Introduction
Globally, gastric cancer (GC) is the third and fifth
most common cause of cancer-associated mortality in men and women,
respectively (1). Patients with GC
usually exhibit no subjective symptoms in early stages, and the
majority of cases are diagnosed only at advanced stages.
Chemotherapy is the first-line treatment for patients with advanced
GC, and platinum-based drugs are the most commonly used (2,3).
However, primary and secondary drug resistance often lead to
treatment failure and reduced survival rates. It has been reported
that alterations in the DNA repair mechanism cause platinum
resistance (4). The nucleotide
excision repair (NER) pathway serves an important role in this
process (5), and miRNAs can regulate
this pathway (6,7). miRNAs are a class of non-coding, short,
single-stranded regulatory RNAs. They do not encode proteins, but
they can bind to the 3′-untranslated region (3′-UTR) of target
mRNAs in a complementary manner and inhibit translation (8). Abnormal miRNAs expression is associated
with drug resistance in numerous types of tumor. For example, in
cisplatin-resistant SKOV3/DDP and A2780/DDP ovarian cancer cell
lines, miR-152 and miR-185 can inhibit cell proliferation and
promote apoptosis by inhibiting DNA methyltransferase 1, and thus,
reverse platinum resistance (9).
miR-508-5p can decrease ATP binding cassette subfamily B member 1
expression by inhibiting zinc ribbon domain-containing 1 to reverse
multi-drug resistance of GC (10).
In addition, miR-17-5p can render colon cancer cells insensitive to
platinum-based treatment by inhibiting the expression of phosphate
and tensin homolog (PTEN) (11).
It has previously been reported that
miR-200c-3p-induced reversal of drug resistance and inhibition of
proliferation in SGC7901/DDP GC cells is associated with the
induction of E-cadherin, PTEN and B-cell lymphoma-2 (Bcl-2)
associated X protein (BAX) protein expression, as well as the
inhibition of protein kinase B (Akt) pathway activation and
downregulation of Bcl-2 protein expression (12). Additionally, miRNAs are associated
with DNA repair. Notably, miR-890 can inhibit the expression of
mitotic arrest deficient 2 like 2, WEE1 G2 checkpoint kinase and
XPC complex subunit, DNA damage recognition and repair factor, to
delay DNA repair caused by ionizing radiation (IR), and as a
result, prostate cancer cells exhibit increased sensitivity to IR
(13). miR-145 can inhibit the
activity of RAD18 E3 ubiquitin protein ligase to restrain DNA
repair and reverse 5-flurouracil resistance of colon cancer cells
(14). A previous study demonstrated
that miR-192 in HepG2.2.15 liver cancer cells can inhibit the NER
pathway through targeted regulation of ERCC excision repair 3,
TFIIH core complex helicase subunit (ERCC3) and ERCC excision
repair 4, endonuclease catalytic subunit (ERCC4) (7), but whether high miR-200c-3p expression
in GC serves a role in the NER pathway is unknown. Therefore, the
present study investigated the difference between SGC7901/DDP and
SGC7901 cell lines with respect to NER ability, and measured
miR-200c-3p expression. The present study revealed that
upregulation or downregulation of miR-200c-3p expression can
regulate the expression levels of key proteins in the NER pathway,
including ERCC3 and ERCC4.
Materials and methods
Cell culture and lentiviral
transduction
Human SGC7901/DDP and SGC7901 gastric adenocarcinoma
cell lines were purchased from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). Cells were grown in RPMI-1640 medium supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100 U/ml
streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified atmosphere with 5%
CO2. To maintain the phenotype of cisplatin resistance,
SGC7901/DDP cells were cultured in medium supplemented with 800
ng/ml cisplatin (Qilu Pharmaceutical Co., Ltd. Jinan, China).
Lentiviral vectors carrying miR-200c-3p (pLV-miR-200c-3p), negative
control (pLV-miR-200c-3p-NC) and the corresponding viruses
[1×108 plaque forming units (PFU)] were all obtained
from GeneCopoeia (Guangzhou, China). The lentiviruses were
transfected into 293T cells for packaging, which was completed by
GeneCopoeia prior to infecting GC cells. Lentiviral transduction to
induce overexpression or knockdown of miR-200c-3p was performed
according to the manufacturer's protocols. Specifically, cells were
seeded in 12-well plates overnight to reach a density of
1×105 cells/well. Subsequently, cells were transduced at
lentiviral multiplicity of infection 10 PFU/cell. After 72 h of
transduction, SGC7901/DDP cells were cultured in medium
supplemented with 2 µg/ml puromycin for 3 days. Similarly,
lentiviral vector bearing a RNA interference sequence
(5′-UCCAUCAUUACCCGGCAGUAUUA-3′) against miR-200c-3p was used to
knockdown miR-200c-3p expression in SGC7901 cells. Following
transduction, the cells were cultured in medium supplemented with
150 µg/ml puromycin for 5 days. Cell clones that survived the
selection were used in subsequent experiments. The lentiviral
vectors included green fluorescent protein or red fluorescent
protein markers, which were used to monitor transduction efficiency
under a fluorescence microscope. In summary,
SGC7901/DDP-LV-NC-overexpression (NC-OE) cells and
SGC7901/DDP-LV-miR-200c-3p-OE (miR-200c-3p-OE) cells were developed
from SGC7901/DDP cells, whereas SGC7901-LV-miR-200c-3p-knockdown
(miR-200c-3p-KD) cells and SGC7901-LV-NC-KD (NC-KD) cells were
generated from SGC7901 cells.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from GC cell lines using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA
concentration was evaluated at an absorbance of 260 nm using a
NanoDrop-2000 spectrophotometer (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). RT-qPCR was performed on an
Mx3000P qPCR system (Agilent Technologies, Inc., Santa Clara, CA,
USA) with a SYBR Green single-step RT-qPCR kit (Biomics
Biotechnologies Co., Ltd., Nantong, China) according to the
manufacturer's protocols. U6 small nuclear RNA was selected as an
endogenous control. The 25-µl RT-qPCR reaction system included 2 µl
total RNA extract, 2X master mix, miR-200c-specific primers and
U6-specific primers. PCR was performed under the following
conditions: RT at 42°C for 30 min; 95°C denaturation for 10 min; 40
cycles of 95°C denaturation for 20 sec, 60°C annealing for 30 sec
and 72°C extension for 30 sec; subsequently, fluorescent signals
were collected at 72°C. The primer sequences were as follows:
miR-200c, forward 5′-GGCGTAATACTGCCGGGTA-3′, reverse
5′-ATTGCGTGTCGTGGAGTCG-3′; and U6, forward 5′-CTCGCTTCGGCAGCACA-3′
and reverse 5′-AACGCTTCACGAATTTGCGT-3′. Data were analyzed using
the following equation: ΔΔCq=Experimental
(CqmiR-200-3p-Cqu6)-control
(CqmiR-200-3p-Cqu6), and the relative
expression levels of miR-200c-3p were calculated using the
2−ΔΔCq method (15). All
experiments were performed in triplicate.
