Introduction
Lung cancer has the highest levels of morbidity and
mortality of all malignant tumors globally, of which 80–85%
patients are non-small cell lung cancer (NSCLC) (1). NSCLC includes squamous cell carcinoma,
adenocarcinoma, and large cell carcinoma. Compared with small cell
carcinoma, NSCLC has a relatively slow growth rate and relatively
late on-set of metastasis. Common symptoms of NSCLC include
coughing, hemoptysis, chest pain, chest tightness and dyspnea. In
recent years, radiotherapy has become the most common method for
treating lung cancer and has an increasing role in treating
patients with recurrent lung cancer who are unable to undergo
surgery. Unlike small cell lung cancer (SCLC), non-small cell lung
cancer (NSCLC) is insensitive to radiotherapy and has a low
response rate. Therefore, the overall therapeutic effect is
unsatisfactory (2,3). Therefore, knowing how to improve the
therapeutic effect of radiotherapy is of great significance, and
radiosensitization has become a major focus in the field of
radiotherapy. Furthermore, researchers in and outside of China are
currently searching and testing different methods of
radiosensitization. Curcumin is a phenolic compound extracted from
the rootstocks of multiple traditional Chinese medicines (TCMs),
and multiple biological activities of curcumin have been reported,
including antihypertensive and anti-hyperlipidemic effects,
oxidation resistance and immunoregulation (4–6). In
recent years, it has been reported that curcumin has multiple
anti-tumor activities, including inhibiting proliferation and
inducing cell apoptosis in tumors (7–9).
Cisplatin is a common chemotherapeutic agent for lung cancer. A
previous study has demonstrated that treatment with a combination
of curcumin and cisplatin is able to inhibit proliferation and
induce apoptosis in lung cancer (10). However, whether or not curcumin has a
radiosensitization effect on NSCLC tumors is not clear, and to the
best of our knowledge has not been demonstrated previously.
Therefore, the present study was conducted to determine the
radiosensitization effect of a combination of curcumin and
cisplatin on the treatment of NSCLC A549 cells.
Materials and methods
Key reagents and instruments
Curcumin and DMSO were purchased from Sigma-Aldrich,
(Merck KGaA, Darmstadt, Germany). NSCLC A549 cells were purchased
from the Chinese Academy of Sciences (Shanghai, China). Trypsin and
RPMI-1640 medium were obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and methyl thiazolyl
tetrazolium (MTT) was purchased from Beyotime Institute of
Biotechnology (Haimen, China). Rabbit anti-epidermal growth factor
receptor (EGFR) polyclonal (1:1,000; cat. no. 1721100) and rabbit
anti-GADPH (1:2,000; cat. no. 1721011) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA), and newborn calf serum
was purchased from Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd. (Hangzhou, China). The CO2 incubator
with thermostat, medical linear accelerator, automatic enzyme
standard instrument (ELX800; Omega Bio-Tek, Inc., Norcross, GA,
USA), Hoefer mini-VE, ECL chemiluminescence reagent (PerkinElmer,
Inc., Waltham, MA, USA), and nitrocellulose membranes (Omega
Bio-Tek, Inc.) were also used.
Cell culture
A549 cells were inoculated into RPMI-1640 medium
containing 15% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.), and the cells were put into the incubator with a saturated
humidity of 5% CO2 at 37°C. Adherent cells grew well and
were sub-cultured every 3 days. The cells in the logarithmic phase
were used for subsequent experiments.
Irradiation conditions
An electron linear accelerator was used for
irradiating cells in cell culture plates with a water tank below
(height, 5 cm) and a tissue glue above (thickness, ~1.5 cm) the
plates. The source-target distance was 100 cm, and 6MV–X ray
irradiation was administered under a 10×10 cm irradiation field,
with a 200 cGy/min dose rate. The cells were irradiated by
different doses of X-ray (0–10 Gy) according to experimental
requirements and then cultured in the incubator with a saturated
humidity of 5% CO2 at 37°C for 24 h.
MTT assay
The cells in the logarithmic phase were inoculated
into 96-well culture plates at a concentration of
6.0×107/l (100 µl/well) and cultured in a 5%
CO2 incubator at 37°C for 24 h. Different doses of
curcumin (10, 20, 50, 100 and 200 µmol/l) and cisplatin (1, 2, 5,
10 and 20 mg/l) were added to the culture plates following cell
confluence. Cell cultures without curcumin or cisplatin were
regarded as control groups, while the other cell cultures were the
experimental groups. Following cultivation of the cells at 37°C for
24, 48 and 72 h, 20 µl MTT (2 g/l) was added to each well and
cultured for another 4 h until cultivation was terminated. After
the culture solution was removed, 150 µl DMSO was added to each
well and shaken for 10 min. The absorbance value (ABS) of each well
was determined by the microplate reader at a wavelength of 490 nm.
A total of 6 parallel replicate wells were used for each
concentration, and the experiment was repeated three times. The
following formula was used to calculate cell viability: Cell
viability=(ABS in experimental group/ABS in control group)
×100%.
