Introduction
Colorectal cancer (CRC) is one of the most common
types of gastrointestinal malignancy and is the fourth leading
cause of cancer-associated mortality worldwide (1). The therapeutic effects of traditional
treatment options, including surgery and adjuvant chemotherapy, are
limited and the majority of patients develop drug resistance and
disease recurrence (2,3). Therefore, there is an urgent
requirement to develop novel treatment strategies for CRC. Adoptive
cellular immunotherapy (ACT) has the potential to fulfill this
requirement. The success of ACTs for hematological malignancies has
increased the interest for investigating ACT for the treatment of
solid tumors, including CRC (4,5).
Useful approaches of ACT for the treatment of CRC
include the use of dendritic cell (DC) pulsed with tumor-associated
antigens (TAAs) and cytotoxic T lymphocytes (CTLs) activated using
those DCs (6–9). However, these treatment strategies are
unable to generate sufficient antitumor immune responses. One
possible reason for this is the low immunogenicity of TAAs, which
results in inadequate activity of the host immune system and
treatment failure (10). DCs are the
most potent types of antigen-presenting cells and are critical for
the activation of the immune response (11). DCs are capable of presenting tumor
antigen to naïve T cells in a human leukocyte antigen
(HLA)-restricted manner. However, the low immunogenicity of TAAs
may result in inadequate stimulation of DCs and defective DCs
cannot effectively induce CTLs against tumors. One possible
approach to increase the presentation of TAAs by DCs is to
synthesize α-gal epitopes on tumor cells (12,13).
The α-gal epitope is abundantly presented in
non-primate mammals and New World monkeys, but it is absent in
humans, apes and Old World monkeys (14). However, natural anti-gal antibody has
been identified to be present in human serum in large amounts and
is responsible for the hyperacute rejection of xenotransplantation
(15). Once anti-gal binds to the
α-gal epitope, the Fc portion of anti-gal binds to Fcγ receptor III
on DCs (16). This interaction can
induce effective phagocytosis of DCs and increase the presentation
of TAAs (17). The α-gal epitope is
synthesized by the enzyme α1,3Galantosyltransferase (α1,3GT), which
is present within the Golgi of cells. Galactose is transferred by
α1,3GT from the sugar-donor uridine diphosphate-gal to the acceptor
N-acetyllactosamine residue (Galα1-4GlcNAc-R) to synthesize the
α-gal epitope (18). The expression
of α-gal epitopes on tumor cells can be successfully achieved by
the transduction of a recombinant lentivirus vector expressing the
α1,3GT gene (19).
The present study presents a new therapeutic
strategy for CRC, in which α-gal epitopes were successfully
synthesized on the CRC SW620 cell line and tumor lysate expressing
α-gal epitopes was used to maximize DC phagocytosis and activate
CTLs to kill CRC cells in vitro.
Materials and methods
Cell culture
The human CRC cell line SW620 and 293T cells were
purchased from American Tissue Culture Collection (Manassas, VA,
USA). These cell lines were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 50 U/ml
penicillin, 50 mg/ml streptomycin and 4 mM glutamine in a
humidified incubator with 5% CO2 at 37°C.
Construction of a recombinant
lentivirus vector expressing murine α1,3GT gene
Untransfected cells were termed SW620-normal, SW620
cells transfected with an empty vector were termed SW620-control
and cells transfected with the recombinant lentivirus vector
expressing the α1,3GT gene were termed SW620-α-gal. The coding
sequence (CDS) of the α1,3GT gene was identified from GenBank
(www.ncbi.nlm.nih.gov/nuccore/NM_001145821.2) and
primers for amplification of the CDS of the α1,3GT gene were
designed using Vector NTI (version 10.3; Invitrogen; Thermo Fisher
Scientific, Inc.). pLenti-CMV-mCherry-3FLAG-PGK-Puro was digested
by BamHI-HF and the α1,3GT gene was subcloned into
pLenti-CMV-mCherry-3FLAG-PGK-Puro (Shanghai Heyuan, Shanghai,
China) to construct pLenti-CMV-mCherry-2A-α1,3GT-3FLAG-PGK-Puro.
