Introduction
Lymphoepithelioma-like gastric carcinoma (LELGC) is
a rare type of gastric cancer characterized by lymphocytic
infiltration of the tumor stroma (1). Watanabe et al (2) described LELGC for the first time in
1976 (2). LELGC constitutes 1–4% of
all gastric carcinomas (3,4), is considered to predominantly affect
males and has a more positive prognosis than other types of gastric
carcinoma (5).
LELGC is categorized into 2 subsets: Epstein-Barr
virus (EBV)-positive carcinoma and microsatellite instability
(MSI)-high carcinoma. EBV-positive cancers commonly comprise
increased tumor-infiltrating lymphocytes compared with EBV-negative
cancers. MSI-high cancers also present increased tumor-infiltrating
lymphocytes compared with non-MSI-high cancers. MSI-high status may
result from defective function of DNA mismatch repair enzymes,
including MutL homolog 1 or MutS homolog 2, but rarely MutS homolog
6 (6). Overall, >80% of LELGC is
EBV-positive, whereas the prevalence of MSI-high LELGC is 7–39%,
depending on geographical location (1).
In the present study, 2 cases of LELGC in male
patients were examined. The diagnosis and prognosis of LELGC are
discussed.
Case report
Case 1
A 65-year-old man reporting epigastric discomfort
for 1 month underwent diagnostic esophagogastroduodenoscopy (EGD)
at The Third Affiliated Hospital of Soochow University and The
First People's Hospital of Changzhou (Changzhou, China) in August
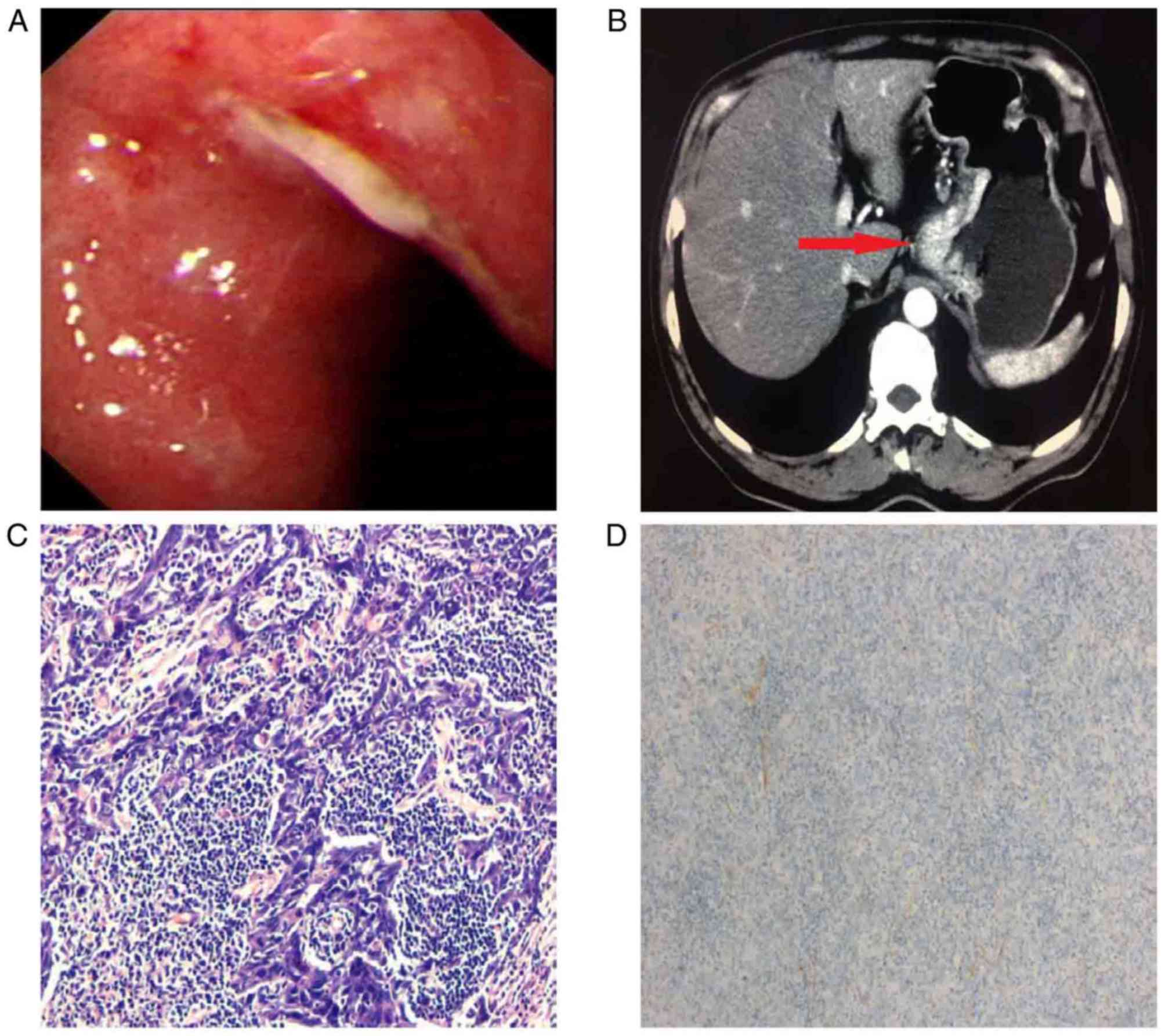
2016. An ulcerated round lesion of ~4.0 cm was detected in the
lesser curvature of the cardia (Fig.