In vitro drug sensitivity test
SGC7901/DDP, SGC7901/DDP-LV-miR-200c-3p-OE,
SGC7901/DDP-LV-NC-OE, SGC7901, SGC7901-LV-miR-200c-3p-KD and
SGC7901-LV-NC-KD cells were suspended at a density of
1×105 cells/ml and seeded in 96-well plates, with each
well containing 100 µl RPMI-1640 medium. After 24 h, freshly
prepared cisplatin was added to SGC7901/DDP,
SGC7901/DDP-LV-miR-200c-3p-OE and SGC7901/DDP-LV-NCscells at
concentrations of 40, 20, 2, 0.2, 0.02 and 0.002 µg/ml; cisplatin
was added to SGC7901, SGC7901-LV-miR-200c-3p-KD and
SGC7901-LV-NC-KD at concentrations of 4, 2, 0.2, 0.02, 0.002 and
0.0002 µg/ml. A total of 48 h after the addition of cisplatin,
original medium was replaced with 180 µl fresh medium, and 20 µl
MTT (5 mg/ml) was added to each well. Cells were then cultured for
4 h at 37°C in a humidified atmosphere with 5% CO2,
after which, the supernatant in the well was discarded.
Subsequently, 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to each well to dissolve crystals at
72°C for 10 min. Absorbance (490 nm) was measured using a
spectrophotometer. The half maximal inhibitory concentration
(IC50) of cisplatin was calculated according to relative
cell survival. Each experiment was performed in triplicate.
Western blotting
Total protein was extracted from cells using
radioimmunoprecipitation assay (Beyotime Institute of
Biotechnology, Shanghai, China) lysis buffer containing protease
inhibitor. Protein concentration was assessed using a Bicinchoninic
Acid Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The primary antibodies against ERCC3 (1:2,000 dilution; cat.
no. ab27317) and ERCC4 (1:1,000 dilution; cat. no. ab76948) (both
from Abcam, Cambridge, UK) used were rabbit anti-human polyclonal
antibodies. β-actin served as a loading control. Equal amounts of
proteins (80 µg) per group were loaded and separated by 10 or 8%
SDS-PAGE, transferred onto polyvinylidene fluoride membranes, and
blocked with 5% non-fat milk for 1.5 h at room temperature.
Membranes were incubated with the ERCC3, ERCC4 or β-actin (1:1,000
dilution; cat. no. ab8227; Abcam) primary antibodies overnight at
4°C. Membranes were washed three times with TBS-0.1% Tween (TBST;
10 min/wash) and then incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000 dilution; cat.
no. 7074S; Shanghai Youningwei Biotechnology Co. Ltd., Shanghai,
China) overnight at 4°C. Membranes were washed a further three
times with TBST and signal detection was performed using a Super
Enhanced Chemiluminescence Plus Detection reagent (Thermo Fisher
Scientific, Inc.). Protein expression levels were normalized to
β-actin, and the fold changes were calculated by densitometry
(Tanon 2500 Fully Automatic Digital Gel Image Analysis system;
Tanon Science and Technology Co., Ltd., Shanghai, China).
