vincristine is an
anti-neoplastic agent from the vinca alkaloid family that is
commonly used for acute lymphoblastic leukemia (3), whereas PTX has been isolated from the
bark of Taxus brevifolia and is primarily used as a
treatment for breast cancer (4).
Ampelopsin (AMP), also termed dihydromyricetin, is a flavonoid
(5) isolated from the tender stems
and leaves of the Chinese medicinal herb Ampelopsis
grossedentata (6). Previous
studies have reported that AMP possesses anti-cancer activities in
various cancer cell lines, including B16 melanoma cells (7), HepG2 human hepatoma cell line (8), MCF-7 and MDA-MB-231 breast cancer cells
(6), PC-3 human prostate cancer
cells (9) and A2780 ovarian cancer
cells (10), which indicates that
AMP is a promising anti-cancer agent that exhibits low toxicity
(11). AMP sodium (AMP-Na) is an AMP
sodium salt with higher solubility and stability compared with AMP
(12). The acute systemic toxicity
of AMP-Na administered intravenously was determined to be more than
two times lower compared with that of AMP in a previous unpublished
study; the upper tolerance dose in mice was about 2,000 mg/kg
(13). However, whether the
anti-cancer activity of AMP-Na is altered compared with that of AMP
has remained to be fully elucidated. Our previous study has
demonstrated that AMP-Na significantly inhibited the proliferation
of EJ and sarcoma 180 cells in vitro and in vivo
(12); the present study
investigated the effects of AMP-Na, alone and in combination with
other drugs, on human lung adenocarcinoma cell lines, and
investigated the underlying mechanism of action.
Materials and methods
Cell lines and reagents
The human lung adenocarcinoma SPC-A-1 and A549 cell
lines were purchased from the Cell Bank of the Chinese Academy of
Sciences. They were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal calf serum
(Sigma-Aldrich; Merck KGaA) and antibiotics (50 UI/ml penicillin G
and 50 µg/ml streptomycin; Beijing Solarbio Science &
Technology Co., Ltd.) at 37°C in a humidified atmosphere containing
5% CO2.
AMP-Na lyophilized powder (Guangdong Taihe
Technology, Ltd., Guangdong, China) was dissolved in a mixture of
phosphate buffers at pH 6.8 and 6.5 at the final ratio of 1:1.5
[AMP-Na was alkaline (pH 7.6–7.8) when dissolved with pH 6.8
buffer; therefore, the phosphate buffer at pH 6.5 was used to
adjust pH value] at the final concentration of was 4 mg/ml. The
stock solution was prepared with sterile filtered PBS (pH 6.8–7.0)
and diluted with normal saline solution to the desired
concentrations. MTT was purchased from Sigma-Aldrich (Merck KGaA),
the Annexin V-FITC Apoptosis Detection kit was from Beyotime
Institute of Biotechnology and the rat monoclonal tubulin (cat. no.
ab6161) and FITC-labeled rabbit anti-mouse immunoglobulin (Ig)G
antibodies (cat no. ab6730) were purchased from Abcam.
Cell viability assay
Cell viability was determined using the MTT
colorimetric assay (14). Cells in
the logarithmic growth phase were seeded in a 96-well plate at
4.5×103 cells/well, incubated for 24 h and treated with
a range of concentrations of AMP-Na (12.5, 25, 50 or 100 µg/ml) for
48, 72 or 96 h. To investigate the effects of AMP-Na combined with
different chemotherapeutic drugs, the SPC-A-1 cells were treated
with AMP-Na (50 µg/ml) combined with carboplatin (6.25–100 µg/ml;
Qilu Pharmaceutical Co., Ltd.), 5-fluorouracil (5-FU; 3.125–50
µg/ml; Jinghua Pharmaceutical Group Co., Ltd.) or PTX (3.125–50
µg/ml; Sichuan Shenghe Pharmaceutical Co., Ltd.) for 48 h prior to
adding 10 µl MTT at 37°C for 4 h. The reaction product, formazan,
was dissolved using 100 µl 10% SDS (14) and the absorbance at 570 nm was read
using an EL800 microplate reader (Bio-Tek Instruments, Inc.). The
experiments were performed in triplicate. Cell viability was
calculated as follows: Cell viability (%) =
Atreated/Acontrol ×100%, where A is
absorbance. The coefficient of drug interaction (CDI) was
calculated as follows: CDI = AB/(A × B), where AB is the absorbance
ratio of the combination groups to the control group, and A or B
are the absorbance ratios of the single agent groups to the control
group. A CDI value <1, =1 or >1 was considered to indicate
that drugs were synergistic, additive or antagonistic, respectively
(15).
Colony formation assay
The SPC-A-1 and A549 cells were trypsinized to
obtain single-cell suspensions and seeded into 6-mm incubation
plates at a density of 250 cells/well. Cells were treated with
AMP-Na (25 or 50 µg/ml), cultured for 15 days and subsequently
fixed with methanol and glacial acetic acid (ratio, 7:1) for 25 min
at 25°C and stained with 0.1% crystal violet for 25 min at 25°C.
The colonies that contained >50 cells were counted using
Image-pro plus 6.0 (Media Cybernetics, Inc.).
Scratch wound healing assay
A scratch wound healing assay was used to evaluate
the migratory ability of SPC-A-1 and A549 cells. Cells
(5×105/ml) were cultured in 12-well plates for 24 h.
Straight scratches of equal width were made in the monolayer of
cells using a 200 µl pipette tip, and the monolayers were washed
twice with PBS to remove debris. Following incubation with 50 µg/ml
AMP-Na for 24 h, images were captured using a CKX41 inverted light
microscope (Olympus Corporation). And the gap width was measured
using Image-pro plus 6.0.
Cell apoptosis analysis by flow
cytometry
Following culture in 6-well plates at
2×105 cells/well for 24 h, SPC-A-1 cells were treated
with 100 µg/ml AMP-Na for 48 h, collected in 1.5-ml centrifuge
tubes, washed twice with cold PBS and re-suspended in binding
buffer. A total of 2 µl Annexin V-FITC and propidium iodide were
added separately, followed by incubation in the dark for 15 min and
analysis using a flow cytometer.
Cell cycle distribution analysis by
flow cytometry
SPC-A-1 cells were seeded at 5×105
cells/flask in a 100-ml culture flask for 24 h, treated with 100
µg/ml AMP-Na for 48 h, harvested and fixed in ice-cold 75% ethanol
at −20°C overnight. Following washing with PBS, cells were fixed
with 500 µl staining solution containing propidium iodide (50
µg/ml) and RNase (50 µg/ml; Jiangsu KeyGEN BioTECH Corp., Ltd.) and
incubated for 30 min in the dark at room temperature. Flow
cytometric analysis was performed and the cell proportion in each
phase was calculated using a Cell FIT DNA Analysis system (BD
Biosciences).
