Introduction
As a malignant tumor of the digestive tract with
quite a common occurrence, colorectal cancer (CRC) has the third
highest incidence worldwide (1).
Without a clarified etiology, CRC is currently considered to be a
consequence of a common coordination effect of eating habits,
living habits, environment, genetic and other factors (2). Due to the developed economy,
accelerated life rhythm, and the changed living environment, as
well as a common diet characterized with low fiber, high fat, and
high protein, the incidence and mortality of CRC are increasing
each year. With few obvious symptoms in the early stage except for
discomfort and indigestion, CRC develops more symptoms at the
middle and late stage when the distant metastasis of cancer cells
occurs, including stool bleeding, abdominal pain, intestinal
obstruction, change of bowel habit, weight loss or fever (3). Due to the lack of specific clinical
manifestations in the early stage of CRC, patients often receive no
correct treatment in the optimal treatment period because of
misdiagnosis and missed diagnosis. Some patients even start seeking
medical help at the middle and late stages of cancer, losing the
chance of radical surgery and receiving unsatisfactory efficacy
from routine treatment (4). Studies
have revealed disappointing 5-year survival rate of CRC, only
approximately 65%, along with a poor prognosis with a recurrence
rate of 37% (5).
As a type of cytokine produced by a variety of cells
and acting on a variety of cells, the interleukin family plays a
pivotal role in the differentiation, maturation, proliferation,
activation, immune regulation, and mediated inflammatory response
of immune cells, involved in a variety of physiological and
pathological reactions (6).
Interleukin 10 (IL-10) has a two-way
immunomodulatory effect, mainly negative immunomodulatory effects
(7). Mainly produced by monocytes,
activated B cells, and keratinocytes, IL-10 is located on
chromosome 1 of the human body and has a molecular weight of 35–40
kDa, in the main form of a dimer (8). IL-10 can not only inhibit the synthesis
of a variety of cytokines such as various inflammatory factors and
growth factors, and promote the secretion of anti-inflammatory
factors in the body, but also can inhibit effector molecules to
achieve tumor immunosuppression, being able to be produced by
various types of solid tumors and hematopoietic tumors (9).
Interleukin 18 (IL-18), a 1.1 kb-long cytokine that
is located on chromosome 11 (11q22.2–22.3), consists of 6 exogenous
factors and 5 inclusions, with 157 amino acids and a molecular
weight of 18.3 kDa (10). As an
inflammatory cytokine that is mainly produced by macrophages, T
cells, B cells, osteoblasts, glial cells, and other cells, IL-18
plays a vital role in the inflammatory response of cells (11). Previous findings have shown that
overexpression of the IL-18 protein can reduce the viability of
tongue squamous cell carcinoma cells by inducing apoptosis to
importantly inhibit the growth and development of tongue squamous
cell carcinoma cells (12). However,
the role of IL-18 in the occurrence and development of diseases is
controversial. It has been previously shown that the expression of
IL-18 in the endometrium of female patients with polycystic ovary
syndrome is significantly increased compared with that in normal
women, with higher expression of IL-18 in the uterus of overweight
patients with polycystic ovary syndrome than in the uterus of
polycystic ovary syndrome patients with normal weight (13). Thus IL-18 may play opposite roles in
different tumors or diseases.
Considering the unclear role of IL-10 and IL-18 in
CRC, this study investigated the expression of IL-10 and IL-18 in
serum of patients with CRC and explored the clinical relationship
between IL-10 and IL-18 and the clinical stage, tumor
differentiation and post-treatment prognosis of CRC patients, as
well as the value of IL-10 and IL-18 in evaluating the prognosis of
patients with CRC, with the purpose of providing references for
clinical practice.
Patients and methods
Clinical baseline data
This study used a retrospective analysis of the
medical records of 146 patients with CRC admitted to Binzhou
Medical University Hospital (Binzhou, China) from January 2011 to
May 2013 and the physical examination data of 82 healthy volunteers
who underwent a physical examination during the same period. A
total of 146 patients (84 males and 62 females, with an average age
of 56.43±16.43 years) who received colonoscopy before the treatment
and were confirmed by pathology as CRC patients were enrolled in
the study group, 82 healthy volunteers (44 males and 38 females,
with an average age of 54.32±15.69 years) were enrolled in the
control group. This study was conducted after the approval by the
Medical Ethics Committee of Binzhou Medical University Hospital,
and both the patients and their families signed the informed
consent form.
Inclusion and exclusion criteria
The inclusion criteria were: patients with the
first-listed diagnosis of CRC, patients who were older than or
equal to 18 years, patients who received regular follow-up after
the surgery, patients who did not undergo radiotherapy and
chemotherapy before admission, patients who were willing to be
re-examined in Binzhou Medical University Hospital regularly after
discharge.
The exclusion criteria were: those who were treated
in Binzhou Medical University Hospital with reoccurred CRC, those
with diabetes, those with immune disease, those with cardiovascular
and cerebrovascular diseases, those who suffered from intestinal
obstruction complicated with intestinal bleeding before the
treatment.
Main reagents and instruments
IL-10 ELISA kit (Shanghai Haling Biological
Technology Co., Ltd., Shanghai, China; article number: HL10289);
IL-18 ELISA kit (Shanghai Yuanmu Biotechnology Co., Ltd., Shanghai,
China; article number: YM-QP10207); fully-automatic quantitative
enzyme standard instrument (Anthos Labtec Instruments GmbH,
als-Siezenheim, Austria; article number: anthos2010); and
UV-visible spectrophotometer (UV1700; Runqee Instrument Technology
Co., Ltd., Shanghai, China) were used in the present study.