Colony formation experiment
According to the MTT results, concentrations of
curcumin (10 µmol/l) and cisplatin (1 mg/l) with mild cytotoxicity
were selected for subsequent experiments. Single-cell suspensions
of A549 cells in the logarithmic phase were produced and inoculated
into 24-well plates at 200 cells/well. Each plate included the
following four groups: Single irradiation, curcumin + irradiation,
cisplatin + irradiation, and curcumin + cisplatin + irradiation.
After 24 h of transfection, the cells were irradiated by different
doses of X-ray (0, 2, 4, 6, 8 and 10 Gy) for another 10 days of
cultivation, fixed with absolute ethyl alcohol for 15 min and
stained with 0.1% crystal violet for 20 min. The number of clones
>50 was counted under an inverted microscope in order to
calculate the cloning efficiency (CE), as follows: CE (%)=(mean
clone formation for the treatment group/inoculated cell number)
×100%. Surviving fraction (SF)=(CE in the irradiated group/CE in
the non-irradiated group) ×100%. The experiments were repeated
three times for calculating the average value.
According to the multi-target single-hit model
[SF=1-(1-e−D/D0)N], a cell survival curve was
drawn for calculating the sensitization enhancement ratio (SER).
The equation for SER is as follows: SER=D0 in the
control group/D0 in the experimental group. Based on the
equation, D0 refers to the required dose of the curve
index reduced by 63% (D0=1/k), and Dq refers
to the threshold dose of cell damage
(Dq=D0.lnN). The experiment was repeated
three times.
Scratch wound assay
A549 cells in the four treatment groups were
inoculated into 96-well plates at a density of 1×105/ml
and irradiated with 4 Gy X-ray. The cells formed into a cell
monolayer. A line was drawn along the bottom of the culture plate
using a pipette tip. The marginal area and relative distance of the
scratch was captured and determined under an inverted microscope.
After the culture solution was replaced with serum-free RPMI-1640
medium, the cells were cultured according to the requirements of
the groups. Afterwards, an image of the area of the wounded region
lacking cells was captured and calculated. Values of the area of
the wounded region lacking cells prior to and following treatment
were compared. The experiment was repeated three times.
Matrigel assay for assessing
invasion
A Matrigel assay was performed by using a Transwell
chamber (Qiagen GmbH, Hilden, Germany) with pore size of 8.0 µm.
The Transwell chamber was coated with 60 µl 0.8% liquid Matrigel at
37°C for incubation 4–5 h. A549 cells in the four treatment groups
irradiated by 4 Gy X-ray were inoculated into RPMI-1640 medium
containing 15% FBS, and the cells were placed into an incubator
with a saturated humidity of 5% CO2 at 37°C for 24 h.
They were then washed three times with Dulbecco's modified Eagle's
medium (Thermo Fisher Scientific, Inc.) and suspended in culture
medium containing 10% FBS (5×105/ml). The cell
suspension (200 ml) was added to the upper chamber of the Transwell
chamber, and 600 ml culture medium containing 10% FBS was added
into the lower chamber. Subsequently, the cells were cultured in a
5% CO2 incubator at 37°C for 24 h. The cells in the
upper chamber were scrapped with a cotton swab then the culture
medium containing 10% FBS was inverted, and dried at 37°C for 12 h.
The cells were put in a 24-well plate, which were stained with 0.1%
crystal violet (500 µl) were washed with phosphate buffer solution
(PBS) following incubation at 37°C for 30 min. A total of four
visual fields were observed with a confocal microscope (×63 oil
immersed optics; DC 300F, Leica Microsystems GmbH, Wetzlar,
Germany), and images for calculating the number of cells that
migrated across the membranes. The experiment was repeated three
times.
Western blot analysis of signaling
proteins
A549 cells in the four treatment groups irradiated
with 4 Gy of X-rays and cultured for 24 h were added to RIPA lysis
buffer (Hunan Sunshine Bio-Tech, Co., Ltd., Changsha, China) for 30
min and centrifuged at 12,000 r/min for 10 min at 37°C in a
pre-cooled Eppendorf tube. The supernatant was collected for
detection of protein concentration using Nanodrop 2000
spectrophotometer (Nanodrop Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Then, an isopycnic loading buffer was
re-added to the Eppendorf tube and bathed in boiling water for 5
min. Loading buffer (20–50 µl) was added. The proteins were
separated by 12% SDS polyacrylamide gel electrophoresis, initially
at 80 V and then at 100 V. The proteins were subsequently
transferred into polyvinylidene difluoride membranes. Following
incubation in 1% bovine serum albumin at room temperature for 2 h,
the appropriate primary rabbit antibodies (1:500; cat no. SC-1616;
Santa Cruz Biotechnology, Inc.) were added. The membranes were
incubated with the primary antibodies at room temperature for 2 h
and with the secondary antibody (cat no. SC-2054; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. The membrane was
washed three times with Tris-buffered saline with Tween (TBST) for
5 min. The membranes were visualized with electrochemiluminescence
(ECL; cat no. NCI4106; Thermo Fisher Scientific, Inc.) reagent. A
single group was performed three times with X-film exposure,
developed, and photographic fixed. GADPH was used as a loading
control. The optical density of the target bands was detected using
Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA) and using the following equation: The final result=gray
value of each band/internal reference.