1A). A pathological examination of a specimen collected with a
forceps biopsy indicated that this lesion was a highly atypical
hyperplasia and possibly a malignant tumor. A computed tomography
(CT) scan revealed that the lesion was a tumor with local
ulceration and perigastric lymphadenectasis (Fig. 1B).
Based on the preoperative evaluation, a proximal
gastrectomy was performed. The macroscopic lesion was round, with a
diameter of ~4.0 cm, and occurred in the cardia and lesser
curvature of the stomach. Pathological analysis of the
postoperative specimen determined that the lesion was
morphologically an ulcerated LELGC invading the subserosal layer,
with poor differentiation and lymphovascular invasion (Fig. 1C). In situ hybridization (ISH)
performed as previously described (7) revealed that the tumor was negative for
Epstein-Barr-encoded RNA (EBER) (Fig.
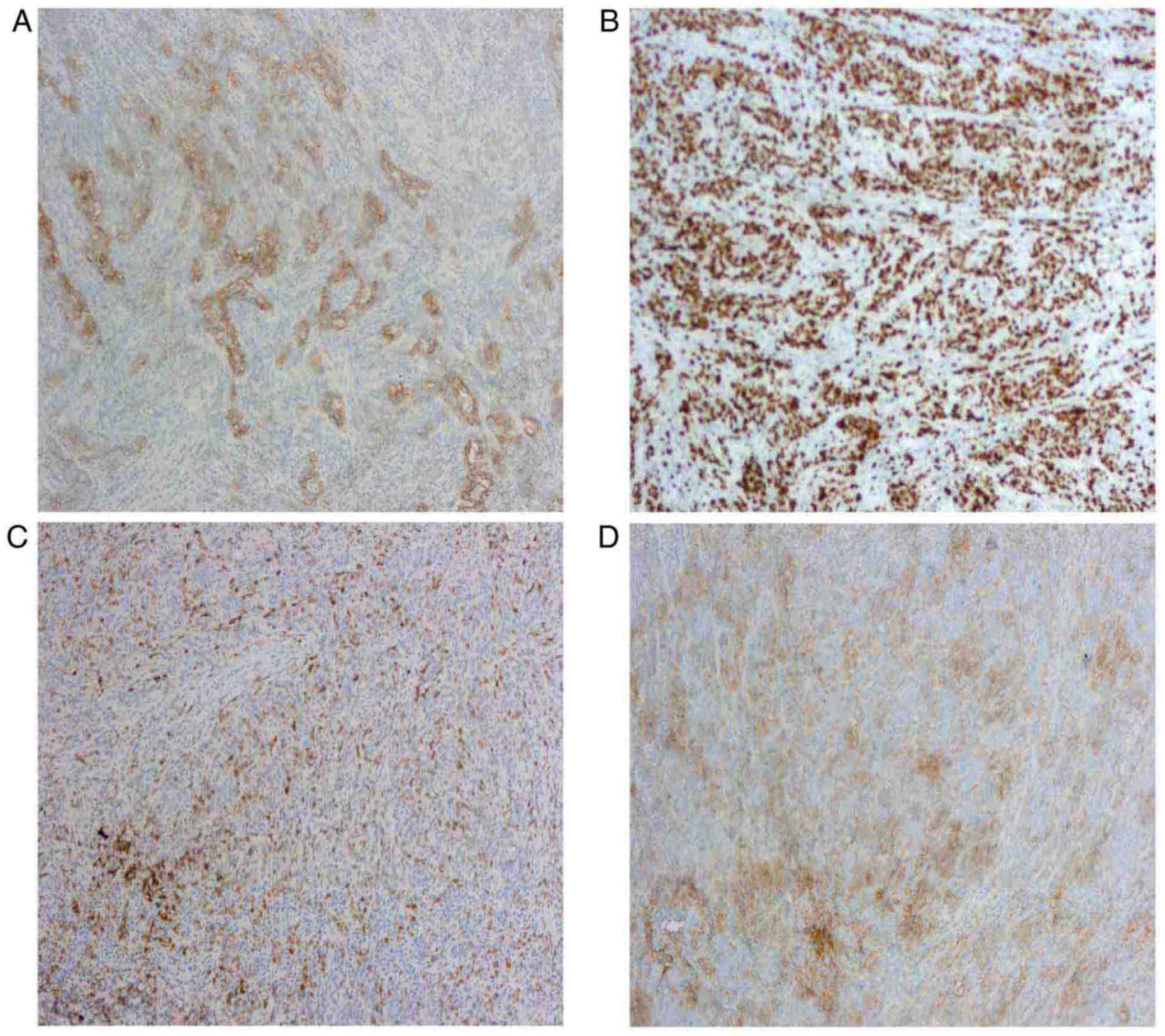
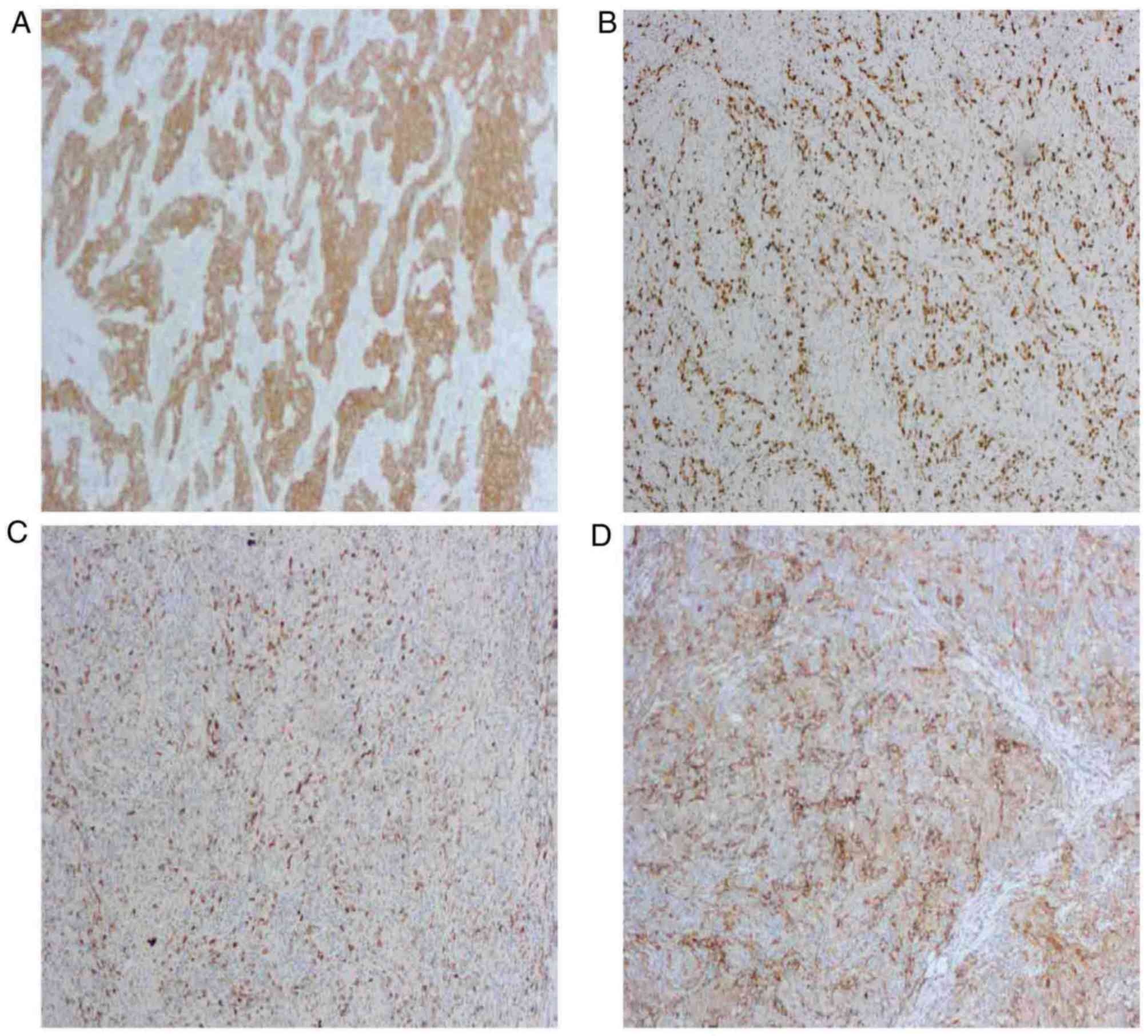
1D). Immunohistochemical analysis of the postoperative specimen
demonstrated that the tumor was positive for receptor
tyrosine-protein kinase erbB-2 (c-erbB-2), proliferation marker
Ki-67, programmed cell death 1 (PD-1) and programmed cell death 1