Statistical analysis
SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used for data processing. All experiments were performed
in triplicate, and all data are expressed as the means ± standard
deviation. A Student's t-test was used to compare differences
between two groups and one-way analysis of variance with a
Student-Newman-Keuls post hoc test was used to compare differences
among three or more groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-200c-3p, ERCC3 and
ERCC4 in GC cell lines
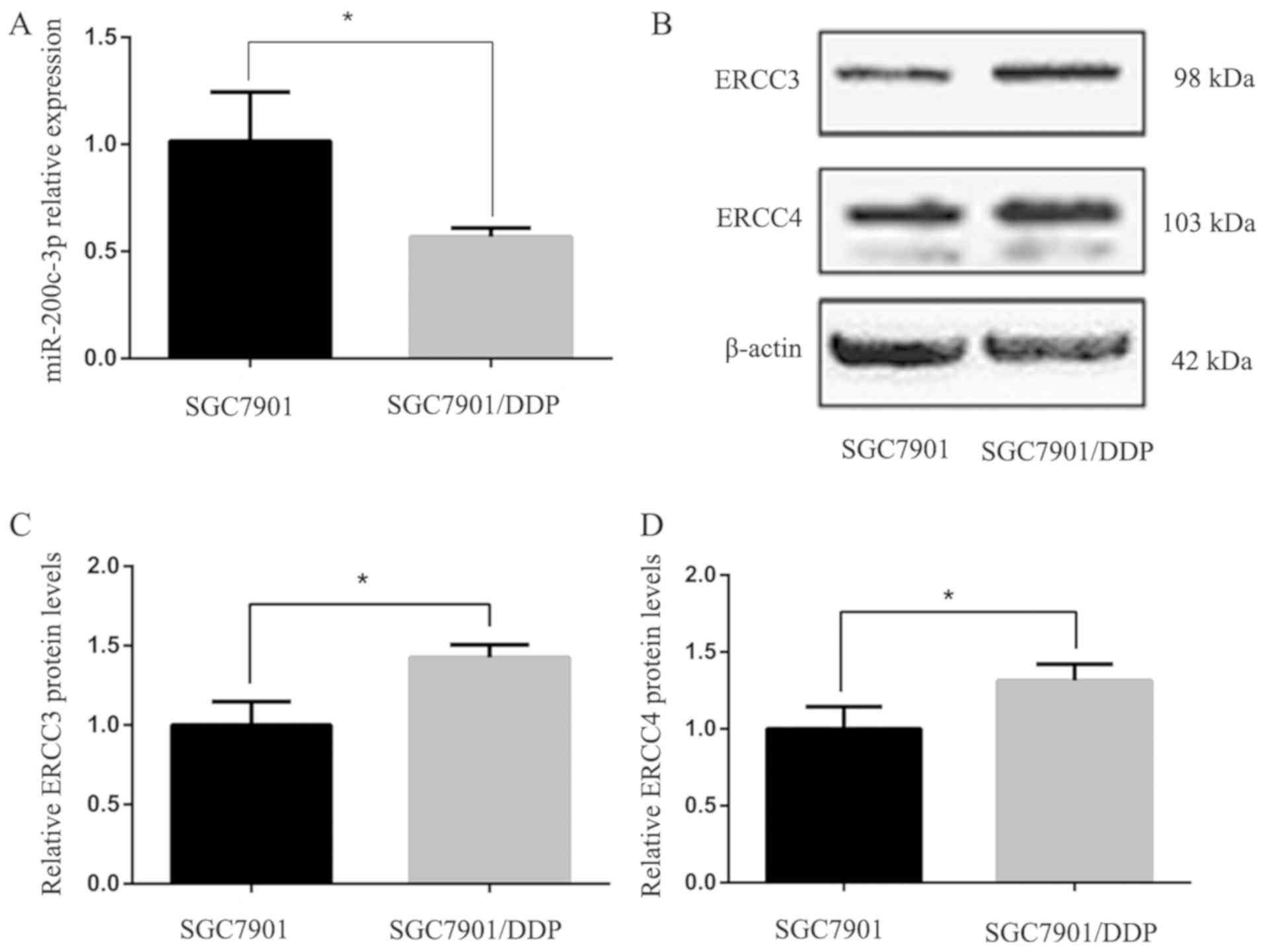
The expression levels of miR-200c-3p in
drug-resistant SGC-7901/DDP cells were significantly lower than in
the parent cell line SGC-7901 (P<0.05; Fig. 1A). Western blot analysis of ERCC3 and
ERCC4 protein expression in SGC-7901 and SGC-7901/DDP cells
indicated that ERCC3 and ERCC4 protein expression was significantly
higher in SGC7901/DDP cells than in SGC7901 cells (P<0.05;
Fig. 1B-D).
Modifying miR-200c-3p expression
levels with lentiviral vectors
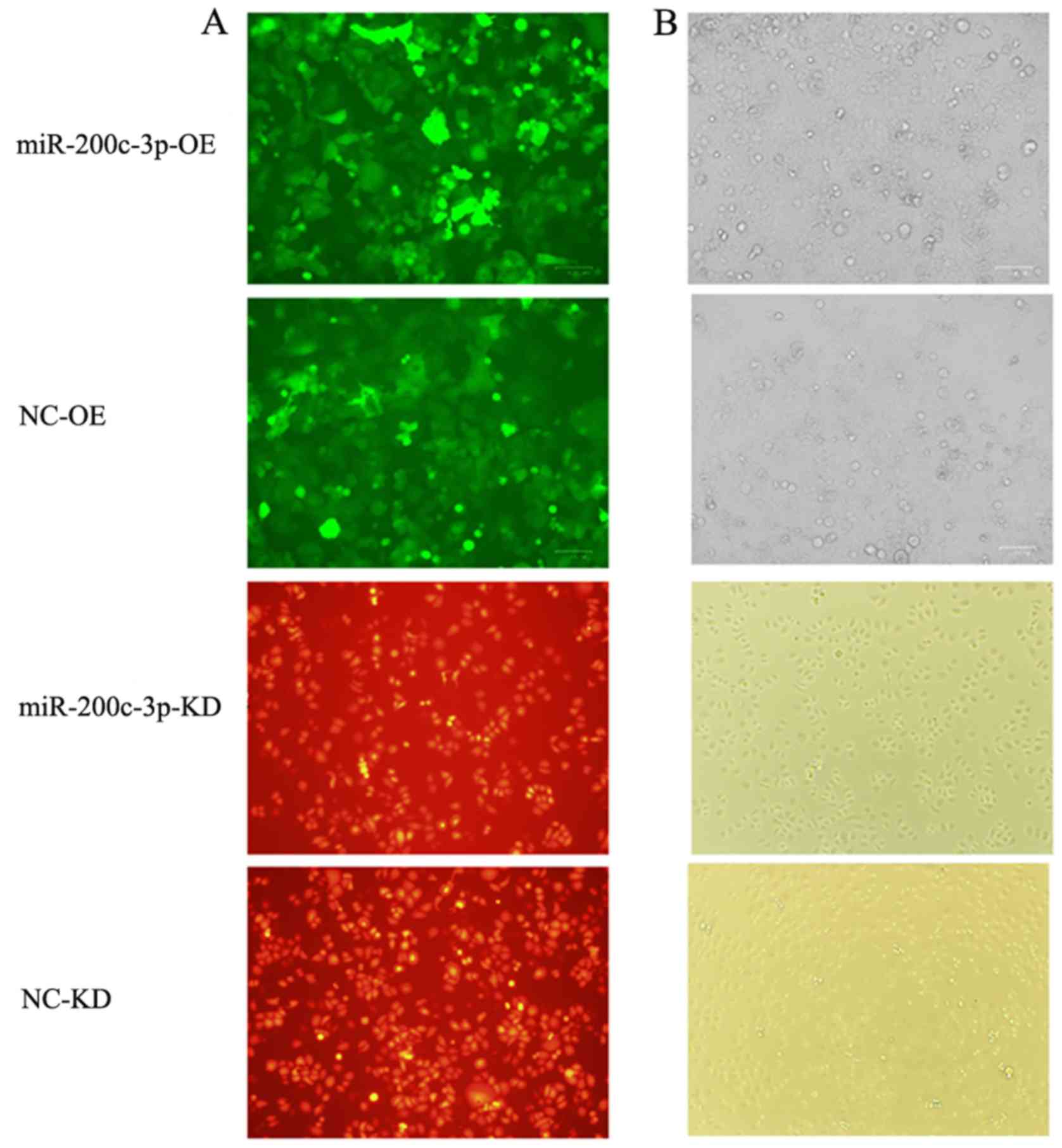
Fluorescence microscopy confirmed that
SGC7901/DDP-LV-miR-200c-3p-OE cells with upregulated miR-200c-3p
and SGC7901/DDP-LV-NC-OE cells transduced with an empty vector
exhibited green fluorescence, whereas SGC7901-LV-miR-200c-3p-KD
cells with downregulated miR-200c-3p and SGC7901-LV-NC-KD cells
transduced with an empty vector exhibited red fluorescence
(Fig. 2), suggesting that vectors
carrying the target gene were successfully transduced.