Statistical data analysis
SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA)
was used for data analysis. The data are expressed as the mean ±
standard deviation. The differences between two groups were
compared using a paired t-test, and the differences between
multiple groups were compared using one-way analysis of variance
with Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibitory effect of curcumin on A549
NSCLC cells
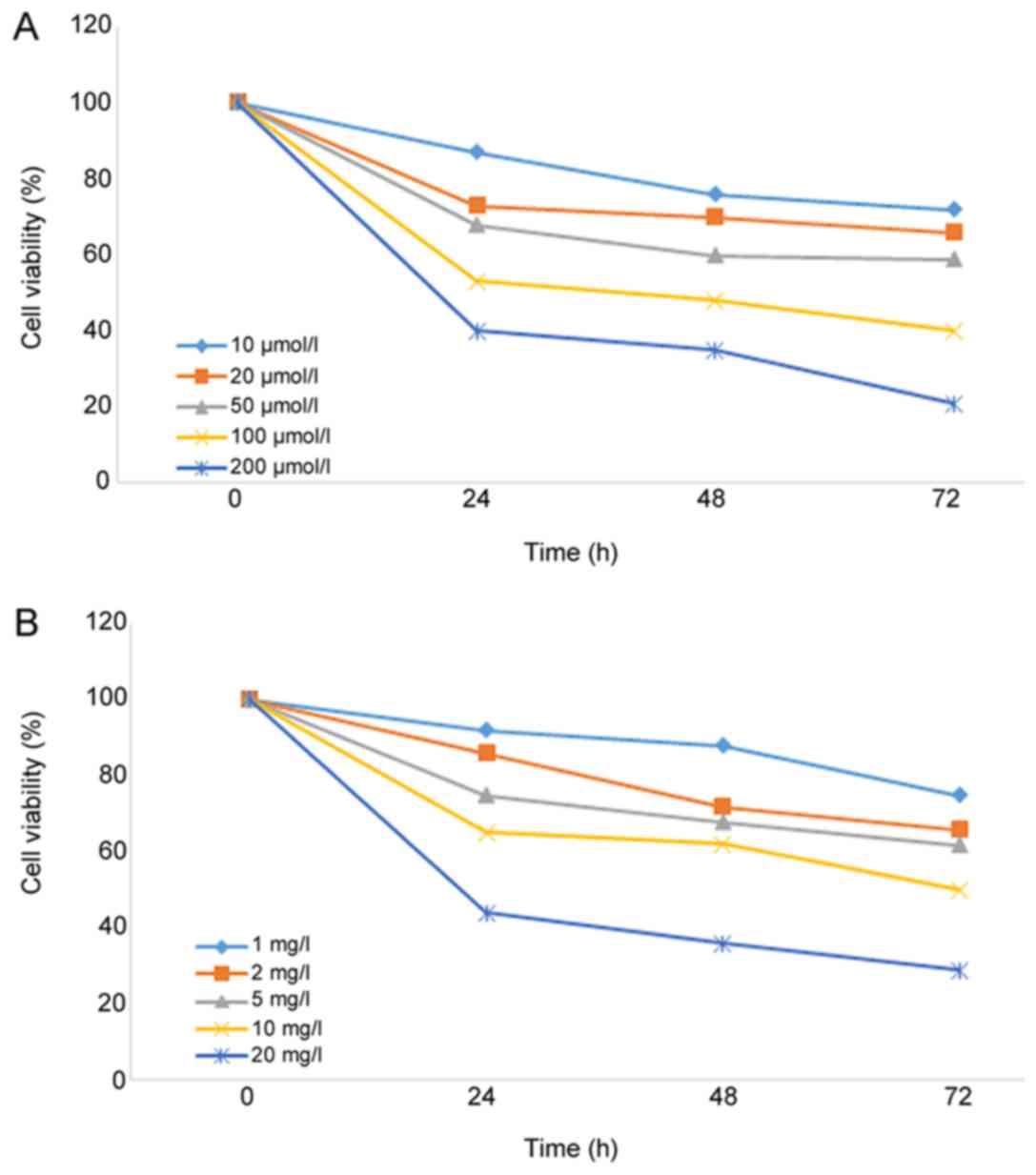
As shown in Fig. 1,
the viability of A549 cells was significantly decreased (P=0.037)
following treatment with different concentrations of curcumin or
cisplatin for 24 h, and the inhibitory effect was dose and
time-dependent. The IC20 values of curcumin and
cisplatin on A549 cells were 15.74 µmol/l and 1.25 mg/l,
respectively. To reduce the toxic effects of curcumin and cisplatin
on A549 cells in the radiosensitization experiments, 10 µmol/l
curcumin and 1 mg/l cisplatin were used.