ligand 1 (PD-L1) (Fig. 2A-D), and
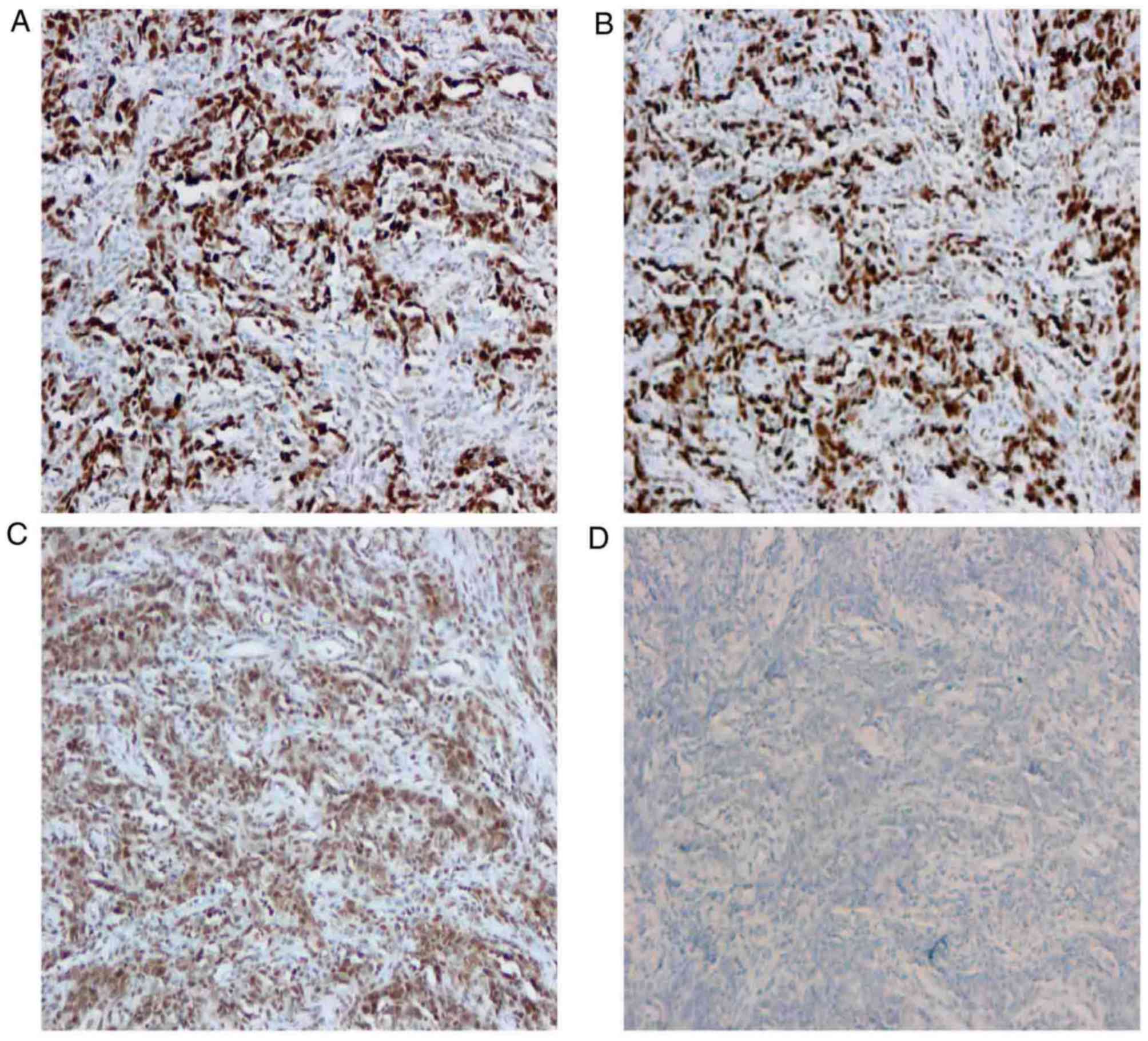
the Ki-67 staining index was ~90%. Further immunohistochemical
analysis demonstrated that the postoperative specimen was positive
for DNA mismatch repair proteins MutS homolog 2, MutS homolog 6 and
MutL homolog 1 (Fig. 3A-C), and
negative for mismatch repair endonuclease PMS2 (Fig. 3D), which indicated that the tumor was
MSI-positive. The resected margins of the specimen were tumor-free
and no lymph-node metastasis was detected. The patient was followed
up for 18 months and recovered well after surgery, and no sign of
local recurrence was detected. This case was one of the only two
cases of LELGC treated at The Third Affiliated Hospital of Soochow
University and The First People's Hospital of Changzhou during the
past several decades, and the MSI characteristics of the tumor were
firstly investigated.
Case 2
A 27-year-old man experiencing epigastric pain and a
hypodynamic impulse for 1 month, with occasional melena, was
admitted to The Third Affiliated Hospital of Soochow University and
The First People's Hospital of Changzhou (Changzhou, China) in