RT-qPCR confirmed that the expression levels of
miR-200c-3p were significantly higher in
SGC7901/DDP-LV-miR-200c-3p-OE cells compared with in SGC-7901/DDP
and SGC7901/DDP-LV-NC-OE cells (P<0.0001; Fig. 3A). Following lentiviral transduction,
miR-200c-3p was significantly downregulated in
SGC7901/DDP-LV-miR-200c-3p-KD cells compared with in SGC7901 and
SGC7901-LV-NC-KD cells (P<0.0001; Fig. 3B). Therefore, the expression levels
of the target gene were altered in cells following lentiviral
transduction.
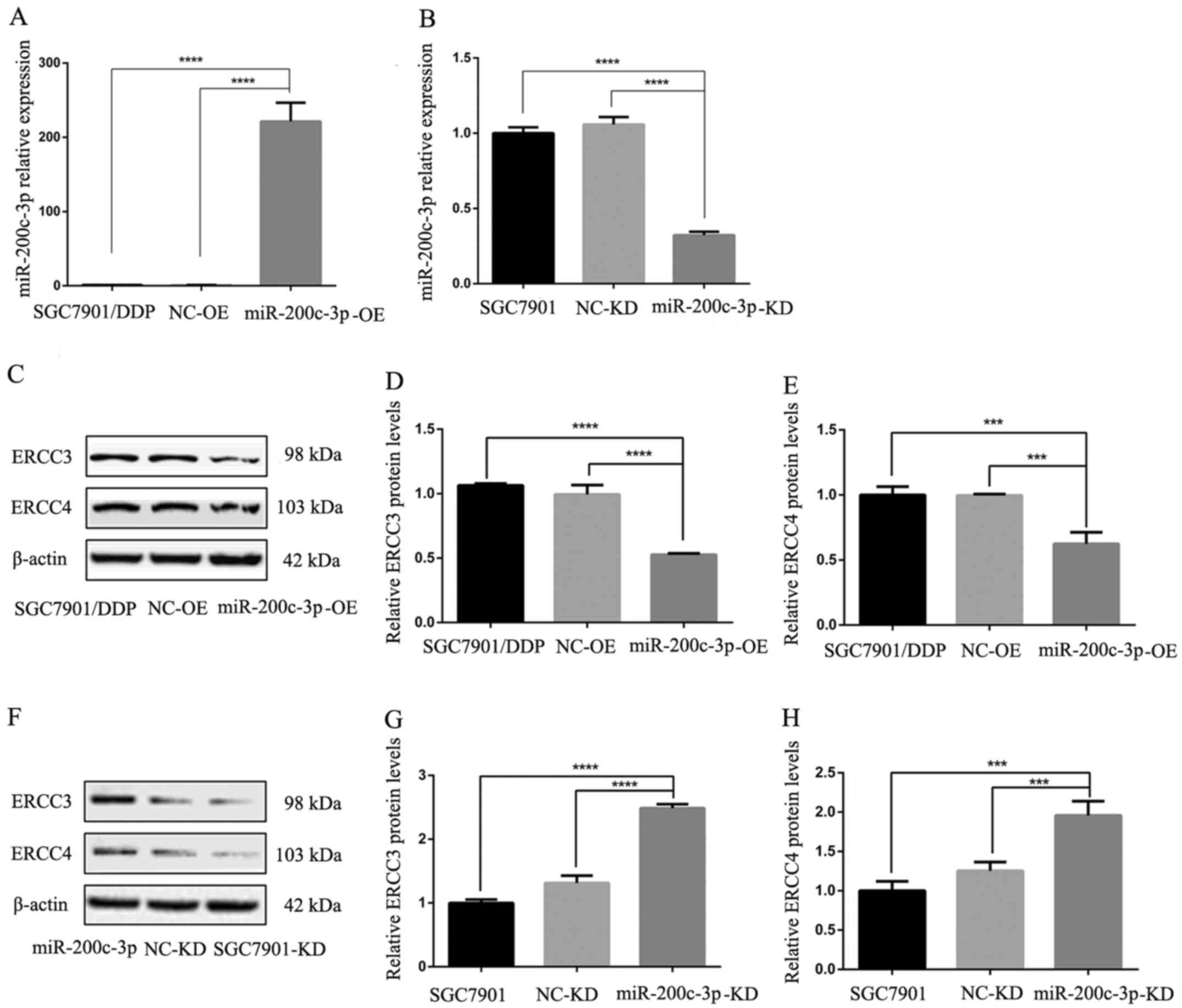 | Figure 3.Reverse transcription-quantitative
polymerase chain reaction revealed the expression levels of
miR-200c-3p, ERCC3 and ERCC4 in cells following lentiviral
transduction. ERCC3 and ERCC4 protein expression in gastric cancer
cells was detected by western blot analysis. (A) Upregulation of
miR-200c-3p in SGC7901/DDP-LV-miR-200c-3p-OE cells compared with in
SGC7901/DDP and SGC7901/DDP-LV-NC-OE cells. (B) Downregulation of
miR-200c-3p in SGC7901-LV-miR-200c-3p cells compared with in
SGC7901 and SGC7901-LV-NC-KD cells. (C) Western blot analysis
detected lower ERCC3 and ERCC4 expression in
SGC7901/DDP-LV-miR-200c-3p-OE cells compared with in SGC7901/DDP
and SGC7901/DDP-LV-NC-OE cells. (D) Relative expression levels of
ERCC3 in each cell type. (E) Relative expression levels of ERCC4 in
each cell type. (F) Western blot analysis detected higher ERCC3 and
ERCC4 expression in SGC7901-LV-miR-200c-3p-KD cells compared with
in SGC7901 and SGC7901-LV-NC-KD cells. (G) Relative expression
levels of ERCC3 in each cell type. (H) Relative expression levels
of ERCC4 in each cell type. ***P<0.001, ****P<0.0001. ERCC3,
ERCC excision repair 3, TFIIH core complex helicase subunit; ERCC4,
ERCC excision repair 4, endonuclease catalytic subunit; KD,
knockdown; LV, lentivirus; miR-200c-3p, microRNA-200c-3p; NC,
negative control; OE, overexpression. |
ERCC3 and ERCC4 expression
post-transduction
Following lentiviral infection of the
cisplatin-resistant SGC7901/DDP GC cell line, ERCC3 and ERCC4
expression levels in SGC7901/DDP-LV-miR-200c-3p-OE cells were
significantly lower than in SGC7901/DDP and SGC7901/DDP-LV-NC-OE
cells (P<0.0001 and P<0.001, respectively; Fig. 3C-E). These findings indicated that
ERCC3 and ERCC4 expression were decreased following upregulation of
miR-200c-3p. Following lentiviral infection of SGC7901 GC cells,
the expression levels of ERCC3 and ERCC4 were significantly
increased in SGC7901-LV-miR-200c-3p-KD cells compared with in