This recombinant lentivirus vector was transfected at a
concentration of 800 ng/µl into packaging 293T cells using HitransG
P transfection reagent (Shanghai GeneChem). High titers of
recombinant lentivirus vector were amplified, purified and stored.
Finally, SW620 cells were transfected with the recombinant
lentivirus vector expressing the α1,3GT gene at a multiplicity of
infection of 200 using HitransG P tranfection reagent (Genechem,
shanghai, China), which generated SW620-α-gal epitopes. An empty
vector (pLenti-CMV-mCherry-3FLAG-PGK-Puro) was used for
transfection of the control group. The subsequent experiments were
performed 72 h after transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the SW620-normal,
SW620-control and SW620-α-gal cells using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized
using RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) at 42 °C for 60 min and 70°C for 5 min, according
to the manufacturer's protocol. RT-qPCR was carried out using
Applied Biosystems® ViiA™ 7 system, and subsequently
amplified using SYBRGreen PCR Master mix (Beyotime Institute of
Biotechnology) and 0.5 µM each of the sense and antisense primers.
PCR amplification was performed with an initial denaturation at
95°C for 5 min, followed by 40 cycles of 30 sec at 95°C, 30 sec at
60°C and 30 sec at 72°C. β-actin was chosen as the reference gene
with the following primer pairs: Forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The primers for α1,3GT were as
follows: Forward, 5′-TCTGAGAAGAGGTGGCAGGA-3′ and reverse,
5′-GGTGAACTTCTCGGGACTGG-3′. Relative gene expression data was
analyzed by the 2−ΔΔCq method (20).
DC and CTL preparation
The present study was approved by the Ethics
Committee of Chinese People's Liberation Army General Hospital
(Beijing, China). Written informed consent was obtained from all
volunteers (two males; 28 and 31 years old, respectively) and blood
collection took place in October 2017. DCs and CTL were prepared as
previously described (21). Briefly,
peripheral blood mononuclear cells (PBMCs) were obtained from the
peripheral blood of the healthy volunteers (20 ml) using density
gradient centrifugation (1,500 × g for 20 min at 37°C). PBMCs were
cultured in serum-free Cellix 901 medium (Xinminglitai, Beijing,
China) for 3 h. The adherent cells were cultured in Cellix 901
medium containing granulocyte-macrophage colony-stimulating factor
(1,000 U/ml) and recombinant human interleukin-4 (1,000 U/ml). On
day 8, the DCs were co-cultured with SW620-normal, SW620-control
and SW620-α-gal cell lysates in Cellix 901 and 10% human AB group
serum (Gibco; Thermo Fisher Scientific, Inc.) overnight in an
incubator with 5% CO2 at 37°C. On day 9, the DCs were
harvested, washed and resuspended. The harvested DCs were
co-cultivated with non-adherent PBMCs separated from the second
collection of the same volunteer's blood for 14 days to harvest
CTLs. The CTLs activated by DCs cultured with SW620-normal,
SW620-control and SW620-α-gal cell lysates were referred to as
CTL-normal, CTL-control and CTL-α-gal, respectively.
Flow cytometric analysis
The expression of α-gal epitopes on SW620-α-gal
cells was evaluated by flow cytometry. Firstly, ~1×106
α-1,3GT-transfected cells were suspended in PBS and incubated with
fluorescein isothiocyanate (FITC)-BS-IB4 lectins (Sigma-Aldrich;
Merck KGaA), which specifically bind to α-gal epitopes. Phenotypic
analysis was performed by flow cytometry. The following antibodies
were used: Anti-CD80 (cat. no. 557227; 1:40), anti-CD8 (cat. no.
557086; 1:20) and anti-CD127 conjugated with phycoerythrin (cat.
no. 561028; 1:20); anti-CD83 (cat no. 551073, 1:40), anti-CD56
(cat. no. 565139; 1:20) and anti-CD25 conjugated with
allophycocyanin (cat. no. 560987; 1:20); anti-CD86 (cat no. 557343,
1:40) and anti-CD4 conjugated with FITC (cat no. 555346, 1:20); and
anti-HLA-DR (cat no. 552764, 1:40) and anti-CD3 conjugated with
peridinin chlorophyll protein complex (cat no. 552851, 1:20). All
the antibodies were purchased from BD Biosciences. CTL cells were
evaluated using anti-CD3, anti-CD4, anti-CD8, anti-CD56, anti-CD25
and anti-CD127. DC maturation was evaluated using anti-CD80,
anti-CD83, anti-CD86 and anti-HLA-DR. Briefly, ~1×105
cells were stained with the appropriate antibodies in FACS buffer
for 30 min at 4°C in the dark. All analyses were performed with a
FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) and
CellQuest software (version 1.0; BD Biosciences).