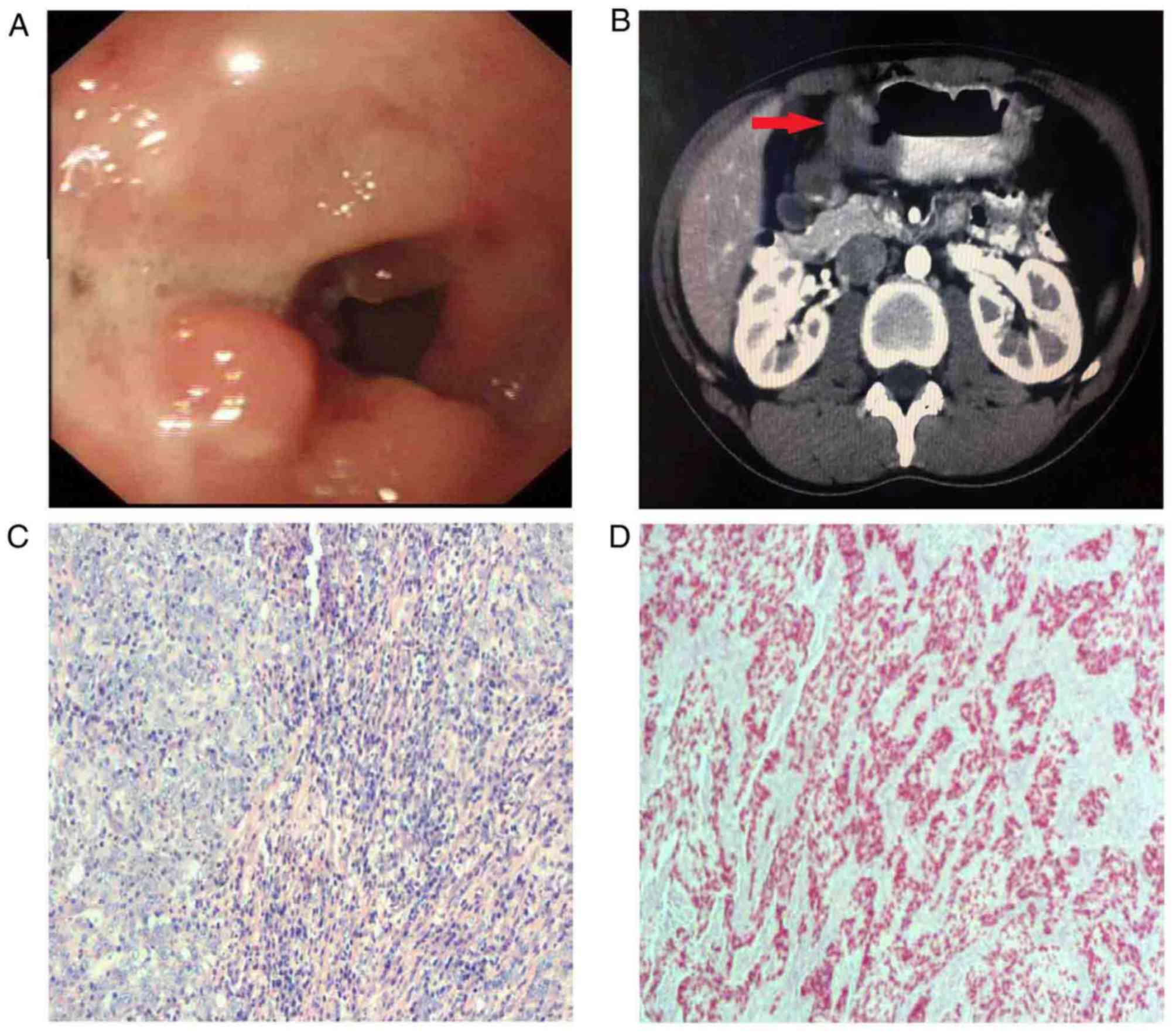
November 2017. EGD revealed irregular apophyses in the posterior
wall of the anterior pyloric region, invading the duodenum, with
surface ulceration (Fig. 4A).
Pathological examination of a specimen collected with a forceps
biopsy confirmed the presence of a gastric antrum cancer, which was
considered to be a poorly differentiated adenocarcinoma, but no
Helicobacter pylori infection was detected. An enhanced
abdominal CT scan identified thickening of the stomach wall in the
gastric antrum, with peripheral lymphadenectasis, indicating a
malignant tumor (Fig. 4B).
The patient was transferred to Zhongshan Hospital
affiliated to Fudan University, (Shanghai, China) where better
treatment was available. Following a full preoperative evaluation,
a distal gastrectomy was performed. A bulging mass with a central
ulcerated lesion ~6.5×5.0 cm2 in size was removed from
the lesser curvature of the gastric antrum. Pathological analysis
of the postoperative specimen revealed that the lesion had invaded
the serous layer, with dense lymphocytic infiltration (Fig. 4C). ISH demonstrated that the tumor
cells were positive for EBER (Fig.
4D). Immunohistochemical analysis of the specimen demonstrated
that it was positive for pan cytokeratin, Ki-67, PD-1 and PD-L1
(Fig. 5A-D), as well as DNA excision
repair protein ERCC1, c-erbB-2, MET proto-oncogene, type II
topoisomerase (TOPOII), β-tubulin, interleukin-9, vimentin, keratin
20 and keratin 19, but that it was negative for FYN proto-oncogene,
neural adhesion molecule 1, glycoprotein hormone α, tumor protein
p63 and keratin 7. The ERCC1, Ki-67, MET and TOPOII staining
indices were ~90, 60, 90 and 3%, respectively. Morphologically and
immunohistochemically, the lesion was characterized as LELGC. The
resected margins were tumor-free and no lymph-node metastasis was
detected. Following surgery, the patient was treated with tegafur,
gimeracil and oteracil potassium. This medicine was taken at the
dose of 40 mg, twice daily for three weeks and stopped for one
week, with a cycle of four weeks and for a total of one year. The
patient recovered well during the 4-month follow-up after surgery,
with no sign of local recurrence. In addition, during the
follow-up, serum analysis revealed that the patient was positive
for EBV antigen.