SGC7901 and SGC7901-LV-NC-KD cells (P<0.0001 and P<0.001,
respectively; Fig. 3F-H). These
results indicated that the expression levels of ERCC3 and ERCC4
were increased following miR-200c-3p knockdown; therefore,
miR-200c-3p may inhibit ERCC3 and ERCC4 protein expression.
Alterations in cisplatin
resistance
Cisplatin resistance was evaluated by calculating
the IC50 value of DDP relative to untreated cells
(Fig. 4).
SGC7901/DDP-LV-miR-200c-3p-OE cells exhibited decreased cisplatin
resistance compared with SGC7901/DDP and SGC7901/DDP-LV-NC-OE
cells, and the differences were statistically significant
(P<0.0001). These findings indicated that drug resistance was
decreased following upregulation of miR-200c-3p. The
IC50 for SGC7901-LV-miR-200c-3p-KD cells was greater
than in SGC7901 and SGC7901-LV-NC-KD cells, and the differences
were statistically significant (P<0.0001). These findings
indicated that cisplatin resistance was increased following
downregulation of miR-200c-3p. Therefore, upregulation of
miR-200c-3p may reverse cisplatin resistance of SGC7901/DDP cells
by inhibiting expression of the target proteins ERCC3 and ERCC4 in
the NER pathway.
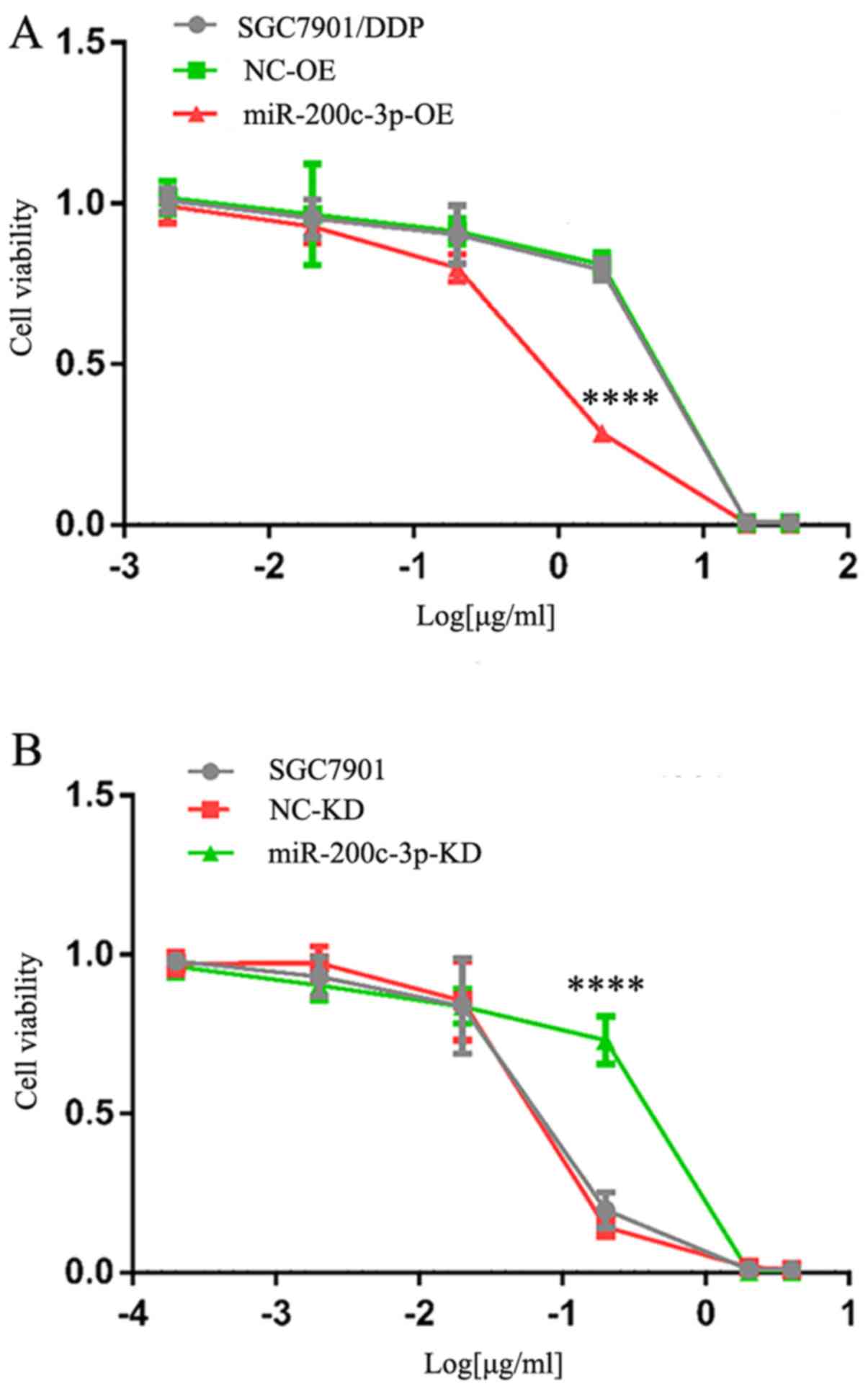 | Figure 4.Drug resistance of SGC7901/DDP cells
following upregulation of miR-200c-3p and 48 h cisplatin treatment
was determined using an MTT assay. (A) Cells were treated with 40,
20, 2, 0.2, 0.02 and 0.002 µg/ml cisplatin. IC50 of
cisplatin was 7.63±0.25, 7.25±0.23 and 1.56±0.12 µg/ml in
SGC7901/DDP, SGC7901/DDP-LV-NC-OE and SGC7901/DDP-LV-miR-200c-3p-OE
cells. ****P<0.0001 vs. SGC7901/DDP and SGC7901/DDP-LV-NC-OE
cells. (B) Cells were treated with 4, 2, 0.2, 0.02, 0.002 and
0.0002 µg/ml cisplatin. IC50 of cisplatin was 0.21±0.01,
0.20±0.003 and 0.59±0.04 µg/ml in SGC7901, SGC7901-LV-NC-KD and
SGC7901-LV-miR-200c-3p-KD cells. ****P<0.0001 vs. SGC7901 and
SGC7901-LV-NC-KD cells. IC50, half-maximal inhibitory
concentration; KD, knockdown; LV, lentivirus; miR, microRNA; NC,
negative control; OE, overexpression. |
Discussion
miR-200c is a member of the miR-200 family, which is
located on chromosome 12 (12p13.31). It has previously been
reported that miR-200c is abnormally expressed in tumor tissues,
and the majority of studies have suggested that low miR-200c is
associated with poor prognosis (16–18). It
is likely that miR-200c can inhibit the metastatic and invasive
potential of tumors by inhibiting epithelial-mesenchymal transition
(EMT) (19,20). Along with its important functions in