Inhibitory effect of X-ray irradiation
on A549 cells following treatment with a combination of curcumin
and cisplatin
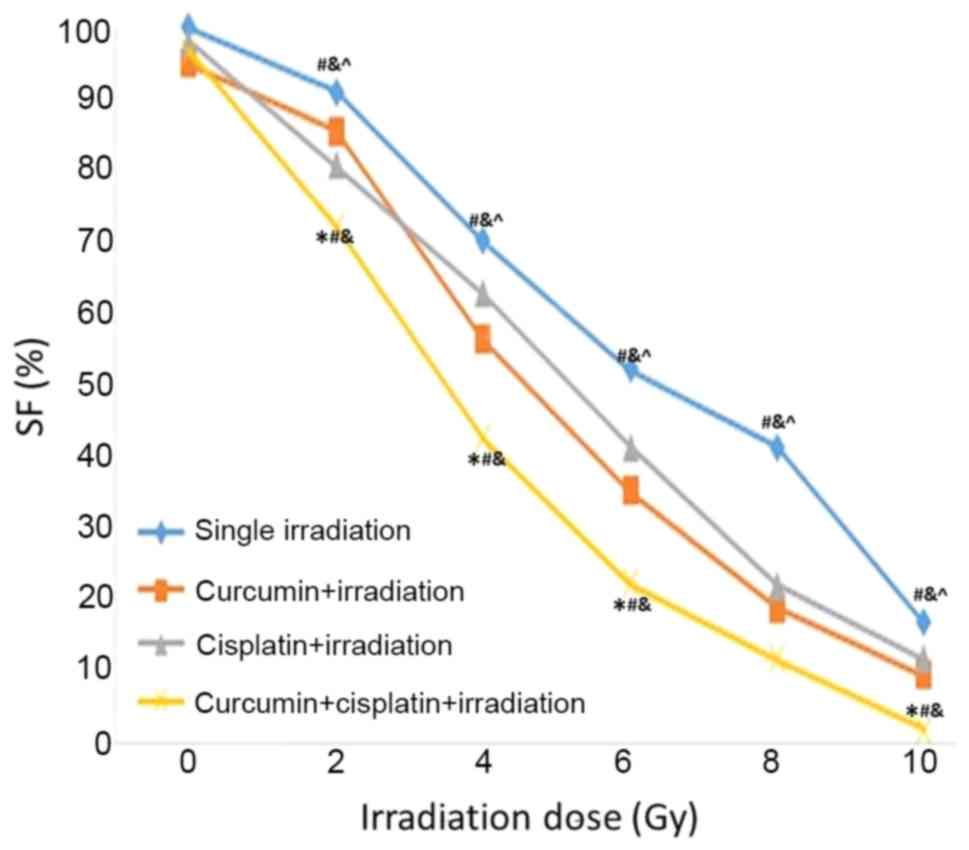
Following treatment with curcumin, cisplatin, or a
combination of curcumin and cisplatin for 24 h, A549 cells were
irradiated with different doses of X-rays (0, 2, 4, 6, 8 and 10
Gy). It was demonstrated that SF value was lower in the curcumin +
cisplatin + irradiation group compared with the other three
treatment groups at 2~10 Gy. The SF value was lower in the curcumin
+ cisplatin + irradiation group compared with the curcumin +
irradiation group at 4–10 Gy. The SF value was lower in the
cisplatin + irradiation group compared with the single irradiation
group 2–10 Gy (P<0.05; Fig. 2).
The SER values for the curcumin + irradiation, cisplatin +
irradiation, and curcumin + cisplatin + irradiation groups were
1.24, 1.31 and 1.96, respectively (Table
I and Fig. 2).
 | Table I.Survival values (%) of A549 cells in
four treatment groups irradiated by different doses of X-rays. |
Table I.
Survival values (%) of A549 cells in