Histology and immunohistochemistry
examinations
Specimens from case 1 and case 2, including
gastroscopic biopsy specimens and postoperative specimens, were
sent for analysis. The postoperative specimen from case 2 was
analyzed in the Zhongshan Hospital affiliated to Fudan University
(Shanghai, China), whereas the other specimens from cases 1 and 2
were all analyzed at The Third Affiliated Hospital of Soochow
University and The First People's Hospital of Changzhou (Changzhou,
China). All specimens were analyzed following the same protocol in
the two hospitals. Specimens were fixed in 10% formalin for 24 h at
room temperature and embedded in paraffin. Sections were cut into
4-mm thick slices. For histological examination, sections were
subjected to routine deparaffinization using xylene (twice for 10
min) and rehydration with ethanol (twice for 5 min each, including
95% ethanol for 5 min and 70% ethanol for 5 min (Shanghai Sangong
Pharmaceutical Co., Ltd., Shanghai, China). Sections were washed in
distilled water for 1 min and stained with 99% hematoxylin and
eosin for 10 min at room temperature (Shanghai Sangong
Pharmaceutical Co.), and mounted with xylene-based mounting medium
(Shanghai Sangong Pharmaceutical Co.). For immunohistochemical
examination, sections were microwaved for 10 min in 0.01 mol/l
citrate buffer (Shanghai Yu Bo Biological Technology Co. Ltd.,
Shanghai, China) for antigen retrieval, and allowed to cool for 30
min. Endogenous peroxidase activity was quenched by incubating
sections with 3% hydrogen peroxide (Shanghai Yu Bo Biological
Technology Co. Ltd.) in methanol for 10 min. Non-specific binding
was blocked by incubation with 5% bovine serum albumin (Shanghai
Acme Biochemical Co. Ltd., Shanghai, China) diluted in PBS (Beijing
TransGen Biotech Co. Ltd.) for 10 min at room temperature. Sections
were washed three times with PBS, and incubated at 4°C overnight
with murine antihuman monoclonal antibodies. Antibodies used for
case 1 were as follows: Anti-erbB-2 (1:200; Clone EP1045Y; Abcam,
Cambridge, UK), anti-ki-67 (1:200; Clone SP6; Abcam), anti-PD-1
(1:200; Clone SP269; Abcam), anti-PD-L1 (1:200; Clone 73–10;
Abcam), anti-MSH2 (1:200; Clone 3A2B8C; Abcam), anti-MSH6 (1:200;
Clone EPR20316; Abcam), anti-MLH1 (1:200; Clone EPR3894; Abcam) and
anti-PMS2 (1:200; Clone EPR3947; Abcam). Antibodies used for case 2
were as follows: Anti-pan cytokeratin (1:200; Clone C-11; Abcam),
anti-Ki-67 (1:200; Clone EPR3610, Abcam), anti-PD-1 (1:200; Clone
NAT105, Abcam) and anti-PD-L1 (1:200; Clone 28–8, Abcam). Sections
were then incubated with an anti-mouse immunoglobulin G2b-PE
peroxidase for 30 min at room temperature (1:1,000; F1032;
SouthernBiotech, Birmingham, AL, USA). Signal was established
following incubation with 3,3-diaminobenzidine tetrahydrochloride
diluted in Tris-HCl buffer (pH 7.6) containing 0.02% hydrogen
peroxide for 10 min at room temperature (Shanghai XY Biotechnology
Co. Ltd.). Sections were finally counterstained with hematoxylin
and mounted as aforementioned. Sections that were not stained with
primary antibodies represented the negative controls. All sections
were observed using an Olympus BX43 microscope (magnification, ×100
or ×200; Olympus Corporation, Tokyo, Japan).
Discussion
LELGC is a type of gastric carcinoma with typical
clinicopathological characteristics (8,9),
including a chiseled tumor margin, dense lymphocytic infiltration
with the number of infiltrating lymphocytes exceeding the number of
tumor cells, obscure cytoplasmic borders, a syncytial growth
pattern with a poorly formed glandular structure and no desmoplasia
(10). LELGC is also known as
‘gastric carcinoma with lymphoid stroma (2), and has been reported to occur in
various organs, including the stomach, salivary gland, thymus,
larynx, lung, esophagus, cervix and skin (1).
At present, there are several similar studies
concerning LELGC; however, the current study presents novel
information and therefore remains of significant value. During the
past several decades, these were the only 2 cases of LELGC on
record in The Third Affiliated Hospital of Soochow University and
The First People's Hospital of Changzhou, indicating the rarity of
this type of gastric cancer. For the EBV-negative patient 1,
further immunohistochemical analysis was performed to investigate
the MSI characteristics of the tumor. Additionally, the EBV antigen
was tested in serum from EBV-positive patient 2. The therapeutic
strategies for EBV-associated LELGC are also discussed in the
present study.
EBV has an etiological association with LELGC and
the prevalence of EBV-positive gastric cancer is reportedly 8.29%
(11,12). EBV infection is also associated with
nasopharyngeal carcinomas. However, the mechanism by which EBV
contributes to the carcinogenesis of the gastric mucosa remains
unknown (13–15). The presence of EBV in tumor cells was
confirmed with ISH targeting EBER. In the present study, the tumor
cells in case 1 were EBV-negative and MSI-positive, whereas those
in case 2 were EBV-positive. In the postoperative follow-up review,
patient 2 was also found to be positive for serum EBV antigen.
The clinical symptoms of LELGC are usually similar
to those of conventional gastric carcinoma, and include abdominal
pain, loss of appetite and weight loss (10,13).
However, LELGC, unlike conventional gastric carcinoma, is usually
located in the proximal stomach and occurs predominantly in males
(5). LELGC is also easily confused
macroscopically with submucosal tumors, as it often presents as an
ulcerated tumor with a thickened gastric wall (5,8).
Therefore, a definitive diagnosis may be difficult based on an
endoscopic biopsy prior to surgery. The presentation of LELGC on CT
can also vary, appearing as a thickened focal mucosa, apparent
thickening of the gastric wall with contrast enhancement or a bulky
mass (16). Therefore, it is
difficult to distinguish LELGC from lymphoma, gastrointestinal
stromal tumors, glomus tumors and neurogenic tumors with CT alone
(4,5). However, an accurate diagnosis can be
achieved based on the histological characteristics of the dissected
specimen following curative surgery or endoscopic submucosal
dissection (ESD). Fukayama and Ushiku (17) concluded that the diagnosis of
EBV-positive LELGC should be based on dysplastic epithelial cells
and the detection of EBER with ISH. Shinozaki-Ushiku et al
(18) reported that
immunohistochemistry with an antibody directed against cytokeratin
and EBER-targeting ISH detected EBV-associated LELGC. The 2
patients in the current study presented with histologically dense
lymphoid cell infiltration of the stroma, which is consistent with
the pathological features of LELGC. Patient 2 was also positive for
EBER and met the diagnostic criteria for EBV-positive LELGC.