EMT, tumor invasion and metastasis, its role in tumor drug
resistance has been explored. Studies (21–23)
describing multiple solid tumors have demonstrated that tubulin β3
(TUBB3) is involved in the regulation of sensitivity to
microtubule-targeting chemotherapeutic drugs (e.g. paclitaxel and
vincristine). miR-200c can directly target the 3′-UTR of TUBB3 mRNA
to inhibit expression of TUBB3 at the post-transcriptional level
(24), and thus increase the
sensitivity of tumor cells to drugs, including paclitaxel and
vincristine. Zhou et al (25)
reported that miR-200c inhibited migration and invasion to reverses
trastuzumab resistance by targeting zinc finger E-box binding
homeobox (ZEB)1 and ZEB2. Several studies have indicated that
miR-200c is associated with cisplatin resistance. The transcription
factor AP-2α (AP-2) gene can increase sensitivity to cisplatin
chemotherapy by promoting apoptosis. In the endometrium,
miR-200b/200c/429 can bind to the 3′-UTR of the AP-2 gene, block
protein expression and enhance cisplatin resistance (26). In esophageal cancer, miR-200c can
inhibit expression of protein phosphatase 2 scaffold subunit Ab and
promote activation of the downstream Akt pathway, which leads to
cisplatin resistance (27). Chen
et al (12) reported that
drug resistance reversal and inhibition of proliferation by
miR-200c in SGC7901/DDP cells is associated with induction of
E-cadherin, PTEN and BAX protein expression, inhibition of Akt
pathway activation, and downregulation of Bcl-2 protein expression.
In addition, it has been reported that miR-200c can enhance the
sensitivity of GC to cisplatin through direct targeted regulation
of ZEB2 (28). Furthermore, Chang
et al (29) demonstrated that
miR-200c directly targets Rho family GTPase 3 (RhoE), and
downgraded RhoE expression may enhance the sensitivity of
SGC7901/DDP cells. Notably, these results suggested that miR-200c
may serve a dual role in tumor chemosensitivity, which may be
associated with the complex mechanisms of miR-200c. However, it has
not been established whether miR-200c can regulate the NER pathway.
Our previous study (30)
demonstrated that miR-200c-3p is upregulated in tissues and plasma
samples of patients with advanced GC, and is associated with
improved efficacy of platinum-based chemotherapy and prognosis of
patients. In order to further explore the mechanism by which
miR-200c-3p promotes the sensitivity of platinum-containing
chemotherapy in GC, the present study was conducted.