Experimental methods
Collection of experimental
specimens
All participants in the experiment were fasted for
more than 8 h in the night before blood collection. Elbow venous
blood (5 ml) on an empty stomach was extracted the next morning and
stored in the refrigerator for 12 h. After the refrigeration, the
blood sample was taken out and placed at 30°C for 25 min at 4°C,
then was centrifugalized at the speed of 800 × g for 5 min at 4°C.
Ten minutes of standing followed the end of the centrifugation to
wait for the stratification of the blood sample. Then the
supernatant liquid was carefully collected and stored at −20°C.
Determination of IL-10 and IL-18
expression levels
In this experiment, the expression levels of IL-10
and IL-18 in the serum of patients in the study group and the
control group were determined by enzyme-linked immunosorbent assay
(ELISA).
The blood sample to be tested and the kit were taken
out from the refrigerator, and thawed at room temperature at 30°C.
Next, the ELISA plate was taken out, and 50 µl of the standard
product was added into the standard well, 10 µl of the sample and
40 µl of the sample dilution solution were added into the sample
well, 100 µl of the chromogenic antibody was added to each well
except the blank well. After that, the ELISA plate was put in a
water bath at 37°C for 60 min and then taken out, and 50 µl of the
washing solution was added to each well and was removed after 1 min
of standing, such washing was repeated 5 times. Afterwards, 50 µl
of the enzyme standard solution was added to each well (except the
blank well) which was then put in the shaking water bath for 15 min
at 37°C. Following that, the color developing agent was added to
each well (except the blank well) to perform the color reaction for
15 min in the dark. Finally, the reaction was terminated by the
addition of a stop solution. The absorbance of each well was
measured at a wavelength of 450 nm 10 min after the end of the
reaction. The ratio of the absorbance of the sample to the
absorbance of the standard product was calculated according to the
absorbance corresponding to the concentration of the standard
product, and the samples with a ratio of its absorbance to the
absorbance of the standard product was ≤99% were determined as
qualified for the experiment. The measured absorbance and the
linear regression equation were calculated by the automatic
microplate reader, and the concentration of the sample was
measured.
Observation indicators and follow-up
methods of the study group
After the surgical operation and the discharge from
hospital, patients in the study group were followed up for 60
months. For patients who were in good health and could maintain
contact, the follow-up was mainly performed by telephone; for
patients with poor communication by telephone interview, such as
those of senior age, in poor health or died during the follow-up,
follow-up was performed by visiting or consulting their families as
appropriate. To record the recurrence situation of the disease,
patients in the study group were required to receive B-scan
ultrasonography, colonoscopy, and CT examinations every 3 months in
the first year after the discharge, every 6 months in the second
year after the discharge, and once a year in the third year after
the discharge. No examinations in the hospital were required three
years after the discharge.
Statistical analysis
Statistical analysis was performed by SPSS 17.0
(Beijing Strong-Vinda Information Technology Co., Ltd., Beijing,
China), and the expression levels of IL-10 and IL-18 were expressed
as the mean ± standard deviation (mean ± SD). The t-test was used
to compare the measurement data between groups, the Chi-square test
was used to compare the count data between groups, while the
variance test and LSD post hoc test were used to compare the
measurement data of multi-group. The Kaplan-Meier method and the
log-rank test were used for survival analysis. The difference was
statistically significant at P<0.05.
Results
Baseline data
No statistical differences of the baseline data
between the study group and the control group were identified
including the age, sex, body mass index, smoking status, alcohol
abuse, fasting blood glucose, marriage history, Hb value, RBC value
and PLT value (P>0.05) (Table
I).
 | Table I.Comparison of general baseline data
between the study group and the control group [n (%)] (mean ±
SD). |
Table I.
Comparison of general baseline data
between the study group and the control group [n (%)] (mean ±
SD).