In the present study, the 2 patients chose
laparotomy for the treatment of their advanced-stage tumors. A
proximal gastrectomy was performed in patient 1 as the lesion was
large and had invaded the subserosa. It was necessary to dissect at
least 3 cm into the transhiatal esophagus and to further confirm
the negative margin by frozen section. In patient 2, the lesion was
in the gastric antrum, so a distal gastrectomy was performed.
However, other surgical methods have been reported in previous
studies. According to Lee et al (8), EBV-associated early LELGC was diagnosed
by ESD, with a favorable long-term outcome. Chen et al
(1) also reported that laparoscopic
surgery can be used for the diagnosis and treatment of LELGC, and
that ESD can be considered for early EBV-positive LELGC,
particularly in patients with a serious comorbidity or a high
surgical risk. In the present study, in addition to surgical
resection, postoperative chemotherapy was administered to patient
2, who is currently on tegafur, gimeracil and oteracil potassium
treatment. Patient 1 rejected our advice to receive chemotherapy.
This chemotherapy strategy was in accordance with the therapeutic
strategies for EBV-associated lymphoepithelioma-like carcinoma
reported in the study by Tse and Kwong (19), which concluded that EBV-targeted
therapy is important and that the prophylactic use of antiviral
drugs is effective in reducing the occurrence of EBV-positive
lymphoproliferative diseases. Geng and Wang (20) reported several similar therapeutic
strategies for lymphoproliferative diseases, including novel
antivirals, immunotherapy and gene- or pathway-targeted
therapies.
As aforementioned, patients with LELGC have a
relatively more positive prognosis than those with conventional
gastric carcinoma. Tak et al (21) reported that postoperative recurrence
or metastasis tended to occur less in patients with LELGC than in
patients with poorly differentiated gastric carcinomas. Park et
al (22) demonstrated that the
5-year survival rate of patients with LELGC was higher than that of
patients with non-LELGC (97.7 vs. 89.4%). A similar conclusion was
drawn in the study by Nakamura et al (23), which revealed that the 5-year
survival rate of patients with LELGC following surgical treatment
was higher than that of patients with conventional adenocarcinoma
(84 vs. 58%). In the present study, patient 1 appeared to exhibit
symptoms of reflux owing to the proximal gastrectomy. Fortunately,
the reflux could be well controlled by medication. Overall, the 2
patients underwent laparotomy and exhibited good outcomes during
follow-up, despite the deep invasion of the tumor cells. However,
the follow-up period was short, and the long-term effects of this
treatment require further examination. A number of studies have
investigated why patients with LELGC exhibit a higher survival rate
than patients with other forms of gastric cancer. Lee et al
(8) reported that LELGC is
characterized by dense lymphocytic infiltration of the tumor
stroma, which could be associated with the positive prognosis. A
massive lymphocytic reaction could prevent the spread of the tumor
through the gastric wall (24,25).
Song et al (10) previously
reported that the 12-year disease-free survival rate of LELGC
patients was ~95%, and suggested that the extensive infiltration of
lymphocytes contributed to low tumor metastasis and an improved
prognosis.
In conclusion, 2 cases of LELGC, a rare stomach
neoplasm that is associated with EBV infection, are described in
the present study. It is difficult to distinguish LELGC from
ordinary gastric carcinoma with an endoscopic biopsy, as the
stromal lymphocytic infiltration is dense. A diagnosis of LELGC
should be established based on the pathological, histological and
immunohistochemical analyses of the postoperative specimen. LELGC
generally has a more positive prognosis than other types of
EBV-positive gastric carcinomas or conventional gastric
carcinomas.
Acknowledgements
The authors would like to thank Dr Zhaoli Li
(Department of Pathology, The Third Affiliated Hospital of Soochow
University and The First People's Hospital of Changzhou, Changzhou,
China) for her assistance in immunohistochemical staining
analyses.
Funding
The present study was supported by a Changzhou
Municipal Scientific Research grant (no. CE20125020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC, YW and ZT wrote the manuscript and analyzed the
clinicopathological data. JX and YQ performed the follow-up and
collected the clinicopathological data of the patients. YW and ZT
assisted HC to revise the manuscript, and YW assisted to fund the
study.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Third Affiliated Hospital of Soochow University.
Patient consent for publication
Written consent for publication was provided by the
patients included in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen M, Yin L, Yao Y, Wang L, Xu G, Zhang
X, Lv Y, Sun QI, Fan X and Zou X: Lymphoepithelioma-like gastric
carcinoma in a patient with rectal laterally spreading tumor: A
case report. Oncol Lett. 11:2491–2496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe H, Enjoji M and Imai T: Gastric
carcinoma with lymphoid stroma. Its morphologic characteristics and
prognostic correlations. Cancer. 38:232–243. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corvalan A, Ding S, Koriyama C, Carrascal
E, Carrasquilla G, Backhouse C, Urzua L, Argandona J, Palma M,
Eizuru Y and Akiba S: Association of a distinctive strain of
Epstein-Barr virus with gastric cancer. Int J Cancer.