The present study revealed that miR-200c-3p was
significantly downregulated in a drug-resistant SGC7901/DDP GC cell
line compared with in the SGC7901 cell line, and that ERCC3 and
ERCC4 in the NER pathway may be two targets of miR-200c-3p.
Upregulation of miR-200c-3p decreased ERCC3 and ERCC4 expression in
a SGC7901/DDP cell line and inhibited the NER pathway, decreasing
cisplatin resistance. Downregulation of miR-200c-3p increased ERCC3
and ERCC4 expression in a SGC7901 GC cell line and may enhance the
NER pathway, enhancing cisplatin resistance. These results
suggested that upregulation of miR-200c-3p may inhibit ERCC3 and
ERCC4 protein expression to interfere with the NER pathway in the
SGC7901/DDP cell line, in order to reverse cisplatin resistance.
ERCC3 and ERCC4 are important proteins in the NER pathway, and
deletion or mutation of either can modify their activities
(31–34). ERCC3 is located on human autosome
2q21 and encodes a protein with DNA helicase activity (35). Relying on the function of DNA ATPase
and helicase, it can repair structure-distorting DNA damage and DNA
around the RNAP II transcription promoter; therefore, it is an
essential gene in the NER pathway, and in gene transcription
generally. ERCC4 and ERCC excision repair 1, endonuclease
non-catalytic subunit can form a heterodimer, which is a
structure-specific endonuclease that can cut the DNA strand at the
5′-end of impaired DNA to facilitate removal of impaired DNA
segments. However, the NER pathway involves multiple genes and each
gene has a different function (36).
In the present study, overexpression of miR-200c-3p only regulated
two genes in the NER pathway, which would not completely inhibit
the pathway. Our present study (37)
indicated that ERCC1 might also be involved in the regulation of
cisplatin resistance in gastric cancer. In addition, there are
certain limitations of the present study. For example, only a
single cell line (SGC7901/DDP) and a single drug (cisplatin) were
investigated. The absence of direct binding luciferase assays to
reveal the interaction between miR-200c-3p and ERCC3/4 was another
limitation of the present study. In addition, no rescue
experiments, such as upregulation or downregulation of ERCC3/4,
were performed to confirm that miR-200c-3p reverses cisplatin
resistance by regulating ERCC3/4. Therefore, further studies should
be conducted to address these limitations and elucidate the
detailed molecular mechanism.
In conclusion, to the best of our knowledge, the
present study is the first to report that miR-200c-3p may reverse
cisplatin resistance in a SGC7901/DDP GC cell line by inhibiting
ERCC3 and ERCC4 protein expression, thus interfering with the NER
pathway. This study provided novel insights into the mechanism
underlying the regulatory effects of miR-200c-3p on cisplatin
resistance.
Acknowledgements
Not applicable.
Funding
This study was supported by the Projects of Foreign
Science and Technology Cooperation of Anhui Province (grant no.
1604b0602027).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ML and KG conceived the hypothesis and study design.
ML and MG wrote the manuscript and analyzed data. XX performed the
experiments. YZ, JN and PL were involved in experimental design and
gave important intellectual suggestions for the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F, Allum W, Carneiro F, Mitry E,
Tabernero J, Tan P, Van Cutsem E, van de Velde C and Cervantes A:
Unmet needs and challenges in gastric cancer: The way forward.
Cancer Treat Rev. 40:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lordick F, Lorenzen S, Yamada Y and Ilson
D: Optimal chemotherapy for advanced gastric cancer: Is there a
global consensus? Gastric Cancer. 17:213–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Basourakos SP, Li L, Aparicio AM, Corn PG,
Kim J and Thompson TC: Combination platinum-based and DNA damage
response-targeting cancer therapy: Evolution and future directions.
Curr Med Chem. 24:1586–1606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crosby ME, Kulshreshtha R, Ivan M and
Glazer PM: MicroRNA regulation of DNA repair gene expression in
hypoxic stress. Cancer Res. 69:1221–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie QH, He XX, Chang Y, Sun SZ, Jiang X,
Li PY and Lin JS: MiR-192 inhibits nucleotide excision repair by
targeting ERCC3 and ERCC4 in HepG2.2.15 cells. Biochem Biophys Res
Commun. 410:440–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu
G, Zhao R, Huang H, Wang X, Qiao Y, et al: MiR-152 and miR-185
co-contribute to ovarian cancer cells cisplatin sensitivity by
targeting DNMT1 directly: A novel epigenetic therapy independent of
decitabine. Oncogene. 33:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li
K, Zhou L, Sun Y, Li M, Zhou J, et al: miR-508-5p regulates
multidrug resistance of gastric cancer by targeting ABCB1 and
ZNRD1. Oncogene. 33:3267–3276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang L, Li H, Wang L, Hu J, Jin T, Wang J
and Yang BB: MicroRNA-17-5p promotes chemotherapeutic drug
resistance and tumor metastasis of colorectal cancer by repressing
PTEN expression. Oncotarget. 5:2974–2987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Zuo J, Liu Y, Gao H and Liu W:
Inhibitory effects of miRNA-200c on chemotherapy-resistance and
cell proliferation of gastric cancer SGC7901/DDP cells. Chin J
Cancer. 29:1006–1011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hatano K, Kumar B, Zhang Y, Coulter JB,
Hedayati M, Mears B, Ni X, Kudrolli TA, Chowdhury WH, Rodriguez R,
et al: A functional screen identifies miRNAs that inhibit DNA
repair and sensitize prostate cancer cells to ionizing radiation.
Nucleic Acids Res. 43:4075–4086. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu RL, Dong Y, Deng YZ, Wang WJ and Li
WD: Tumor suppressor miR-145 reverses drug resistance by directly
targeting DNA damage-related gene RAD18 in colorectal cancer.