Ultra-microstructure analysis
SPC-A-1 cells were seeded at 5×105
cells/flask in a 100-ml culture flask for 24 h. Following treatment
with 100 µg/ml AMP-Na for 48 h, cells were harvested, fixed with
2.5% glutaraldehyde for 2 h at 4°C, washed with PBS three times,
fixed in 1% osmium tetroxide for 1 h at 4°C, dehydrated in graded
ethanol, and embedded in araldite. The ultrathin sections of cells
(50–70 nm) were prepared and stained with 1% (w/v) uranyl acetate
and lead citrate. Transmission electron microscopy (TEM; JEOL,
Ltd.) was used to analyze the cell ultra-microstructure.
Immunofluorescence intensity
analysis
Following treatment with 1 ml AMP-Na at a range of
concentrations (12.5, 25, 50 and 100 µg/ml) for 48 h, SPC-A-1 cells
were collected, washed twice with PBS, fixed with 3.7%
paraformaldehyde for 15 min at 4°C, and treated with 0.3% Triton
X-100 for 10 min on ice. Cells were incubated with the primary rat
monoclonal anti-tubulin antibody (1:400) on ice for 40 min, washed
twice with cold PBS, stained with the FITC-labeled rabbit anti-rat
IgG antibody (1:200) on ice for 40 min and post-fixed with 1%
paraformaldehyde at 4°C for at least 30 min. Cells were washed
again, re-suspended in PBS and analyzed by flow cytometry.
Laser confocal microscopic
analysis
SPC-A-1 cells were seeded in 100-ml culture flasks
(5×105 cells/flask). Polylysine-coated glass coverslips
were embedded in the flasks for 24 h, followed by exposure to
AMP-Na (25, 50 or 100 µg/ml) for 48 h. Coverslips were collected,
washed with PBS and fixed with 3.7% paraformaldehyde for 15 min at
room temperature. Cells were permeabilized with 0.3% Triton X-100
for 5 min on ice, washed three times with PBS and incubated with
the primary rat monoclonal anti-tubulin antibody (1:400) in a
sealed wet incubation chamber at 37°C for 1 h. Coverslips were
washed three times with PBS for 5 min, stained with the
FITC-labeled rabbit anti-mouse IgG antibody (1:100) in a sealed wet
box at 37°C for 1 h, and analyzed by laser confocal microscopy
(Leica TCSSP2; Leica Microsystems Heidelberg GmbH).
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc.).
Differences between two groups were analyzed using a Student's
t-test. For comparison of multiple groups, one-way analysis of
variance followed by Dunnett's post hoc test was used. Values are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
AMP-Na inhibits lung cancer cell
proliferation
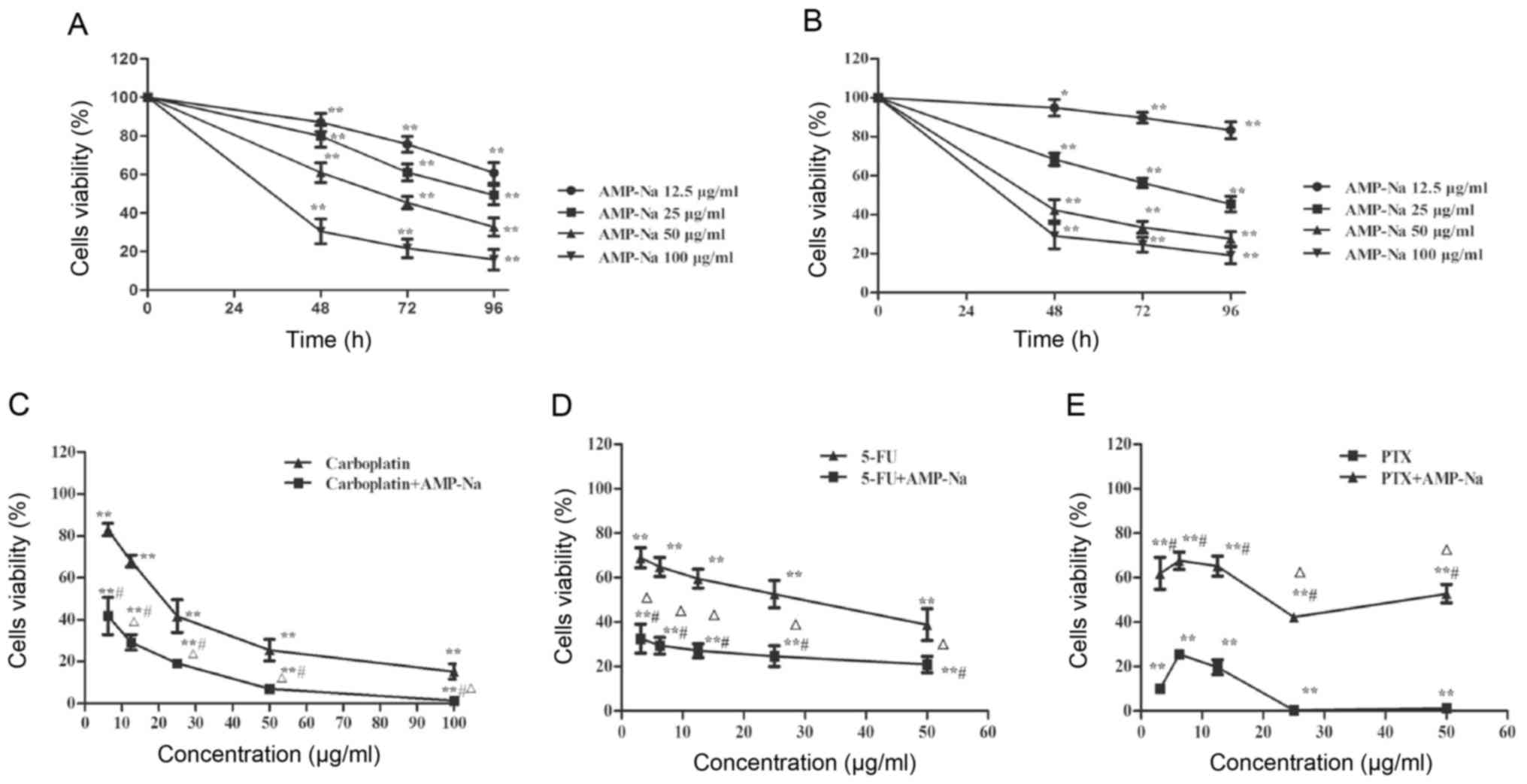
Compared with control group, AMP-Na significantly
reduced the proliferation of SPC-A-1 and A549 cells in a time- and
dose-dependent manner, when used at 12.5–100 µg/ml for 48, 72 or 96
h (Fig. 1A and B). In addition,
combined treatment with AMP-Na (50 µg/ml) and carboplatin or 5-FU
inhibited cell viability synergistically across a wide
concentration range (CDI<1; Fig. 1C
and D), whereas the combination with PTX displayed potential
antagonism as cell viability was significantly enhanced at all
concentrations in the PTX + AMP-Na group compared with the PTX only
group (CDI>1; Fig. 1E). These
results suggested that AMP-Na and PTX may have similar targets.