118:1736–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SW, Shin HC, Kim IY, Kim CJ, Lee JH,
Lee CK and Jeong DJ: Epstein-Barr virus-associated
lymphoepithelioma-like gastric carcinoma presenting as a submucosal
mass: CT findings with pathologic correlation. Korean J Radiol.
11:697–700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Q, Du J and Liu BL:
Lymphoepithelioma-like gastric carcinoma located in the lesser
curvature of the gastric body: A case report and review of the
literature. Mol Clin Oncol. 4:405–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grogg KL, Lohse CM, Pankratz VS, Halling
KC and Smyrk TC: Lymphocyte-rich gastric cancer: Associations with
Epstein-Barr virus, microsatellite instability, histology, and
survival. Mod Pathol. 16:641–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weiss LM and Movahed LA: In situ
demonstration of Epstein-Barr viral genomes in viral-associated B
cell lymphoproliferations. Am J Pathol. 134:651–659.
1989.PubMed/NCBI
|
|
8
|
Lee JY, Kim KM, Min BH, Lee JH, Rhee PL
and Kim JJ: Epstein-Barr virus-associated lymphoepithelioma-like
early gastric carcinomas and endoscopic submucosal dissection: Case
series. World J Gastroenterol. 20:1365–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng N, Hui DY, Liu Y, Zhang NN, Jiang Y,
Han J, Li HG, Ding YG, Du H, Chen JN and Shao CK: Is gastric
lymphoepithelioma-like carcinoma a special subtype of
EBV-associated gastric carcinoma? New insight based on
clinicopathological features and EBV genome polymorphisms. Gastric
Cancer. 18:246–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song HJ, Srivastava A, Lee J, Kim YS, Kim
KM, Ki Kang W, Kim M and Kim S, Park CK and Kim S: Host
inflammatory response predicts survival of patients with
Epstein-Barr virus-associated gastric carcinoma. Gastroenterology.
139:84–92.e82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibata D, Tokunaga M, Uemura Y, Sato E,
Tanaka S and Weiss LM: Association of Epstein-Barr virus with
undifferentiated gastric carcinomas with intense lymphoid
infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol.
139:469–474. 1991.PubMed/NCBI
|
|
12
|
Sousa H, Pinto-Correia AL, Medeiros R and
Dinis-Ribeiro M: Epstein-Barr virus is associated with gastric
carcinoma: The question is what is the significance? World J
Gastroenterol. 14:4347–4351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZH, Zhao JJ and Yuan Z:
Lymphoepithelioma-like gastric carcinoma: A case report and review
of the literature. World J Gastroenterol. 22:3056–3061. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang MS, Kim WH, Kim CW and Kim YI:
Epstein-Barr virus in gastric carcinomas with lymphoid stroma.
Histopathology. 37:309–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang GH, Lee S, Kim WH, Lee HW, Kim JC,
Rhyu MG and Ro JY: Epstein-Barr virus-positive gastric carcinoma
demonstrates frequent aberrant methylation of multiple genes and
constitutes CpG island methylator phenotype-positive gastric
carcinoma. Am J Pathol. 160:787–794. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda E, Akahane M, Uozaki H, Kato N,
Hayashi N, Fukayama M and Ohtomo K: CT appearance of Epstein-Barr
virus-associated gastric carcinoma. Abdom Imaging. 34:618–625.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukayama M and Ushiku T: Epstein-Barr
virus-associated gastric carcinoma. Pathology, Res Pract.
207:529–537. 2011. View Article : Google Scholar
|
|
18
|
Shinozaki-Ushiku A, Kunita A and Fukayama
M: Update on Epstein-Barr virus and gastric cancer (review). Int J
Oncol. 46:1421–1434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tse E and Kwong YL: Epstein Barr
virus-associated lymphoproliferative diseases: The virus as a
therapeutic target. Exp Mol Med. 47:e1362015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geng L and Wang X: Epstein-Barr
virus-associated lymphoproliferative disorders: Experimental and
clinical developments. Int J Clin Exp Med. 8:14656–14671.
2015.PubMed/NCBI
|
|
21
|
Tak DH, Jeong HY, Seong JK, Moon HS and
Kang SH: Comparison of clinical characteristics and prognostic
factors between gastric lymphoepithelioma-like carcinoma and
gastric adenocarcinoma. Korean J Gastroenterol. 62:272–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park S, Choi MG, Kim KM, Kim HS, Jung SH,
Lee JH, Noh JH, Sohn TS, Bae JM and Kim S: Lymphoepithelioma-like
carcinoma: A distinct type of gastric cancer. J Surg Res.
194:458–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura S, Ueki T, Yao T, Ueyama T and
Tsuneyoshi M: Epstein-Barr virus in gastric carcinoma with lymphoid
stroma. Special reference to its detection by the polymerase chain
reaction and in situ hybridization in 99 tumors, including a
morphologic analysis. Cancer. 73:2239–2249. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, Jin L, Xu X, Lin N, Lei B and Shen
H: Pathological and computed tomography findings of
lymphoepithelioma-like gastric carcinoma with epithelioid
granulomas: A case report. Oncol Lett. 5:549–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamura T, Hamada T, Sako T, Makihara K,
Yamada K, Kashima K, Yokoyama S, Hirata K, Hachiya Y, Fukuyama T
and Hirano Y: Lymphoepithelioma-like carcinoma of the stomach with
epithelioid granulomas. Case Rep Gastroenterol. 4:361–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|