| Factors | Study group
(n=146) | Control group
(n=82) | χ2/t | P-value |
|---|
| Age (years) | 56.43±16.52 | 54.32±15.69 | 0.942 | 0.347 |
| Sex |
|
| 0.320 | 0.581 |
| Male | 84 (57.53) | 44 (53.66) |
|
|
|
Female | 62 (42.47) | 38 (46.34) |
|
|
| Body mass index
(kg/m2) |
|
| 0.049 | 0.890 |
|
<24 | 77 (52.74) | 42 (51.22) |
|
|
| ≥24 | 69 (47.26) | 40 (48.78) |
|
|
| Smoking status |
|
| 0.485 | 0.493 |
| Yes | 80 (54.79) | 41 (50.00) |
|
|
| No | 66 (45.21) | 41 (50.00) |
|
|
| Alcohol abuse |
|
| 0.417 | 0.579 |
| Yes | 67 (45.89) | 34 (41.46) |
|
|
| No | 79 (54.11) | 48 (58.54) |
|
|
| Fasting blood sugar
(mmol/l) | 4.35±1.81 | 4.63±1.48 | 1.194 | 0.234 |
| Marriage
history |
|
| 0.453 | 0.591 |
|
Married | 137 (93.84) | 75 (91.46) |
|
|
|
Unmarried | 9 (6.16) | 7 (8.54) |
|
|
| Hb (g/l) | 123.58±18.43 | 126.97±15.63 | 1.405 | 0.161 |
| RBC
(×1012/l) | 4.60±0.38 | 4.49±0.49 | 1.886 | 0.061 |
| PLT
(×109/l) | 216.35±57.26 | 224.89±48.21 | 1.142 | 0.255 |
Expression of IL-10 and IL-18 in the
two groups
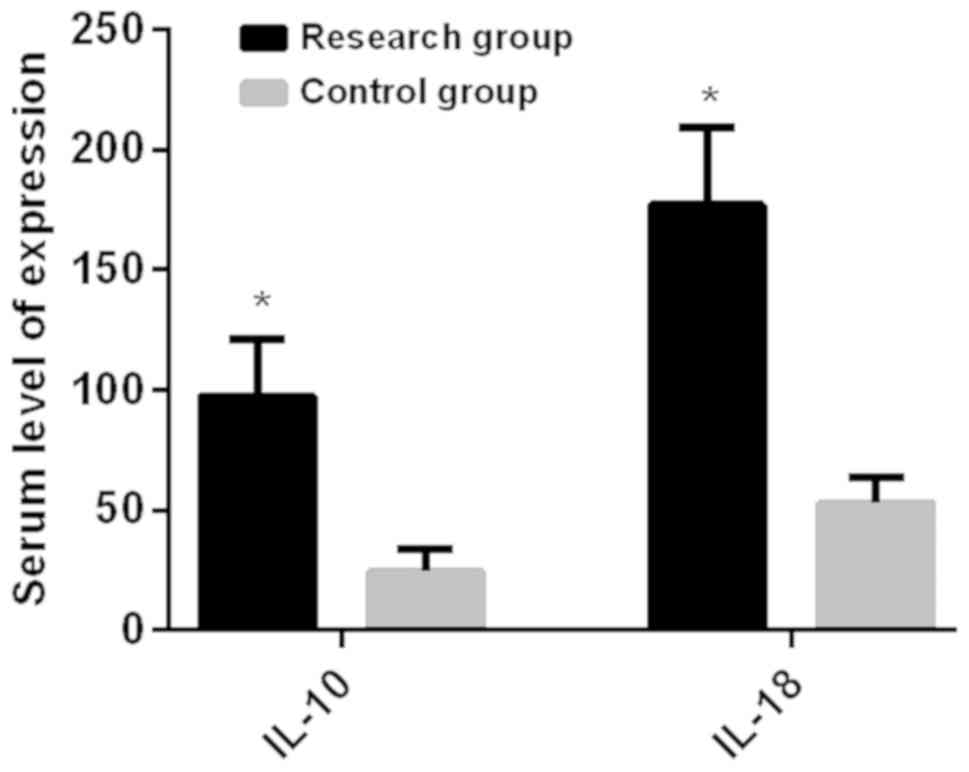
Both the expression level of IL-10 (97.36±23.51
ng/l) and the expression level of IL-18 (176.98±32.34 ng/l) in the
serum of the study group before surgery were higher than those of
the control group which had a serum IL-10 expression level of
(24.53±9.23) ng/l and a serum IL-18 expression level of
(52.93±11.09) ng/l, and the difference was statistically
significant (P<0.05) (Fig.
1).
Relationship between the serum IL-10 and IL-18 and
the clinicopathology of CRC in the study group. According to the
results, the expression of IL-10 and IL-18 in the study group were
not statistically related with factors including age, sex and body
mass index (P>0.05), but were statistically significant for
factors such as the Dukes staging, tumor size, histological grades,
and the distant metastasis of cancer cells (P<0.05) (Table II).
 | Table II.Comparison of expression of IL-10 and
IL-18 in different clinical and pathological conditions in the
study sroup (mean ± SD). |
Table II.
Comparison of expression of IL-10 and
IL-18 in different clinical and pathological conditions in the
study sroup (mean ± SD).