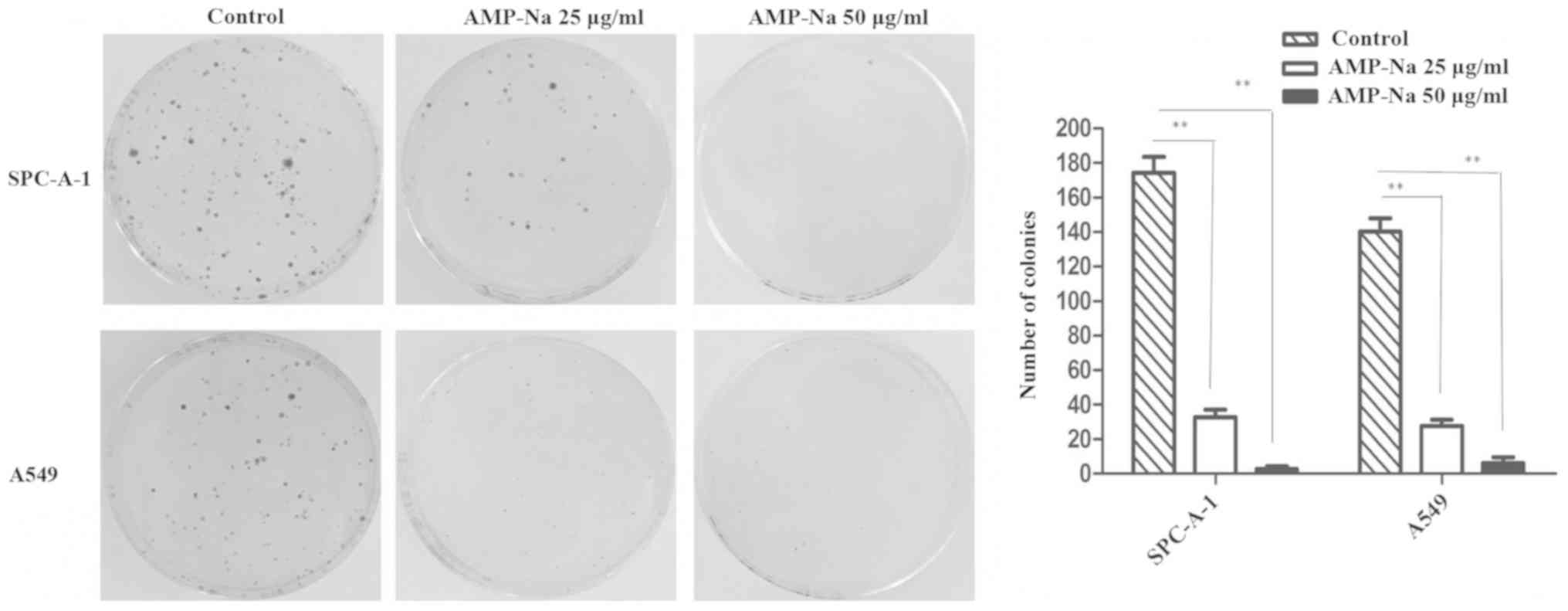
AMP-Na inhibits the colony formation
of lung cancer cells
Following 15 days in culture, a significant decline
in the number and size of the colonies was detected in the SPC-A-1
and A549 cells treated with AMP-Na (25 and 50 µg/ml). These results
suggested that AMP-Na markedly suppressed the colony formation
capabilities of human lung adenocarcinoma cell lines in
vitro (Fig. 2).
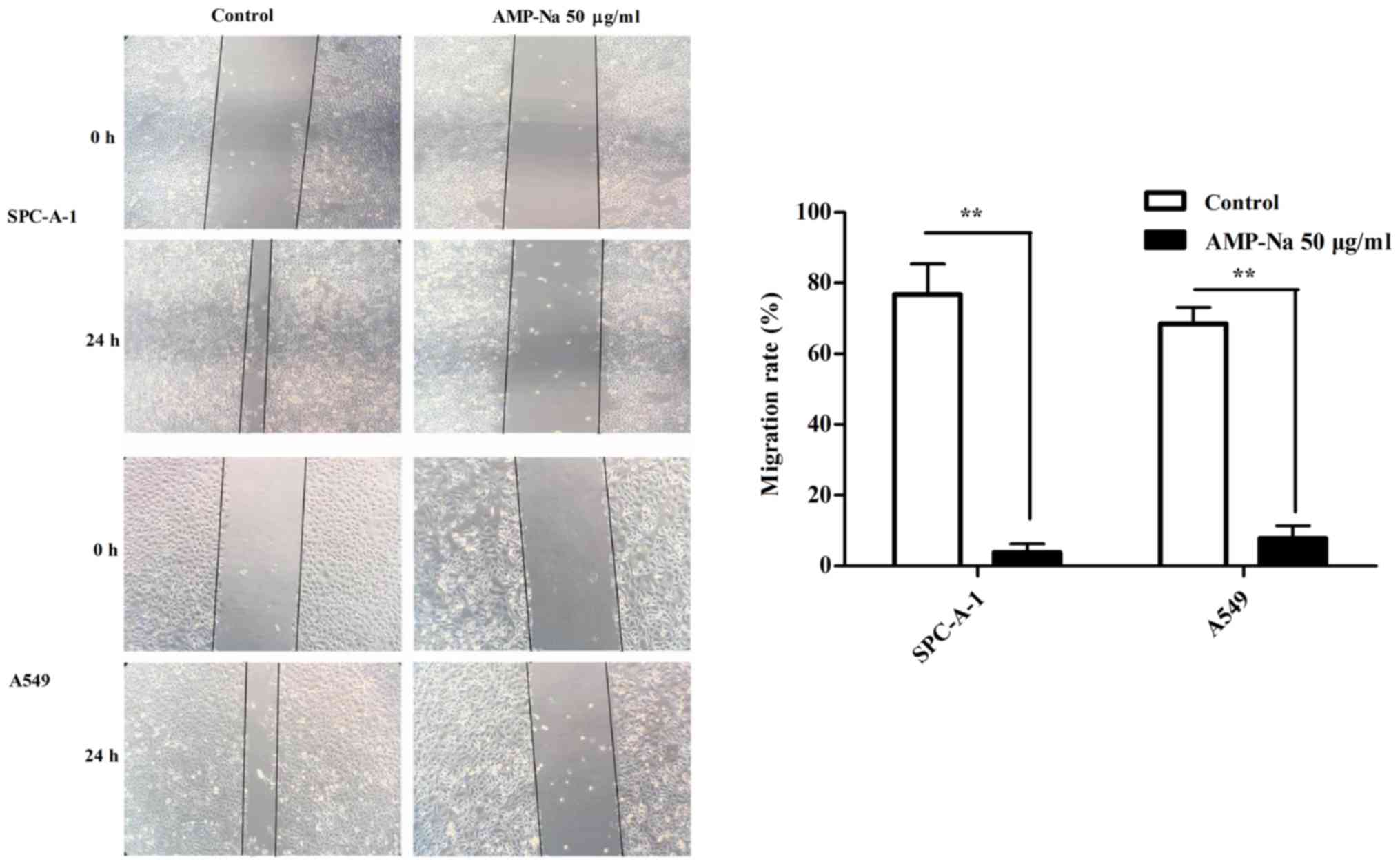
AMP-Na inhibits the migration of lung
cancer cells
A wound healing assay was used to evaluate cell
migration. The SPC-A-1 and A549 cell lines were treated with AMP-Na
(100 µg/ml) for 24 h. The results demonstrated that scratch healing
was significantly reduced in the presence of AMP-Na and cells
treated with AMP-Na exhibited a significantly lower migratory
ability compared with those in the control group (P<0.01;
Fig. 3).