four treatment groups irradiated by different doses of X-rays.
|
| Radiation dose
(Gy) |
|---|
|
|
|
|---|
| Groups | 0 | 2 | 4 | 6 | 8 | 10 |
|---|
| Single irradiation,
mean ± SD | 100.00±1.23 | 91.10±1.69 | 70.22±1.33 | 52.25±1.74 | 41.58±2.28 | 17.08±1.93 |
| Curcumin +
irradiation, mean ± SD | 95.01±3.21 | 85.59±2.05 |
56.35±1.91a |
35.40±2.03a |
19.04±1.53a |
9.61±2.24a |
| Cisplatin +
irradiation, mean ± SD | 97.82±2.04 |
80.78±1.75a |
62.79±2.06a,b |
41.49±1.24a,b |
22.40±1.09a,b |
12.09±1.76a,b |
| Curcumin + cisplatin
and irradiation, mean ± SD | 96.54±2.55 |
72.78±2.64a–c |
42.79±2.64a–c |
22.49±1.83a–c |
9.40±0.84a–c |
2.22±2.02a–c |
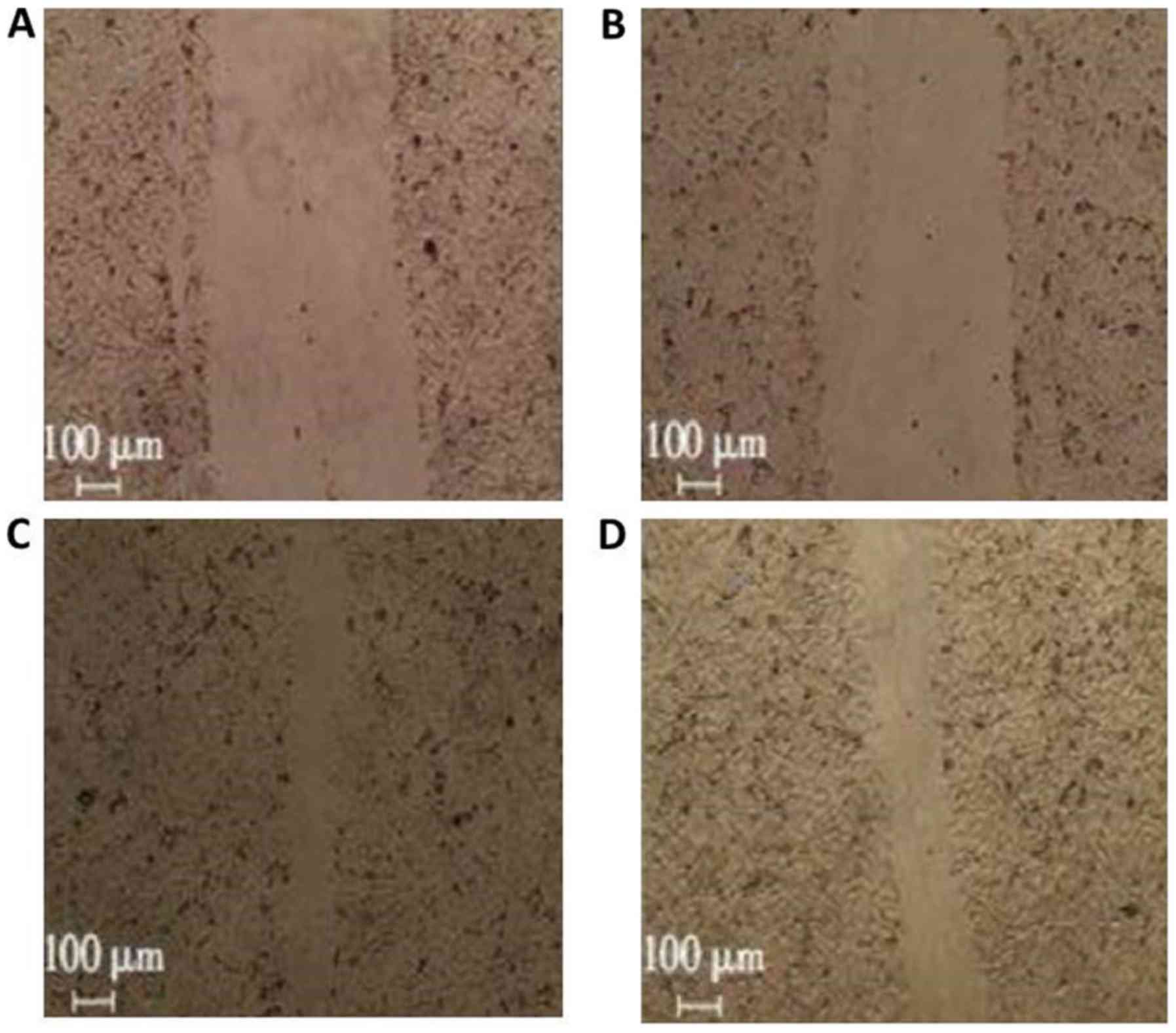
Inhibitory effects of X-ray
irradiation on migration of A549 cells following treatment with a
combination of curcumin and cisplatin
The migration ratios in the curcumin + irradiation,
cisplatin + irradiation, and curcumin + cisplatin + irradiation
groups were 86.0±9.0, 79.0±6.0, and 37.0±5.0%, respectively
(Fig. 3) compared with the single
irradiation group (1.00) (P<0.05; Fig. 2).