| Factor | Study group
(n=146) | IL-10 (ng/l) | t/F | P-value | IL-18 (ng/l) | t/F | P-value |
|---|
| Age (years) |
|
| 1.716 | 0.088 |
| 1.821 | 0.071 |
|
<55 | 67 | 93.45±19.60 |
|
| 170.45±25.81 |
|
|
|
≥55 | 79 | 99.34±21.53 |
|
| 179.02±30.30 |
|
|
| Sex |
|
| 0.899 | 0.370 |
| 1.462 | 0.146 |
|
Male | 84 | 95.11±21.26 |
|
| 172.99±28.35 |
|
|
|
Female | 62 | 98.39±22.48 |
|
| 180.03±29.29 |
|
|
| Body mass index
(kg/m2) |
|
<24 | 77 | 94.83±20.98 | 1.506 | 0.134 | 181.06±28.26 | 1.517 | 0.131 |
|
≥24 | 69 | 100.05±20.82 |
|
| 173.84±29.20 |
|
|
| Dukes staging |
|
| 9.474 | <0.01 |
| 4.406 |
<0.01 |
| Stage A
and B | 53 | 83.88±10.03 |
|
| 169.41±24.77 |
|
|
| Stage C
and D | 93 | 105.82±15.05 |
|
| 187.02±22.30 |
|
|
| Tumor size
(cm) |
|
| 7.138 | <0.01 |
| 7.748 | <0.01 |
|
<4 | 61 | 85.36±11.51 |
|
| 158.94±14.30 |
|
|
| ≥4 | 85 | 103.54±17.33 |
|
| 185.48±23.84 |
|
|
| Histological
grades |
|
| 64.150 | <0.01 |
| 131.000 | <0.01 |
| Well
differentiated | 43 | 82.84±8.99 |
|
| 154.95±10.31 |
|
|
|
Moderately differentiated | 56 |
94.93±13.08a |
|
|
178.43±12.57a |
|
|
| Poorly
differentiated | 47 |
109.84±11.03a |
|
|
196.27±13.05a |
|
|
| Distant
metastasis |
|
| 8.082 | <0.01 |
| 11.340 | <0.01 |
|
Yes | 82 | 106.35±14.52 |
|
| 192.94±16.38 |
|
|
| No | 64 | 87.36±13.51 |
|
| 161.53±16.89 |
|
|
Expression of IL-10 and IL-18 in the
study group before and after the operation
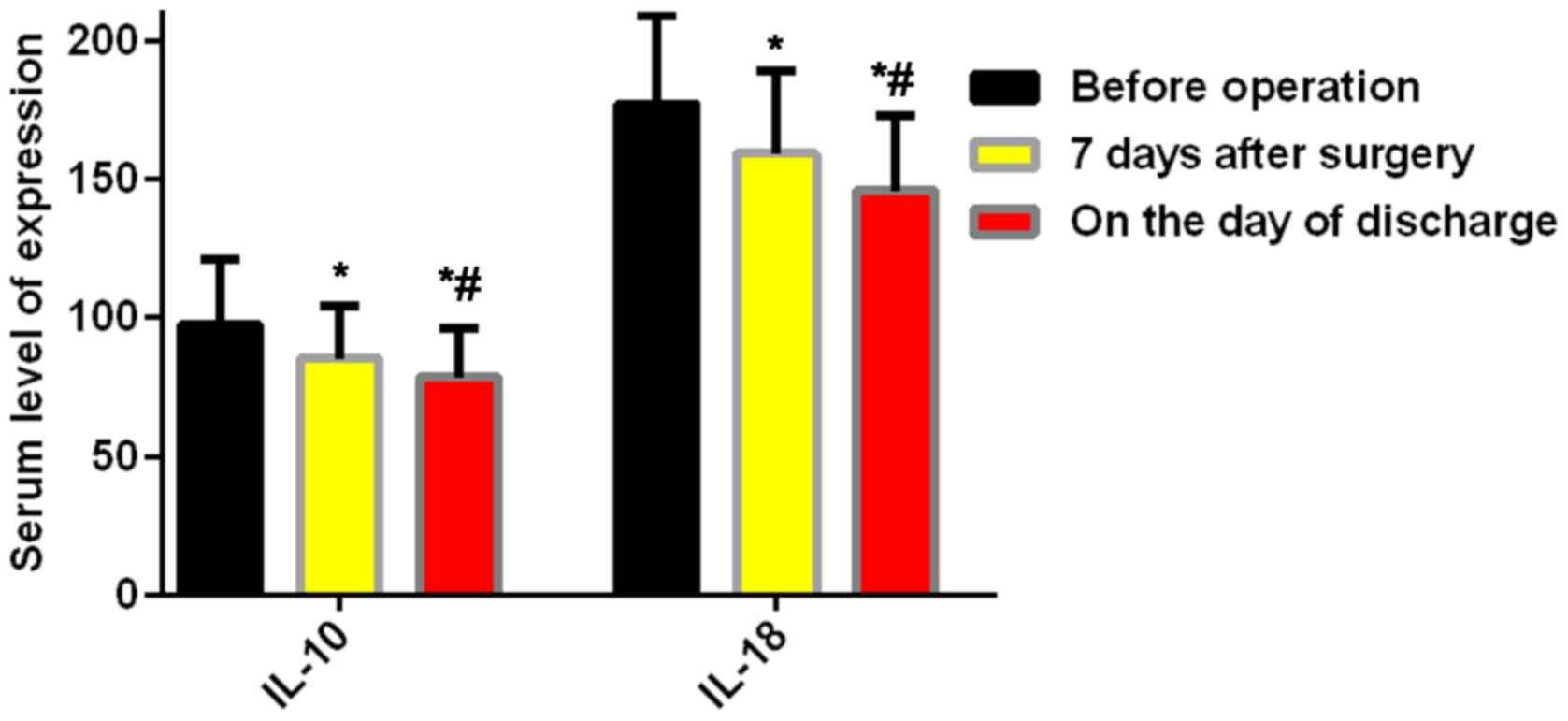
The results presented a gradual decrease of the
expression of IL-10 and IL-18 in the serum of the study group after
the operation, from (97.36±23.51) ng/l of IL-10 before the
operation to (85.23±19.40) ng/l of IL-10 7 days after the
operation, from (176.98±32.34) ng/l of IL-18 before the operation
to (159.34±29.94) ng/l of IL-18 7 days after the operation, and the
differences were statistically significant (P<0.05). In
addition, the expression of IL-10 on the day of discharge
(78.49±17.43 ng/l) and the expression of IL-18 (145.95±26.91 ng/l)
on the day of discharge were statistically lower than the
expression of IL-10 and IL-18 7 days after the operation
(P<0.05) (Fig. 2).