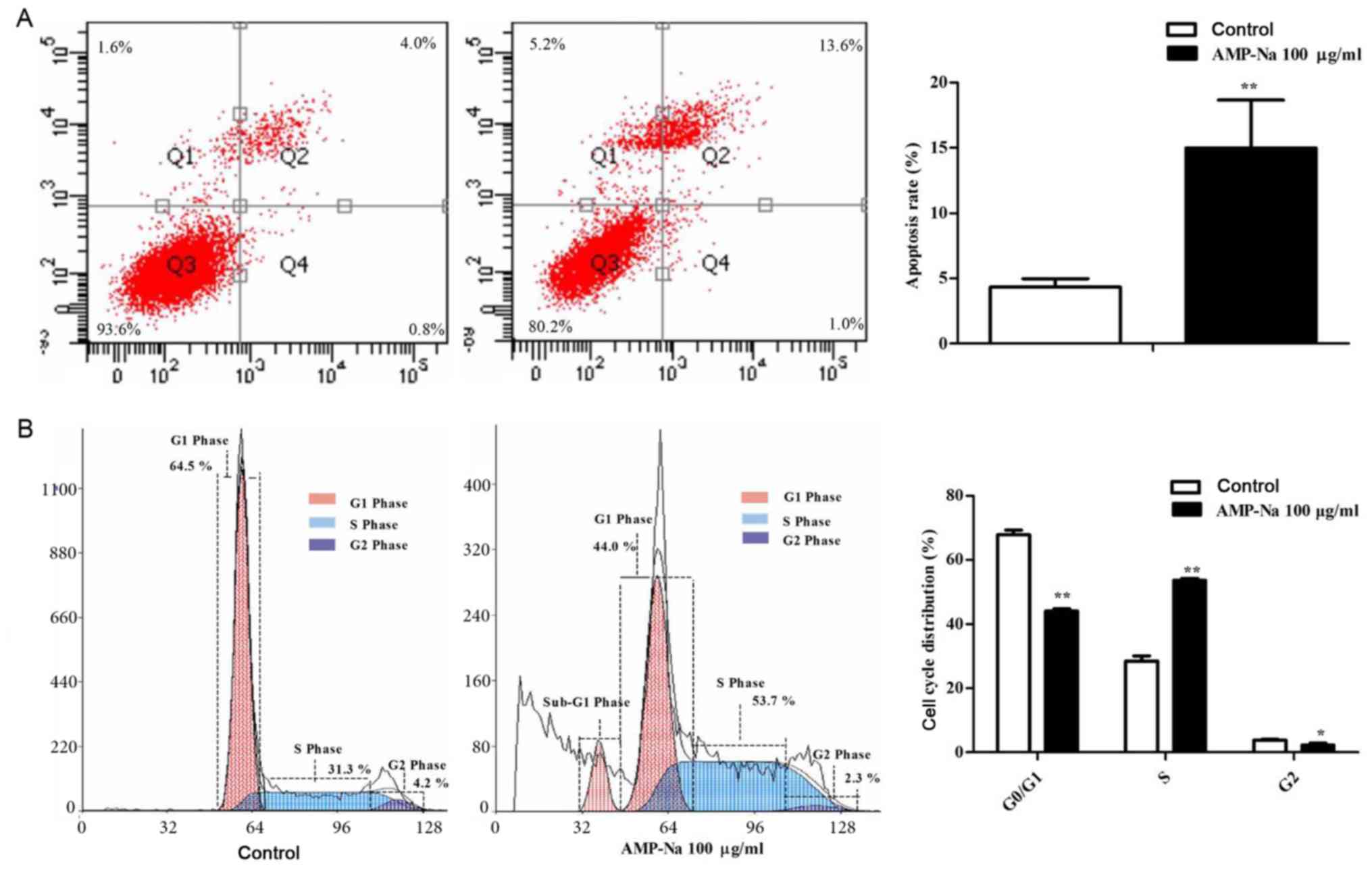
AMP-Na induces apoptosis and cell
cycle arrest in SPC-A-1 cells
The apoptotic rate in the 100 µg/ml AMP-Na-treated
group was higher compared with that in the control group
(14.71±1.10 vs. 4.31±0.56%; P<0.01; Fig. 4A). In addition, following treatment
with 100 µg/ml AMP-Na for 48 h, flow cytometry revealed a peak of
cells in sub-G1 phase (Fig. 4B). In
addition, the number of cells in S-phase was increased in the
AMP-Na-treated group compared with that in the control group
(P<0.01; Fig. 4B), whereas the
number of cells in G2/M-phase and G1-phase were
significantly decreased (P<0.05; Fig.
4B).