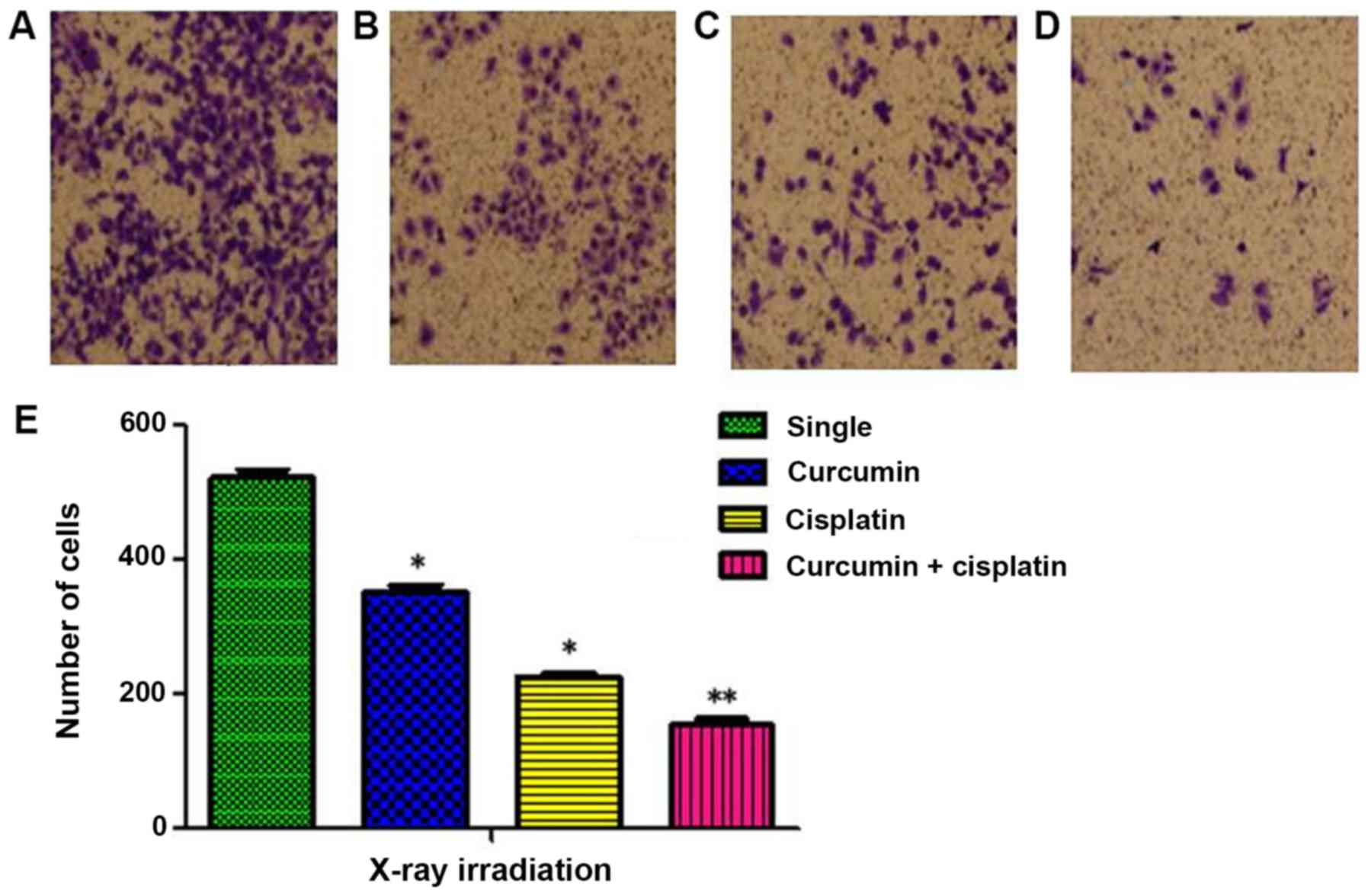
Inhibitory effects of X-ray
irradiation on invasion of A549 cells following treatment with a
combination of curcumin and cisplatin
The number cells invaded through the membrane in the
single irradiation, curcumin + irradiation, cisplatin +
irradiation, and curcumin + cisplatin + irradiation groups were
521±21, 352±17, 229±12, and 154±16, respectively (P<0.05;
Fig. 4).
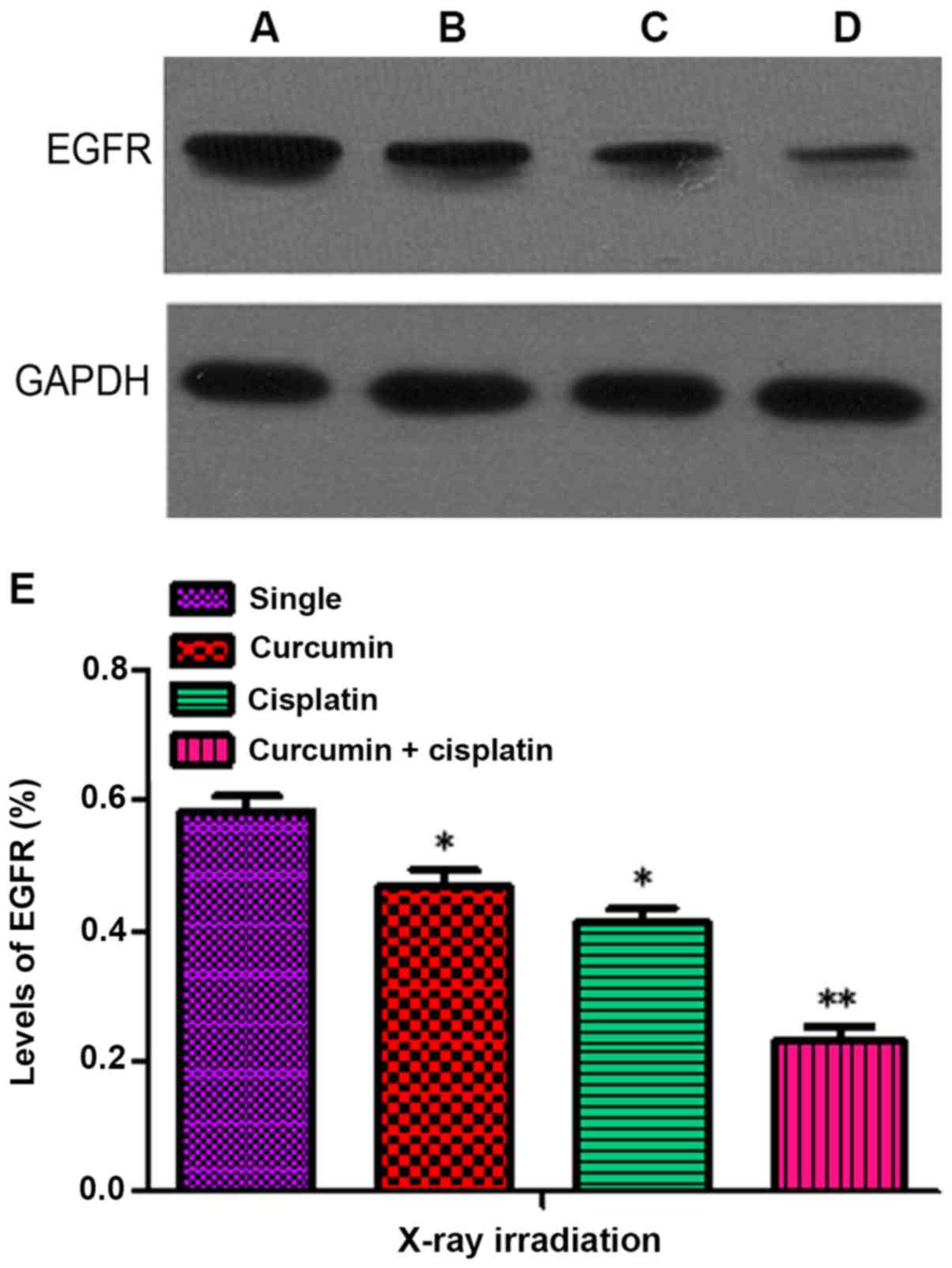
Inhibitory effects of X-ray
irradiation on the expression of EGFR following treatment with a
combination of curcumin and cisplatin
The levels of EGFR in the curcumin + irradiation,
cisplatin + irradiation, and curcumin + cisplatin + irradiation
groups following treatment for 48 h were 0.47±0.04, 0.41±0.04 and
0.23±0.04, respectively, which were significantly lower compared
with the single irradiation group (0.59±0.05; P<0.05). The level
of EGFR expression in the curcumin + cisplatin and irradiation
group was lower compared with the curcumin + irradiation and
cisplatin + irradiation groups (P<0.05; Fig. 5).
Discussion
As the results of the present study demonstrated,
treatment with curcumin was able to inhibit the viability of
humanized NSCLC A549 cells, and that this inhibitory effect was
concentration- and time-dependent, which was consistent with the
results of previous studies (9,10). In
addition, curcumin has a notable inhibitory effect on other tumors
including brain tumors (11),
therefore indicating the broad anti-tumor effect of curcumin. In
order to control the cytotoxicity of curcumin during radiotherapy
(12), lower doses of curcumin that
did not exhibit marked cytotoxicity were selected for subsequent
experiments. The cell survival curve of the A549 cells revealed
that the SF irradiation dose increased with an exponential-reducing
manner, particularly in the dose range of 6–10 Gy and the cell
survival curve which indicated that A549 cells may exhibit
resistance to radiation and have the capacity to undergo DNA repair
when exposed to sub-lethal damage.
The present study also indicated that treatment with