Relationship between the expression
levels of IL-10 and IL-18 and the recurrence of the disease in the
study group
After the discharge, the IL-10 expression of
patients in the study group confirmed by the examination reports to
be reoccurred disease after the operation on the day of the
detection of recurrence of cancer cells was (77.48±27.45) ng/l,
significantly higher than the serum expression level of IL-10 of
patients without reoccurred cancer cells (43.65±13.56) ng/l
according to the last examination reports, and the difference was
statistically significant (P<0.01). The IL-18 expression of
patients with reoccurred disease after the operation on the day of
the detection of recurrence of cancer cells was (153.45±14.54)
ng/l, significantly higher than the serum expression level of IL-18
of patients without reoccurred cancer cells which was (84.37±10.04)
ng/l according to the last examination reports, and the difference
was statistically significant (P<0.05) (Table III).
 | Table III.Relationship between expression
levels and recurrence of IL-10 and IL-18 in the study group (mean ±
SD). |
Table III.
Relationship between expression
levels and recurrence of IL-10 and IL-18 in the study group (mean ±
SD).
| Factor | n | IL-10 (ng/l) | t | P-value | IL-18 (ng/l) | t | P-value |
|---|
| Recurrence |
|
| 9.886 | <0.01 |
| 33.970 | <0.01 |
|
Yes | 58 | 77.48±27.45 |
|
| 153.45±14.54 |
|
|
| No | 88 | 43.65±13.56 |
|
| 84.37±10.04 |
|
|
Relationship between the IL-10
expression level and the survival of patients in the study
group
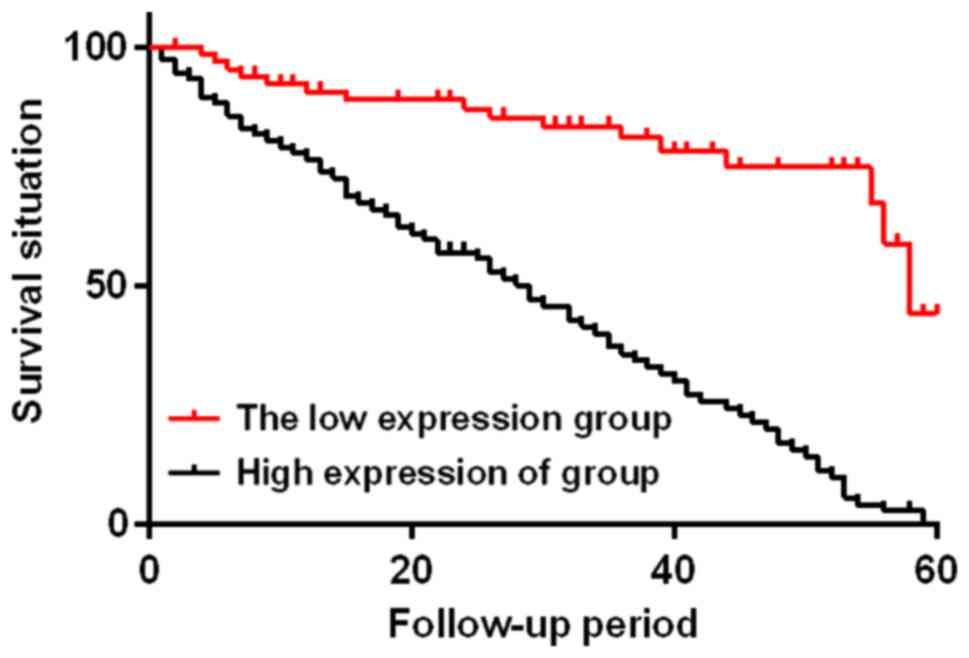
According to the average expression level of serum
IL-10 in the study group on the day of discharge, patients in the
study group were divided into two subgroups, with 69 patients whose
IL-10 expression levels were <75 ng/l in the low IL-10
expression group and 77 patients whose IL-10 expression levels were
≥75 ng/l in the high IL-10 expression group. The two subgroups were
followed up for 60 months. At the end of the follow-up, the low
IL-10 expression group had 16 cases of death, with a median
survival time of 58 months and a mortality rate of 23.18%; the high
IL-10 expression group had 72 cases of death, with a median
survival time of 29 months and a mortality rate of 93.51%. The two
subgroups were statistically different (χ2=49.330,
P<0.01) (Fig. 3).