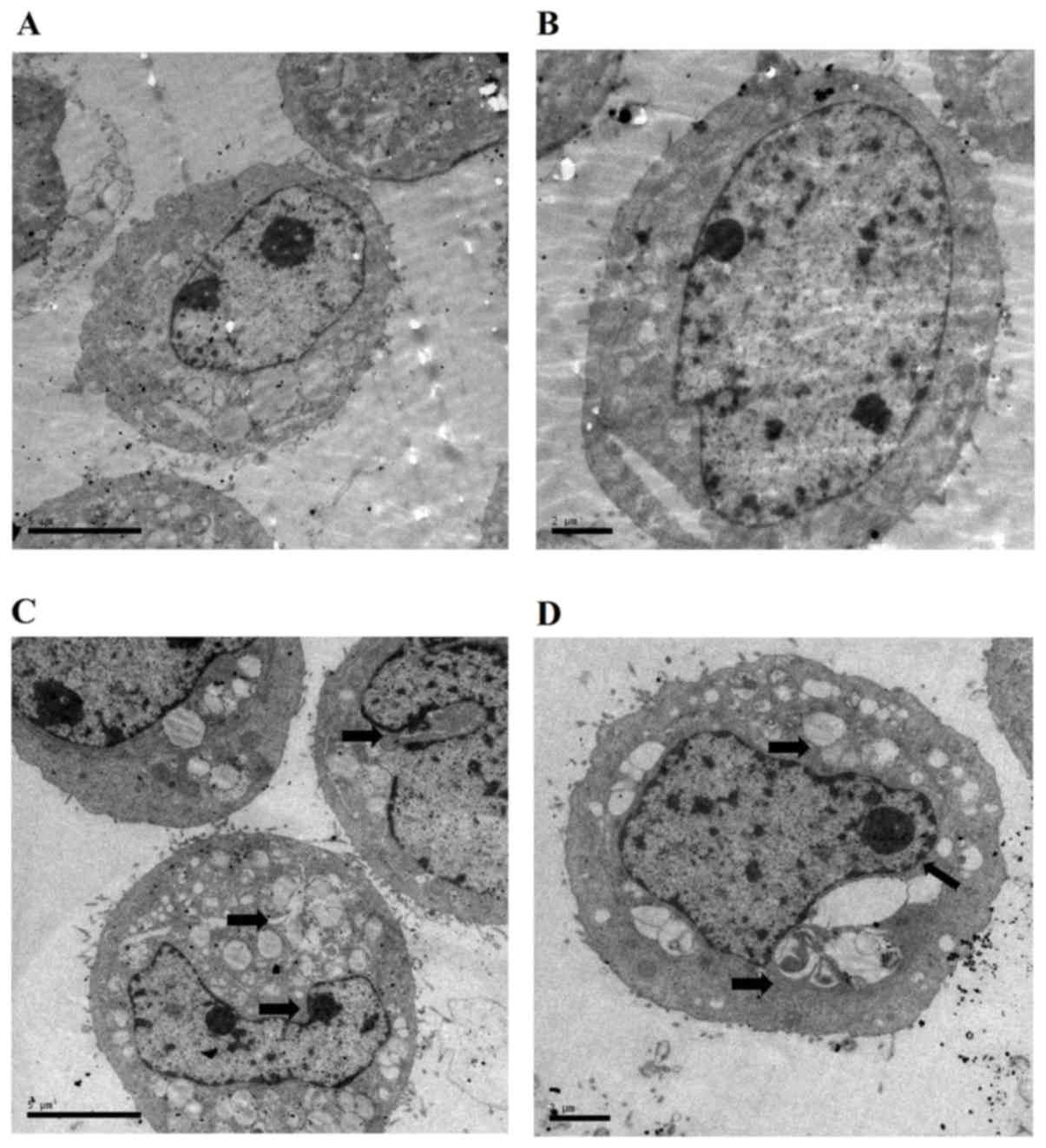
Effect of AMP-Na on SPC-A-1 cell
ultra-microstructure
The cell ultra-microstructure was examined by TEM.
The results demonstrated that cells in the control group had
abundant organelles, intact and distinct cellular ultra-structures
and well-distributed chromatin (Fig.
5A). Of note, treatment with AMP-Na (100 µg/ml) induced a
cytoplasm vacuolization and chromatin margination (Fig. 5B). In addition, nucleus deformation,
nuclear membrane shrinkage and mitochondrial swelling were observed
in the AMP-Na-treated group.
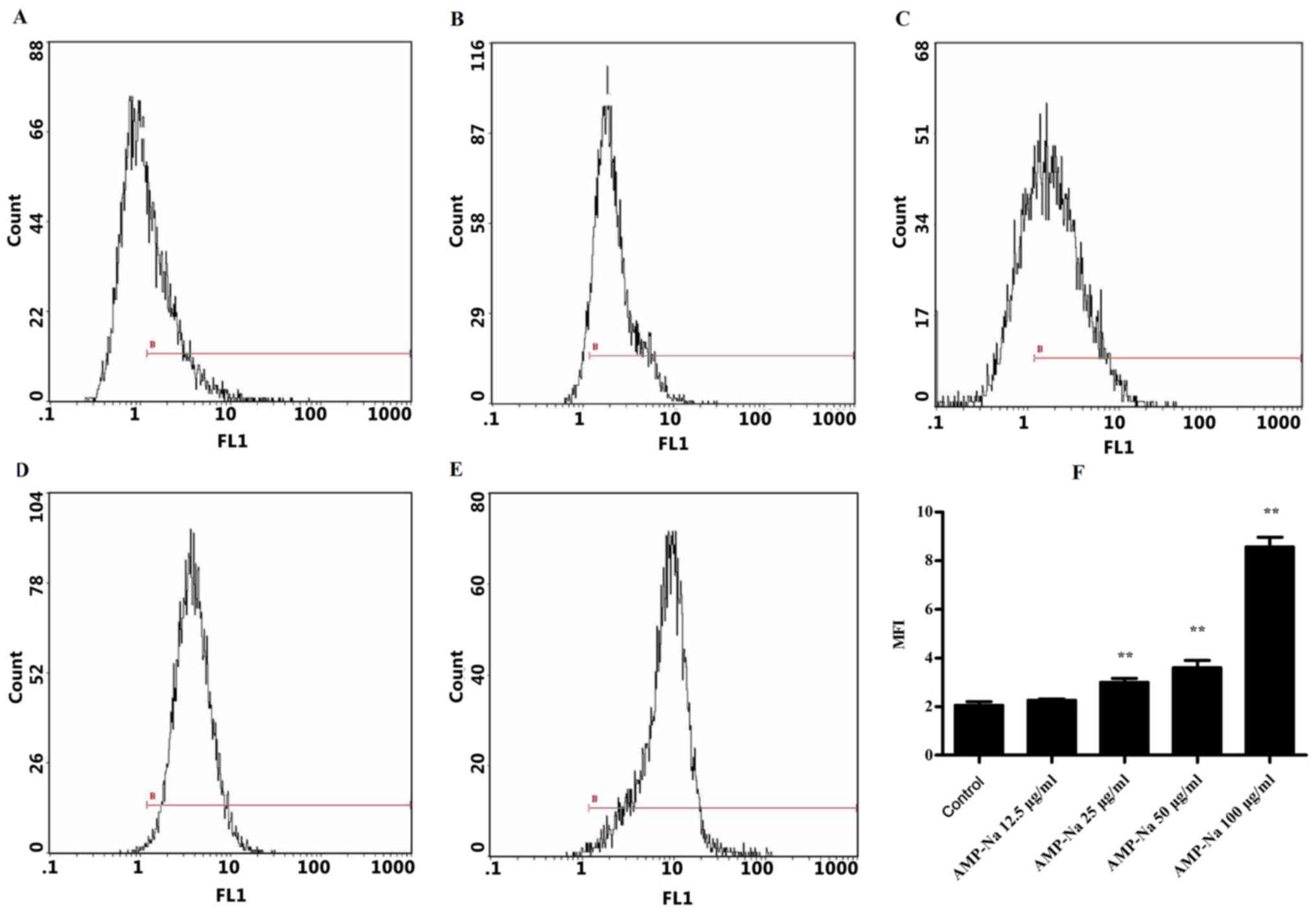
Effect of AMP-Na on microtubulin
immunofluorescence
Flow cytometric analysis was used to assess the
tubulin mean fluorescence intensity (MFI) in the different groups
(Fig. 6). In the control group, the
MFI was 2.08±0.13 following 48-h culture (Fig. 6A and F), whereas in the 12.5 µg/ml
AMP-Na-treated group it was 2.28±0.04 (Fig. 6B and F). No significant difference
was observed in the MFI between these two groups. The tubulin MFI
in cells treated with 25, 50 and 100 µg/ml AMP-Na was 2.96±0.18,
3.62±0.29 and 8.37±0.03, respectively, which was significantly
different compared with that in the control group (P<0.01;
Fig. 6C-F). These results suggested
that AMP-Na may increase the tubulin MFI in a dose-dependent
manner.