curcumin or cisplatin may be able to promote A549 cells death
following X-ray irradiation. It was demonstrated that the
inhibitory effect of using a combination of curcumin and cisplatin
was more effective compared with the treatment of curcumin or
cisplatin alone. The SF value of curcumin + cisplatin + irradiation
was lower (SER, 1.96) compared with that of irradiation and
treatment with either curcumin (SER, 1.24) or cisplatin alone (SER,
1.31), which indicates a reduced resistance to radiation.
Therefore, these findings indicate that treatment with a
combination of curcumin and cisplatin was able to have an evident
radiosensitization effect on A549 cells cultured in
vitro.
Invasion and metastasis are the main causes of
therapeutic failure in lung cancer (13), and radiotherapy is used for treating
metastatic lesions of lung cancer. Therefore, the present study
investigated the effects of curcumin + cisplatin treatment on A549
cells in vitro to evaluate the radiosensitization effects of
curcumin + cisplatin. The results demonstrated that treatment with
a combination of curcumin and cisplatin was able to increase the
inhibitory effect of X-ray irradiation on the migration and
invasion of A549 cells, which is marked by a reduced migration
distance and a reduced number of invaded cells in the curcumin +
cisplatin group, compared with the single irradiation group. These
results indicate that treatment with curcumin + cisplatin was able
to have an overall radiosensitization effect on A549 cells and
therefore may be able to inhibit proliferation, invasion and
metastasis of lung cancer cells.
In recent years, EGFR has been demonstrated to have
an important role in mediating resistance to radiation in malignant
tumors (14,15), and an association between high EGFR
expression and increased resistance of radiation has been revealed
(16). The mechanism of
EGFR-mediated radiation resistance is complex and may be associated
with self-healing when tumor cells become damaged (17). Therefore, reducing the level of EGFR
expression is a common mechanism of action for sensitizers.