Relationship between the IL-18
expression level and the survival of patients in the study
group
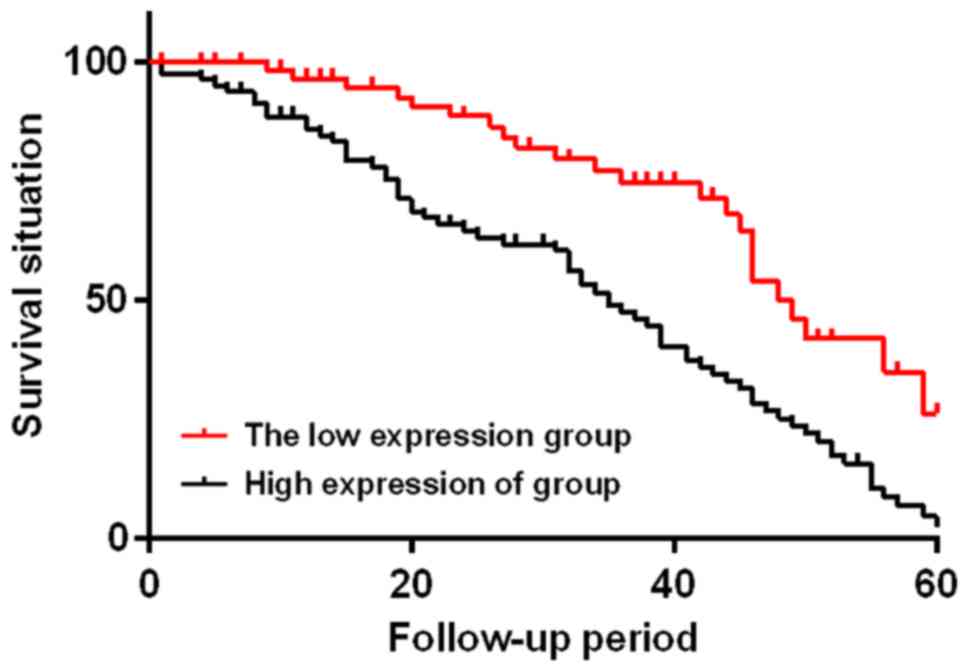
According to the average expression level of serum
IL-18 in the study group on the day of discharge, patients in the
study group were divided into two subgroups, with 55 patients whose
IL-18 expression levels were <150 ng/l in the low IL-18
expression group and 91 patients whose IL-18 expression levels were
≥150 ng/l in the high IL-18 expression group. The two subgroups
were followed up for 60 months. At the end of the follow-up, the
low IL-18 expression group had 21 cases of death, with a median
survival time of 49 months and a mortality rate of 38.18%; the high
IL-18 expression group had 67 cases of death, with a median
survival time of 35 months and a mortality rate of 73.63%. The two
subgroups were statistically different (χ2=15.290,
P<0.01) (Fig. 4).
Discussion
Colorectal cancer (CRC), a very common tumor
disease, has a serious impact on human health due to its high
morbidity and mortality (14). In
recent years, CRC has shown an increase in incidence and a tendency
of a younger age of onset (15),
accounting for more than 15% of all systemic malignant tumors
(16). CRC mostly affects
individuals aged approximately 55 years (17), seriously threatening the safety of
human life with high mortality. Most of the CRC patients start
their treatment at an advanced cancer stage when radiotherapy
brings very limited efficacy due to the patients poor sensitivity
to it, leading to treatment options such as surgical resection.
However, surgical resection, with its huge surgical trauma, is
harmful to the postoperative recovery and survival of patients,
resulting in a low recovery rate and an easy recurrence or
metastasis of cancer cells (18).
Tumor growth is closely related to the regulation of
the bodys immune system (19).
IL-10, an immunosuppressive factor with strong immunosuppressive
effects, mainly performs its function of regulating immune response
through presenting antigens to T cells in various ways (20). In the process of tumor occurrence and
development, IL-10 is of importance as an immunosuppressive factor
by promoting the aggravation of the bodys inflammatory response,
accelerating the occurrence and development of tumors through the
mechanism of immunosuppression, and further advancing tumor
progression by further increasing its expression when the tumor
deteriorates forming a vicious circle (21). IL-18, as a cytokine characterized by
ubiquity and pleiotropy can promote inflammation and immune
stimulation, it has double roles in inhibiting and promoting the
occurrence and development of human immune system diseases
(22). Mainly secreted by monocytes
and macrophages, IL-18 can significantly enhance the bodys immune
function (23). In recent years,
IL-18 has been reported increasingly in literature to be possibly
synthesized by tumor cells, with an especially high expression in
oral cancer patients (24).
This study detected that the levels of IL-10 and
IL-18 in the serum of CRC patients were higher than those in
healthy people, with a statistical difference (P<0.05), which is
similar to the findings of Haghshenas et al (25) who discovered the higher average
expression of IL-18 in gastrointestinal cancer patients compared to
healthy volunteers in the detection of the expression of IL-18 in
the serum of gastrointestinal cancer patients. Galizia et al
(26) discovered the increase of
IL-10 expression in mice which received a subcutaneous injection of
CRC cells, which is similar to the findings of this study. In
further research, our study found the expressions of IL-10 and
IL-18 in CRC patients were not statistically related with factors
including age, gender and body mass index (P>0.05), but were in
statistical relation to factors such as the Dukes staging, tumor
size, histological grades and the distant metastasis of cancer
cells (P<0.05); a gradual decrease of the expression of IL-10
and IL-18 in CRC patients surfaced after surgery and thus the
expression levels of IL-10 and IL-18 in the serum 7 days after the
operation were statistically lower than those before the operation
(P<0.05); in addition, the expression of IL-10 and IL-18 on the
day of discharge were also lower than those 7 days after the
operation, with a statistical difference between them (P<0.05).
Jablonska et al (24) found
that the expression of IL-18 in the serum of patients with oral
squamous cell carcinoma 3 weeks after the surgery was lower than
that before surgery, and the study by Becker et al (27) revealed that patients with colon
cancer had a lower expression of serum IL-10 after the treatment
compared with that before the treatment. This study also found that
the expression of IL-10 in patients with reoccurred CRC after the
operation was statistically significantly higher than that in
patients without recurrence of CRC (P<0.01), similar to the
expression of IL-18 (P<0.01). This study divided patients in the
study group into two subgroups according to the IL-10 expression,
with 69 patients whose IL-10 expression levels were <75 ng/l in
the low IL-10 expression group and 77 patients whose IL-10
expression levels were ≥75 ng/l in the high IL-10 expression group.