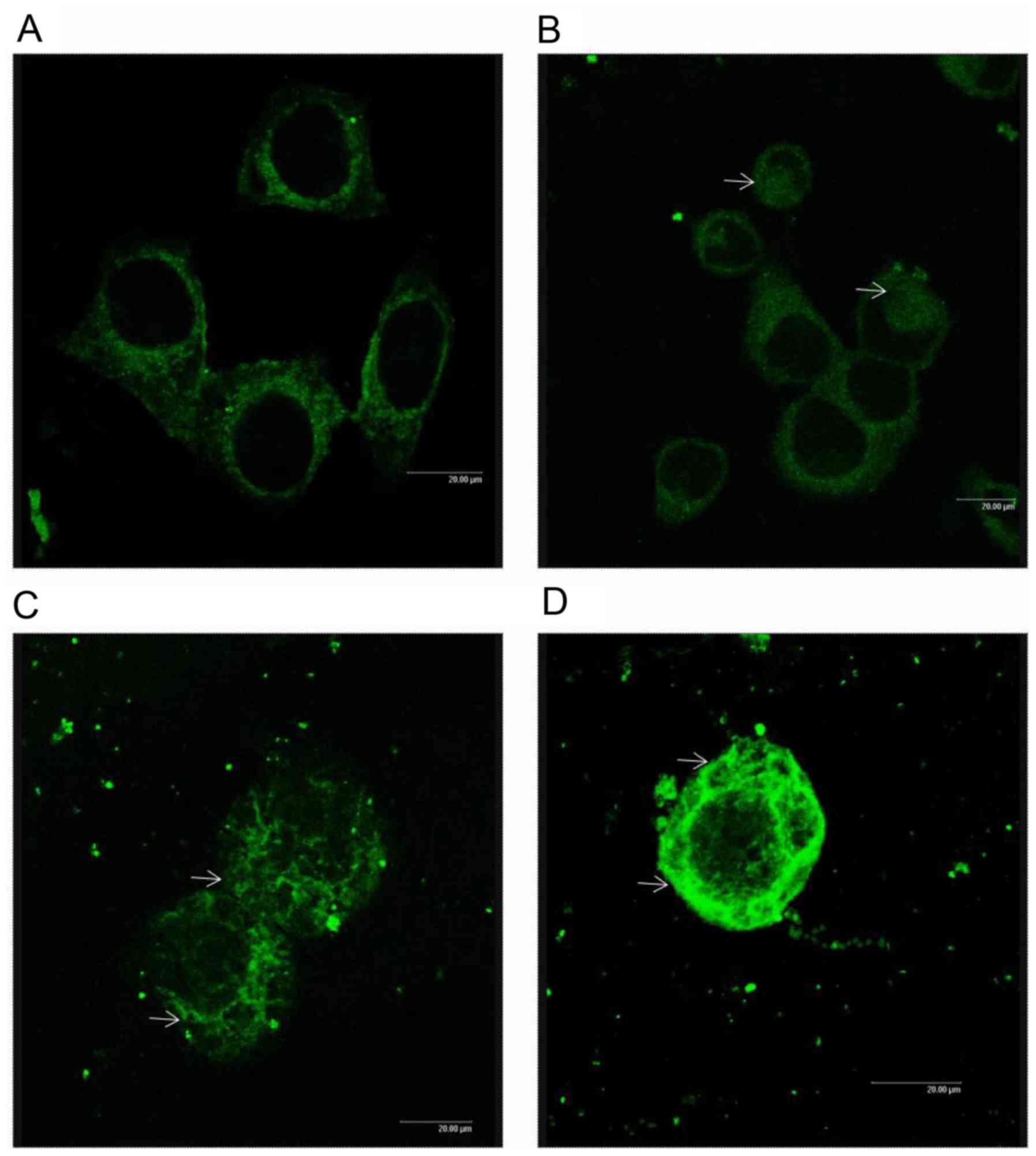
Effect of AMP-Na on tubulin
polymerization in SPC-A-1 cells
Microtubule morphology in cells treated with AMP-Na
was examined by confocal microscopy. Untreated cells exhibited a
normal microtubule network, which radiated from the center to the
cell periphery (Fig. 7A). Treatment
with 25 µg/ml AMP-Na induced a slight tubulin accumulation
(Fig. 7B), whereas treatment with 50
µg/ml AMP-Na caused an abnormal aggregation of tubulin (Fig. 7C). In addition, treatment with 100
µg/ml AMP-Na caused microtubule agglomeration in the cytoplasm
(Fig. 7D).