The present study indicated that curcumin was able
to increase the inhibitory effects of X-ray irradiation on the
expression of EGFR, and the inhibitory effect was increased when
cells were treated with a combination of curcumin and cisplatin,
compared with the single irradiation group. In view of the
signaling pathway associated with EGFR, further experiments with a
combination of curcumin and cisplatin on the associated signaling
pathway should be conducted.
In conclusion, treatment with a combination of
curcumin and cisplatin was able to exert radiosensitization effects
on A549 cells and was able to inhibit the A549 cell proliferation,
and invasion and migration of the tumor. The mechanism of action of
curcumin and cisplatin may be associated with the inhibition of
EGFR-associated signaling pathways. Therefore, treatment with a
combination of curcumin and cisplatin may have promising prospects
in increasing the effects of radiotherapy on NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and YC produced substantial contributions to
conception and design, acquisition of data, and analysis and
interpretation of data. ZS was involved in drafting the manuscript,
revising it critically for important intellectual content and made
substantial contributions to conception and design, acquisition of
data and analysis and interpretation of data. SL provided final
approval of the version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang NN: Investigation on target therapy
and resistance mechanism in non-small cell lung cancer. PhD
dissertation. Peking Union Med College. (Beijing, China). 2016.
|
|
2
|
Zhao H, Gu J, Hua F, Xu H, Li L, Yang B,
Han Y, Liu S and Hong S: A meta-analysis of the timing of chest
radiotherapy in patient with limited-stage small cell lung cancer.
Zhongguo Fei Ai Za Zhi. 13:892–897. 2010.(In Chinese). PubMed/NCBI
|
|
3
|
Kimple RJ: Strategizing the clone wars:
Pharmacological control of cellular sensitivity to radiation. Mol
Interv. 10:341–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang SN, Wang ZC and Li YB: Curcumin
protects human malpighian cell by oxidative damage of ultraviolet
light. Chin J Gerontol. 28:1688–1690. 2008.(In Chinese).
|
|
5
|
Huang HY, Wang Y, Chen FX, Liu JQ, Zhou ZH
and Zhang J: Study on the induction of human tolerogenic denfritic
cells by curcumin. Chin J Immunol. 27:611–615. 2011.(In
Chinese).
|
|
6
|
Song LP: Research progress of curcumin
treating atherosclerosis cardiovascular disease. Med Sci J Cent
South China. 41:417–421. 2013.(In Chinese).
|
|
7
|
Lev-Ari S, Starr A, Katzburg S, Berkovich
L, Rimmon A, Ben-Yosef R, Vexler A, Ron I and Earon G: Curcumin
induces apoptosis and inhibits growth of orthotopic human non-small
cell lung cancer xenografts. J Nutr Biochem. 25:843–850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Xie W, Xie C, Huang C, Zhu J, Liang
Z, Deng F, Zhu M, Zhu W, Wu R, et al: Curcumin modulates
miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7
breast cancer cell proliferation. Phytother Res. 28:1553–1560.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong JM, Park CS, Nam-Goong IS, Kim YS,
Lee JC, Han MW, Choi JI, Kim YI and Kim ES: Curcumin enhances
docetaxel-induced apoptosis of 8505C anaplastic thyroid carcinoma
cells. Endocrinol Metab (Seoul). 29:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao H, Diao LM and Xia D: Effects of
curcumin combined with cisplatin on the proliferation and apoptosis
of human lung cancer cell line A549 in vitro. Med J Wuhan Univ.
29:213–217. 2008.
|
|
11
|
Klinger NV and Mittal S: Therapeutic
potential of curcumin for the treatment of brain tumors. Oxid Med
Cell Longev. 2016:93240852016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jamil QUA, Jaerapong N, Zehl M,
Jarukamjorn K and Jäger W: Metabolism of curcumin in human breast
cancer cells: Impact of sulfation on cytotoxicity. Planta Med.
83:1028–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hassan WA, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 controls cell invasion and
metastasis in small cell lung carcinoma cell lines. Lung Cancer.
86:304–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eke I, Sandfort V, Storch K, Baumann M,
Röper B and Cordes N: Pharmacological inhibition of EGFR tyrosine
kinase affects ILK-mediated cellular radiosensitization in vitro.
Int J Radiat Biol. 83:793–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eke I, Schneider L, Förster C, Zips D,
Kunz-Schughart LA and Cordes N: EGFR/JIP-4/JNK2 signaling
attenuates cetuximab-mediated radiosensitization of squamous cell
carcinoma cells. Cancer Res. 73:297–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rego RL, Foster NR, Smyrk TC, Le M,
O'Connell MJ, Sargent DJ, Windschitl H and Sinicrope FA: Prognostic
effect of activated EGFR expression in human colon carcinomas:
Comparison with EGFR status. Br J Cancer. 102:165–172. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boross P, Lohse S, Nederend M, Jansen JH,
van Tetering G, Dechant M, Peipp M, Royle L, Liew LP, Boon L, et
al: IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol
Med. 5:1213–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|