The two subgroups were followed up for 60 months the low IL-10
expression group had 16 cases of death, with a median survival time
of 49 months and a mortality rate of 23.18%; the high IL-10
expression group had 72 cases of death, with a median survival time
of 29 months and a mortality rate of 93.51%. The two subgroups were
statistically different (χ2=49.330, P<0.01). Such
findings were similar to the study results of Miteva et al
(28), who found that CRC patients
with a high preoperative serum IL-10 levels had low survival rates,
indicating the poor prognosis and survival of CRC patients with
high serum IL-10 levels. Next, patients in the study group were
divided into two subgroups according to the IL-18 expression, with
55 patients whose IL-18 expression levels were <150 ng/l in the
low IL-18 expression group and 91 patients whose IL-18 expression
levels were ≥150 ng/l in the high IL-18 expression group. The two
subgroups were followed up for 60 months, the low IL-18 expression
group had 21 cases of death, with a median survival time of 49
months and a mortality rate of 38.18%; the high IL-18 expression
group had 67 cases of death, with a median survival time of 35
months and a mortality rate of 73.63%. The two subgroups were
statistically different (χ2=15.290, P<0.01), showing
that a high level of serum IL-18 led to a poor prognosis and
survival of CRC patients. Dwivedi et al (21) discovered the ability of IL-10 to
promote tumorigenesis and systemic tumor immune suppression and
speculated that the cause of poor prognosis of patients with high
IL-10 expression might be the IL-10 function of damaging the
activation of the cytotoxic T lymphocyte and inhibiting its
cytolysis activity. Okamoto et al (29) found that serum IL-18 was an
independent prognostic factor for patients with non-small cell lung
cancer, and serum IL-18 expression levels in patients with
non-small cell lung cancer with bone metastases were significantly
higher than those without bone metastases, which is similar to this
study.
This study strictly selected the research subjects
according to the inclusion and exclusion criteria to ensure the
reliability of the results of this study. A 60-month follow-up
study was performed for the prognosis of CRC patients, period,
mainly through monthly telephone interviews, home visits, or
consultations with the patients relatives to guarantee the accuracy
and reality of the result. The accuracy of this study was also
guarded by the precise records of the prognosis of patients
according to the reports of B-scan ultrasonography, colonoscopy,
and CT examinations every 3 months in the first year after the
discharge, every 6 months in the second year after the discharge,
and once a year in the third year after the discharge.
IL-10 and IL-18 have high expression in the serum of
CRC patients and have a close relationship with the Dukes staging,
tumor size, histological grade, and distant metastasis of cancer
cells. IL-10 and IL-18 can be used as indicators to judge the
prognosis of CRC patients, specifically the lower the expression
levels of IL-10 and IL-18, the lower the recurrence rate of cancer,
the better the prognosis and the longer the survival time.
Acknowledgements
Not applicable.
Funding
The study was supported by the Binzhou Science and
Technology Development Plan Project (project no. 2014ZCO107).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
BL drafted the manuscript. BL and FW put forward the
proposition, designed the study and performed ELISA. CM and TH were
responsible for the clinical baseline data collection. LG and HJ
assisted with the collection of the experimental specimens. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of Binzhou Medical University Hospital (Binzhou, China).
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients or
their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ren XL, He GY, Li XM, Men H, Yi LZ, Lu GF,
Xin SN, Wu PX, Li YL, Liao WT, et al: MicroRNA-206 functions as a
tumor suppressor in colorectal cancer by targeting FMNL2. J Cancer
Res Clin Oncol. 142:581–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burden ST, Hill J, Shaffer JL and Todd C:
Nutritional status of preoperative colorectal cancer patients. J
Hum Nutr Diet. 23:402–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasmussen S, Larsen PV, Søndergaard J,
Elnegaard S, Svendsen RP and Jarbøl DE: Specific and non-specific
symptoms of colorectal cancer and contact to general practice. Fam
Pract. 32:387–394. 2015.PubMed/NCBI
|
|
4
|
Cerdán-Santacruz C, Cano-Valderrama O,
Cárdenas-Crespo S, Torres-García AJ and Cerdán-Miguel J: Colorectal
cancer and its delayed diagnosis: Have we improved in the past 25
years? Rev Esp Enferm Dig. 103:458–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zippi M, De Toma G, Minervini G, Cassieri
C, Pica R, Colarusso D, Stock S and Crispino P: Desmoplasia
influenced recurrence of disease and mortality in stage III
colorectal cancer within five years after surgery and adjuvant
therapy. Saudi J Gastroenterol. 23:39–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaudhry H, Zhou J, Zhong Y, Ali MM,
McGuire F, Nagarkatti PS and Nagarkatti M: Role of cytokines as a
double-edged sword in sepsis. In Vivo. 27:669–684. 2013.PubMed/NCBI
|
|
7
|
Liu M, Zhao X and Ma Y, Zhou Y, Deng M and
Ma Y: Transcription factor c-Maf is essential for IL-10 gene
expression in B cells. Scand J Immunol. 88:e127012018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tabibzadeh S, Becker JL and Parsons AK:
Endometriosis is associated with alterations in the relative
abundance of proteins and IL-10 in the peritoneal fluid. Front
Biosci. 8:a70–a78. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamzavi M, Tadbir AA, Rezvani G, Ashraf
MJ, Fattahi MJ, Khademi B, Sardari Y and Jeirudi N: Tissue
expression, serum and salivary levels of IL-10 in patients with
head and neck squamous cell carcinoma. Asian Pac J Cancer Prev.