Discussion
Tumorigenesis is a complex process caused by
dysregulated cell proliferation, cell differentiation and cell
death, which may be caused by various factors, including genetic
alterations and changes in the intracellular environment (16–18). In
the present study, an MTT assay demonstrated that AMP-Na was able
to inhibit the proliferation of lung cancer cell lines in a time-
and dose-dependent manner. Furthermore, treatment with 25 and 50
µg/ml AMP-Na significantly inhibited colony formation by SPC-A-1
and A549 cells. In addition, the wound healing assay demonstrated
that AMP-Na reduced the migratory/metastatic ability of lung cancer
cells. In order to further investigate the anti-tumor effects of
AMP-Na, cell apoptosis was determined using flow cytometry and TEM.
The results of the flow cytometric analysis demonstrated that
following treatment with AMP-Na (100 µg/ml) for 48 h, the SPC-A-1
apoptotic rate was higher compared with that in the control group
(14.71±1.10 vs. 4.31±0.56%; P<0.01). In addition, TEM revealed
morphological characteristics of apoptosis (19,20),
including cytoplasm vacuolization, chromatin margination, nuclear
membrane shrinkage and mitochondrial swelling in AMP-Na-treated
cells.
In the present study, AMP-Na combined with PTX
displayed an antagonistic effect, which suggested that AMP-Na and
PTX may have similar molecular targets. It has been reported that
PTX targets microtubules, and it is known that
microtubule-targeting agents disrupting normal microtubule dynamics
lead to cancer cell apoptosis (21).
Therefore, microtubules represent a crucial target for anti-cancer
therapy (22). Microtubules serve a
crucial role in cell division and in the maintenance of cell shape
(23), particularly in the dynamic
processes of tubulin polymerization and depolymerization during
replication and division (24).
Numerous anti-cancer agents exert their effects through disturbance
of microtubule dynamics, which leads to dysregulation of mitotic
spindles and causes mitotic arrest of cancer cells. For instance,
PTX binds to the microtubule network, stabilizes microtubule
bundles and impairs cellular mitosis in various types of cancer
cell (25,26). By contrast, vinca alkaloids inhibit
microtubule polymerization, which results in mitotic block and
apoptotic cell death (27). The
results of the present study demonstrated that AMP-Na increased
tubulin fluorescence intensity in a dose-dependent manner. Confocal
microscopy revealed that untreated cells exhibited a normal
microtubule network radiating from the center to the cell
periphery, whereas AMP-Na-treated cells exhibited abnormally
distributed microtubules with massive accumulation in the cell
periphery or surrounding the nuclei. The degree of aggregation
increased in a concentration-dependent manner. In the
high-concentration AMP-Na (100 µg/ml) group in particular, the
fluorescence intensity was increased and the extensive aggregation
of tubulin could be seen around the nucleus
A crucial characteristic of traditional anti-cancer
agents is the ability to induce cell cycle arrest (28). Anti-tumor tubulin ligands disturb
microtubule dynamics during the G2/M phase (29,30);
however, due to the mode of interaction between microtubules and
proteins and/or the effectiveness of spindle assembly checkpoints,
cell cycle arrest at G1- or S-phase has also been
reported in various types of cancer cell (31). For instance, Davis et al
(32) revealed that haloacetamido
benzoyl ethyl esters may target tubulin and cause a block of
the G1/S cell cycle transition in cancer cells. In the
present study, AMP-Na induced cell cycle arrest in the S-phase, and
a subsequent decrease in the G1 or G2-phase was observed in SPC-A-1
cells treated with AMP-Na, which suggested that tubulin has
multiple functions and mitosis may not be the specific target for
anti-tubulin agents (33). Early
reports also indicated that microtubule-damaging agents effectively
inhibit cell cycle progression and induce apoptosis without
inducing mitotic arrest (34); the
results of the present study were consistent with these studies.
Tubulin is associated with the downstream apoptotic system, as
tubulin ligands induce bcl-2 phosphorylation by raf-1 kinase, which
results in apoptosis (35). In
addition, the P53-mediated G1/S checkpoint is associated with
S-phase arrest (32).
Tubulin-targeting agents have been previously demonstrated to
arrest the cell cycle in S-phase (32,34,36) and
the S-phase arresting agents have been evaluated for their
potential clinical utility; for example, β-lapachone induced
S-phase arrest (37) and is
currently undergoing phase I clinical trials for the treatment of
solid tumors (https://clinicaltrials.gov; NCT01502800). The present
study suggested that AMP-Na may interfere with the cell cycle by
targeting microtubules, promoting tubulin polymerization and
eventually inducing apoptosis. AMP-Na may have considerable
clinical potential, for example, AMP-Na combined with a
G2/M-phase-arresting drug may enhance the clearance of cancer cells
at different stages of cell cycle.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ and YW conceived and designed the study. LZ and
BZ performed the experiments. LZ and JL analyzed the data. KZ and
SD contributed to the acquisition of data and drafting the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient's consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMP
|
ampelopsin
|
|
CDI
|
coefficient of drug interaction
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hua F, Shang S and Hu ZW: Seeking new
anti-cancer agents from autophagy-regulating natural products. J
Asian Nat Prod Res. 19:305–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nazir HF, AlFutaisi A, Zacharia M,
Elshinawy M, Mevada ST, Alrawas A, Khater D, Jaju D and Wali Y:
Vincristine-induced neuropathy in pediatric patients with acute
lymphoblastic leukemia in Oman: Frequent autonomic and more severe
cranial nerve involvement. Pediatr Blood Cancer. 64:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khongkow M, Olmos Y, Gong C, Gomes AR,
Monteiro LJ, Yague E, Cavaco TB, Khongkow P, Man EP, Laohasinnarong
S, et al: SIRT6 modulates paclitaxel and epirubicin resistance and
survival in breast cancer. Carcinogenesis. 34:1476–1486. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XM, Xie XB, Zhao Q, Wang F, Bai Y,
Yin JQ, Jiang H, Xie XL, Jia Q and Huang G: Ampelopsin induces
apoptosis by regulating multiple c-Myc/S-phase kinase-associated
protein 2/F-box and WD repeat-containing protein 7/histone
deacetylase 2 pathways in human lung adenocarcinoma cells. Mol Med
Rep. 11:105–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng HQ and Liu DY: Anti-invasive and
anti-metastatic effect of ampelopsin on melanoma. Ai Zheng.