Tumour Biol. 36:5011–5019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Ohuchida K, Mizumoto K, Sato N,
Kayashima T, Fujita H, Nakata K and Tanaka M: MicroRNA,
hsa-miR-200c, is an independent prognostic factor in pancreatic
cancer and its upregulation inhibits pancreatic cancer invasion but
increases cell proliferation. Mol Cancer. 9:1692010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li RH, Chen M, Liu J, Shao CC, Guo CP, Wei
XL, Li YC, Huang WH and Zhang GJ: Long noncoding RNA ATB promotes
the epithelial-mesenchymal transition by upregulating the
miR-200c/Twist1 axe and predicts poor prognosis in breast cancer.
Cell Death Dis. 9:11712018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao W and Sun J: MicroRNA-200c promotes
tumor cell proliferation and migration by directly targeting
dachshund family transcription factor 1 by the Wnt/β-catenin
signaling pathway in nasopharyngeal carcinoma. Anticancer Drugs.
30:218–224. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ML, Liang LS and Wang XK: miR-200c
inhibits invasion and migration in human colon cancer cells
SW480/620 by targeting ZEB1. Clin Exp Metastasis. 29:457–469. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adam L, Zhong M, Choi W, Qi W, Nicoloso M,
Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, et al:
miR-200 expression regulates epithelial-to-mesenchymal transition
in bladder cancer cells and reverses resistance to epidermal growth
factor receptor therapy. Clin Cancer Res. 15:5060–5072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HL, Ruan L, Zheng LM, Whyte D, Tzeng
CM and Zhou XW: Association between class III β-tubulin expression
and response to paclitaxel/vinorebine-based chemotherapy for
non-small cell lung cancer: A meta-analysis. Lung Cancer. 77:9–15.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He W, Zhang D, Jiang J, Liu P and Wu C:
The relationships between the chemosensitivity of human gastric
cancer to paclitaxel and the expressions of class III β-tubulin,
MAPT, and survivin. Med Oncol. 31:9502014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duran GE, Wang YC, Moisan F, Francisco EB
and Sikic BI: Decreased levels of baseline and drug-induced tubulin
polymerisation are hallmarks of resistance to taxanes in ovarian
cancer cells and are associated with epithelial-to-mesenchymal
transition. Br J Cancer. 116:1318–1328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cochrane DR, Spoelstra NS, Howe EN,
Nordeen SK and Richer JK: MicroRNA-200c mitigates invasiveness and
restores sensitivity to microtubule-targeting chemotherapeutic
agents. Mol Cancer Ther. 8:1055–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou X, Men X, Zhao R, Han J, Fan Z, Wang
Y, Lv Y, Zuo J, Zhao L, Sang M, et al: miR-200c inhibits
TGF-β-induced-EMT to restore trastuzumab sensitivity by targeting
ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 25:68–76. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Xiao Y, Ding X, Zhuo Y, Ren P, Zhou
C and Zhou J: A miR-200b/200c/429-binding site polymorphism in the
3′ untranslated region of the AP-2α gene is associated with
cisplatin resistance. PLoS One. 6:e290432011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamano R, Miyata H, Yamasaki M, Kurokawa
Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M and
Doki Y: Overexpression of miR-200c induces chemoresistance in
esophageal cancers mediated through activation of the Akt signaling
pathway. Clin Cancer Res. 17:3029–3038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang T, Dong P, Li L, Ma X, Xu P, Zhu H,
Wang Y, Yang B, Liu K, Liu J, et al: MicroRNA-200c regulates
cisplatin resistance by targeting ZEB2 in human gastric cancer
cells. Oncol Rep. 38:151–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang L, Guo F, Wang Y, Lv Y, Huo B, Wang
L and Liu W: MicroRNA-200c regulates the sensitivity of
chemotherapy of gastric cancer SGC7901/DDP cells by directly
targeting RhoE. Pathol Oncol Res. 20:93–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li M, Gu K, Liu W, Xie X and Huang X:
MicroRNA-200c as a prognostic and sensitivity marker for platinum
chemotherapy in advanced gastric cancer. Oncotarget. 8:51190–51199.
2017.PubMed/NCBI
|
|
31
|
Hwang JR, Moncollin V, Vermeulen W, Seroz
T, van Vuuren H, Hoeijmakers JH and Egly JM: A 3′->5′ XPB
helicase defect in repair/transcription factor TFIIH of xeroderma
pigmentosum group B affects both DNA repair and transcription. J
Biol Chem. 271:15898–15904. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lankoff A, Sochacki J, Spoof L, Meriluoto
J, Wojcik A, Wegierek A and Verschaeve L: Nucleotide excision
repair impairment by nodularin in CHO cell lines due to ERCC1/XPF
inactivation. Toxicol Lett. 179:101–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McDaniel LD and Schultz RA: XPF/ERCC4 and
ERCC1: Their products and biological roles. Adv Exp Med Biol.
637:65–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andressoo JO, Weeda G, de Wit J, Mitchell
JR, Beems RB, van Steeg H, van der Horst GT and Hoeijmakers JH: An
Xpb mouse model for combined xeroderma pigmentosum and cockayne
syndrome reveals progeroid features upon further attenuation of DNA
repair. Mol Cell Biol. 29:1276–1290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Poterszman A, Lamour V, Egly JM, Moras D,
Thierry JC and Poch O: A eukaryotic XPB/ERCC3-like helicase in
Mycobacterium leprae? Trends Biochem Sci. 22:418–419. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friedberg EC: How nucleotide excision
repair protects against cancer. Nat Rev Cancer. 1:22–33. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ning J, Jiao Y, Xie X, Deng X, Zhang Y,
Yang Y, Zhao C, Wang H and Gu K: miR-138-5p modulates the
expression of excision repair cross-complementing proteins ERCC1
and ERCC4 and regulates the sensitivity of gastric cancer cells to
cisplatin. Oncol Rep. 41:1131–1139. 2019.PubMed/NCBI
|