14:1681–1685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu SQ, Liang W, Wang GL, Li LY, Wang DL
and Chen C: Polymorphisms of the IL-18 promoter and bronchial
asthma. Mol Med Rep. 6:1385–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cornish J, Gillespie MT, Callon KE,
Horwood NJ, Moseley JM and Reid IR: Interleukin-18 is a novel
mitogen of osteogenic and chondrogenic cells. Endocrinology.
144:1194–1201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Hu M, Wang Y, Sun B, Guo Y, Xu Z,
Li J and Han B: Overexpression of interleukin-18 protein reduces
viability and induces apoptosis of tongue squamous cell carcinoma
cells by activation of glycogen synthase kinase-3β signaling. Oncol
Rep. 33:1049–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long X, Li R, Yang Y and Qiao J:
Overexpression of IL-18 in the proliferative phase endometrium of
patients with polycystic ovary syndrome. Reprod Sci. 24:252–257.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ress AL, Perakis S and Pichler M:
microRNAs and colorectal cancer. Adv Exp Med Biol. 889:89–103.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldvaser H, Purim O, Kundel Y,
Shepshelovich D, Shochat T, Shemesh-Bar L, Sulkes A and Brenner B:
Colorectal cancer in young patients: Is it a distinct clinical
entity? Int J Clin Oncol. 21:684–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng Y and Wu C: Prevalence and trend of
gastrointestinal malignant tumors in the elderly over 75 years old
in China. Zhonghua Wei Chang Wai Ke Za Zhi. 19:481–485. 2016.(In
Chinese). PubMed/NCBI
|
|
17
|
Castells A: Prevention of colorectal
cancer. Med Clin (Barc). 117:69–75. 2001.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hind R, Rew DR and Johnson CD: Surgical
excision alone is adequate treatment for primary colorectal cancer.
Ann R Coll Surg Engl. 74:63–67. 1992.PubMed/NCBI
|
|
19
|
Sio A, Chehal MK, Tsai K, Fan X, Roberts
ME, Nelson BH, Grembecka J, Cierpicki T, Krebs DL and Harder KW:
Dysresgulated hematopoiesis caused by mammary cancer is associated
with epigenetic changes and hox gene expression in hematopoietic
cells. Cancer Res. 73:5892–5904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dwivedi S, Goel A, Natu SM, Mandhani A,
Khattri S and Pant KK: Diagnostic and prognostic significance of
prostate specific antigen and serum interleukin 18 and 10 in
patients with locally advanced prostate cancer: A prospective
study. Asian Pac J Cancer Prev. 12:1843–1848. 2011.PubMed/NCBI
|
|
22
|
Amerio P, Frezzolini A, Abeni D, Teofoli
P, Girardelli CR, De Pità O and Puddu P: Increased IL-18 in
patients with systemic lupus erythematosus: Relations with Th-1,
Th-2, pro-inflammatory cytokines and disease activity. IL-18 is a
marker of disease activity but does not correlate with
pro-inflammatory cytokines. Clin Exp Rheumatol. 20:535–538.
2002.PubMed/NCBI
|
|
23
|
Li X, Ren D, Li Y, Xu J, Liu C and Zhao Y:
Increased cancer risk associated with the −607C/A polymorphism in
interleukin-18 gene promoter: An updated meta-analysis including
12,502 subjects. J BUON. 20:902–917. 2015.PubMed/NCBI
|
|
24
|
Jablonska E, Puzewska W, Grabowska Z,
Jablonski J and Talarek L: VEGF, IL-18 and NO production by
neutrophils and their serum levels in patients with oral cavity
cancer. Cytokine. 30:93–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haghshenas MR, Hosseini SV, Mahmoudi M,
Saberi-Firozi M, Farjadian S and Ghaderi A: IL-18 serum level and
IL-18 promoter gene polymorphism in Iranian patients with
gastrointestinal cancers. J Gastroenterol Hepatol. 24:1119–1122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galizia G, Orditura M, Romano C, Lieto E,
Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C and
De Vita F: Prognostic significance of circulating IL-10 and IL-6
serum levels in colon cancer patients undergoing surgery. Clin
Immunol. 102:169–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Becker C, Fantini MC, Wirtz S, Nikolaev A,
Lehr HA, Galle PR, Rose-John S and Neurath MF: IL-6 signaling
promotes tumor growth in colorectal cancer. Cell Cycle. 4:217–220.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miteva LD, Stanilov NS, Deliysky TS and
Stanilova SA: Significance of −1082A/G polymorphism of IL-10 gene
for progression of colorectal cancer and IL-10 expression. Tumour
Biol. 35:12655–12664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okamoto M, Azuma K, Hoshino T, Imaoka H,
Ikeda J, Kinoshita T, Takamori S, Ohshima K, Edakuni N, Kato S, et
al: Correlation of decreased survival and IL-18 in bone metastasis.
Intern Med. 48:763–773. 2009. View Article : Google Scholar : PubMed/NCBI
|