22:363–367. 2003.(In Chinese). PubMed/NCBI
|
|
8
|
Qi S, Kou X, Lv J, Qi Z and Yan L:
Ampelopsin induces apoptosis in HepG2 human hepatoma cell line
through extrinsic and intrinsic pathways: Involvement of P38 and
ERK. Environ Toxicol Pharmacol. 40:847–854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni F, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Flavonoid ampelopsin inhibits the growth and metastasis of
prostate cancer in vitro and in mice. PLoS One. 7:e388022012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu T, Liu P, Ding F, Yu N, Li S, Wang S,
Zhang X, Sun X, Chen Y, Wang F, et al: Ampelopsin reduces the
migration and invasion of ovarian cancer cells via inhibition of
epithelial-to-mesenchymal transition. Oncol Rep. 33:861–867. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruan LP, Yu BY, Fu GM and Zhu DN:
Improving the solubility of ampelopsin by solid dispersions and
inclusion complexes. J Pharm Biomed Anal. 38:457–464. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Dong S, Cen X, Wang X, Liu X,
Zhang H, Zhao X and Wu Y: Ampelopsin sodium exhibits antitumor
effects against bladder carcinoma in orthotopic xenograft models.
Anticancer Drugs. 23:590–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin H: Study on preparation of
high-water-soluble ampelopsin sodium and its anti-tumor effects
(unpublished PhD thesis). Lanzhou University; 2006
|
|
14
|
Tada H, Shiho O, Kuroshima K, Koyama M and
Tsukamoto K: An improved colorimetic assay for interleukin 2. J
Immunol Methods. 93:157–165. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao SS and Zhen YS: Potentiation of
antimetabolite antitumor activity in vivo by dipyridamole and
amphotericin B. Cancer Chemother Pharmacol. 24:181–186. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez V, Hartmann E, Ott G, Campo E
and Rosenwald A: Pathogenesis of mantle-cell lymphoma: All
oncogenic roads lead to dysregulation of cell cycle and DNA damage
response pathways. J Clin Oncol. 23:6364–6369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bisbis B, Delmas F, Joubès J, Sicard A,
Hernould M, Inzé D, Mouras A and Chevalier C: Cyclin-dependent
kinase (CDK) inhibitors regulate the CDK-cyclin complex activities
in endoreduplicating cells of developing tomato fruit. J Biol Chem.
281:7374–7383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ford HL and Pardee AB: Cancer and the cell
cycle. J Cell Biochem 32–33. (Suppl):166–172. 1999. View Article : Google Scholar
|
|
19
|
Nagasaka A, Kawane K, Yoshida H and Nagata
S: Apaf-1-independent programmed cell death in mouse development.
Cell Death Differ. 17:931–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burgess DJ: Apoptosis: Refined and lethal.
Nat Rev Cancer. 13:792013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Annamalai P, Thayman M, Rajan S, Raman LS,
Ramasubbu S and Perumal P: Ethyl acetate extract from marine sponge
Hyattella cribriformis exhibit potent anticancer activity by
promoting tubulin polymerization as evidenced mitotic arrest and
induction of apoptosis. Pharmacogn Mag. 11:345–355. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marko D, Kemény M, Bernady E, Habermeyer
M, Weyand U, Meiers S, Frank O and Hofmann T: Studies on the
inhibition of tumor cell growth and microtubule assembly by
3-hydroxy-4-[(E)-(2-furyl)methylidene]methyl-3-cyclopentene-1,2-dione,
an intensively coloured Maillard reaction product. Food Chem
Toxicol. 40:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong C, Fan HY, Chen DY, Song XF, Schatten
H and Sun QY: Effects of MEK inhibitor U0126 on meiotic progression
in mouse oocytes: Microtuble organization, asymmetric division and
metaphase II arrest. Cell Res. 13:375–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson L and Jordan MA: Microtubule
dynamics-taking aim at a moving target. Chem Biol. 2:569–573. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maushagen R, Reers S, Pfannerstill AC,
Hahlbrock A, Stauber R, Rahmanzadeh R, Rades D, Pries R and
Wollenberg B: Effects of paclitaxel on permanent head and neck
squamous cell carcinoma cell lines and identification of
anti-apoptotic caspase 9b. J Cancer Res Clin Oncol. 142:1261–1271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo Y, Ji X, Liu J, Li Z, Wang W, Chen W,
Wang J, Liu Q and Zhang X: Moderate intensity static magnetic
fields affect mitotic spindles and increase the antitumor efficacy
of 5-FU and taxol. Bioelectrochemistry. 109:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiu WH, Luo SJ, Chen CL, Cheng JH, Hsieh
CY, Wang CY, Huang WC, Su WC and Lin CF: Vinca alkaloids cause
aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA
damage, mitochondrial dysfunction, and apoptosis in lung
adenocarcinoma cells. Biochem Pharmacol. 83:1159–1171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun B, Wingate H, Swisher SG, Keyomarsi K
and Hunt KK: Absence of pRb facilitates E2F1-induced apoptosis in
breast cancer cells. Cell Cycle. 9:1122–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gamell C, Schofield AV, Suryadinata R,
Sarcevic B and Bernard O: LIMK2 mediates resistance to
chemotherapeutic drugs in neuroblastoma cells through regulation of
drug-induced cell cycle arrest. PLoS One. 8:e728502013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Tanaka M, Krstin S, Peixoto HS and
Wink M: The interference of selected cytotoxic alkaloids with the
cytoskeleton: An insight into their modes of action. Molecules.
21:E9062016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giannakakou P, Sackett D and Fojo T:
Tubulin/microtubules: Still a promising target for new
chemotherapeutic agents. J Natl Cancer Inst. 92:182–183. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davis A, Jiang JD, Middleton KM, Wang Y,
Weisz I, Ling YH and Bekesi JG: Novel suicide ligands of tubulin
arrest cancer cells in S-phase. Neoplasia. 1:498–507. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Komlodi-Pasztor E, Sackett D, Wilkerson J
and Fojo T: Mitosis is not a key target of microtuble agents in
patient tumors. Nat Rev Clin Oncol. 8:244–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheriyamundath S, Mahaddalkar T, Kantevari
S and Lopus M: Induction of acetylation and bundling of cellular
microtubules by 9-(4-vinylphenyl) noscapine elicits S-phase arrest
in MDA-MB-231 cells. Biomed Pharmacotherapy. 86:74–80. 2017.
View Article : Google Scholar
|
|
35
|
Blaqosklonny MV, Ginanakakou P, el-Deiry
WS, Kingston DG, Higgs PI, Neckers L and Foji T: Raf-1/bcl-2
phosphorylatin: A step from microtuble damage to cell death. Cancer
Res. 57:130–135. 1997.PubMed/NCBI
|
|
36
|
Mahaddalkar T, Mehta S, Cheriyamundath S,
Muthurajan H and Lopus M: Tryptone-stabilized gold nanoparticles
target tubulin and inhibit cell viability by inducing an unusual
form of cell cycle arrest. Exp Cell Res. 360:163–170. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Sun X, LaMont JT, Pardee AB and Li
CJ: Selective killing of cancer cells by β-lapachone: Direct
checkpoint activation as a strategy against cancer. PNAS.
100:2674–2678. 2003. View Article : Google Scholar : PubMed/NCBI
|