ELISA
The detection of IL-10 and IL-12 secreted by DCs,
and IL-4 and tumor necrosis factor-α (TNF-α) secreted by CTL cells
was performed using appropriate ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocol.
The following kits were used: Human IL-10 quantikine ELISA kit
(cat. no. S1000B), human IL-12 p70 quantikine ELISA kit (cat. no.
S1200), human IL-4 quantikine ELISA kit (cat. no. S4050) and human
TNF-α quantikine ELISA kit (cat. no. STA00D).
Cytolytic assay
The cytotoxicity of CTL-normal, CTL-control and
CTL-α-gal cells induced by different antigen-pulsed DCs against
normal SW620, SW620-control and SW620-α-gal cells was evaluated
using a lactate dehydrogenase release assay (CytoTox 96®
Non-Radioactive Cytotoxicity assay; Promega Corporation, Madison,
WI, USA), according to the manufacturer's protocol.
Statistical analysis
All experiments were repeated three times. Data are
presented as the mean ± standard deviation. Statistical analyses
were performed with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Differences among groups were compared with one-way analysis of
variance followed by a Fisher's Least Significance Difference post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Generation of a stable SW620 CRC cell
line expressing α-gal epitopes
A recombinant lentivirus vector expressing the
murine α1,3GT gene was constructed and transferred into SW620 cells
to produce SW620-α-gal cells. A total of 72 h after lentivirus
infection, overexpression of α1,3GT mRNA in SW620 cells was
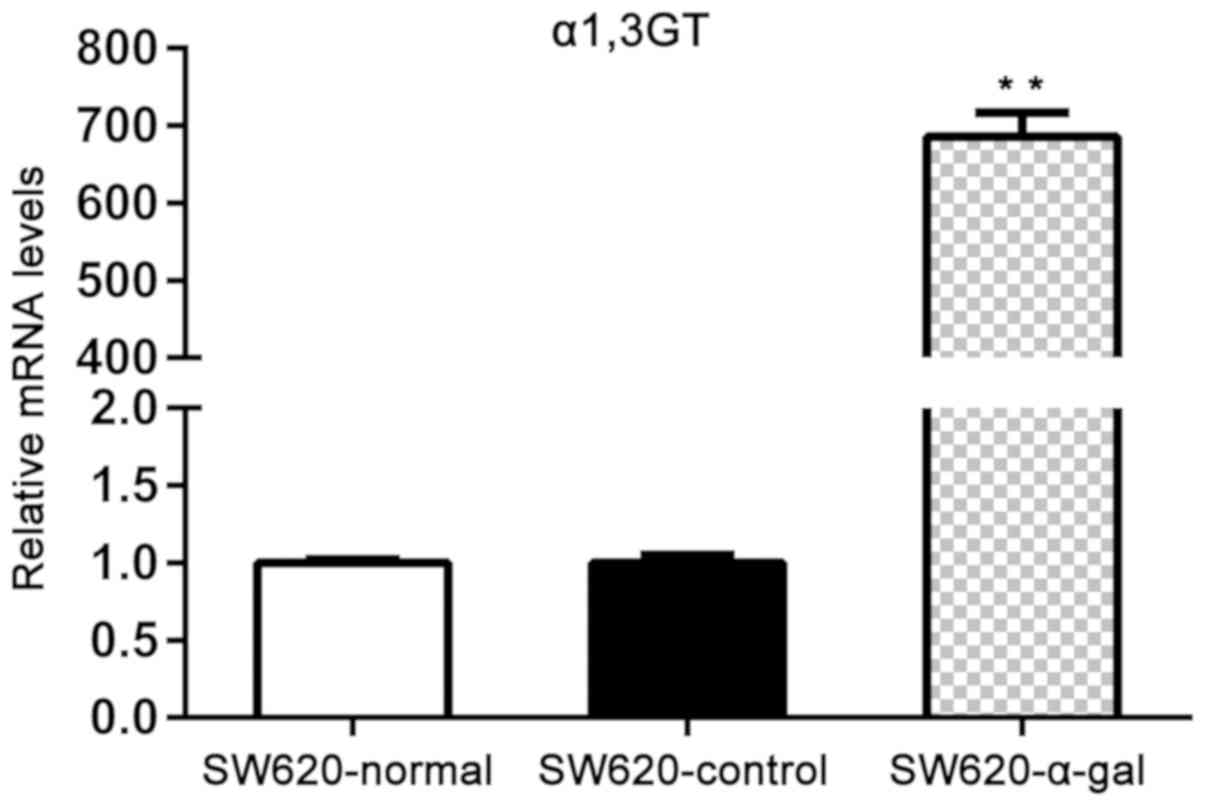
determined by RT-qPCR. As presented in Fig. 1, the expression of α1,3GT mRNA in
SW620-α-gal cells was significantly higher compared with that
observed in SW620-normal and SW620-control cells (P<0.01). The
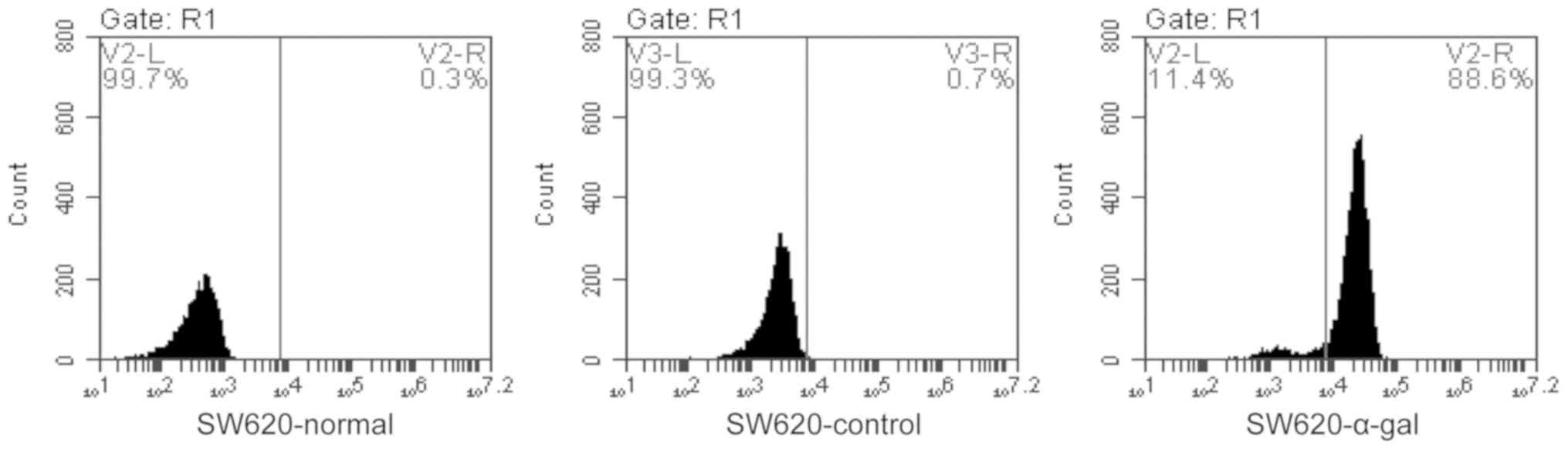
flow cytometry assay demonstrated that >88% of SW620-transfected
cells expressed α-gal epitopes on the surface (Fig. 2).
Effect of pulsing with α-gal
expressing tumor lysate on DC maturation and cytokine
production
Changes in DC immunophenotype and cytokine
production were detected by flow cytometry and ELISA to evaluate
the effects of α-gal expressing tumor lysate on DC maturation. DCs
treated with SW620-normal cell lysate, SW620-control cell lysate
and SW620-α-gal cell lysate were referred to as DC-normal,
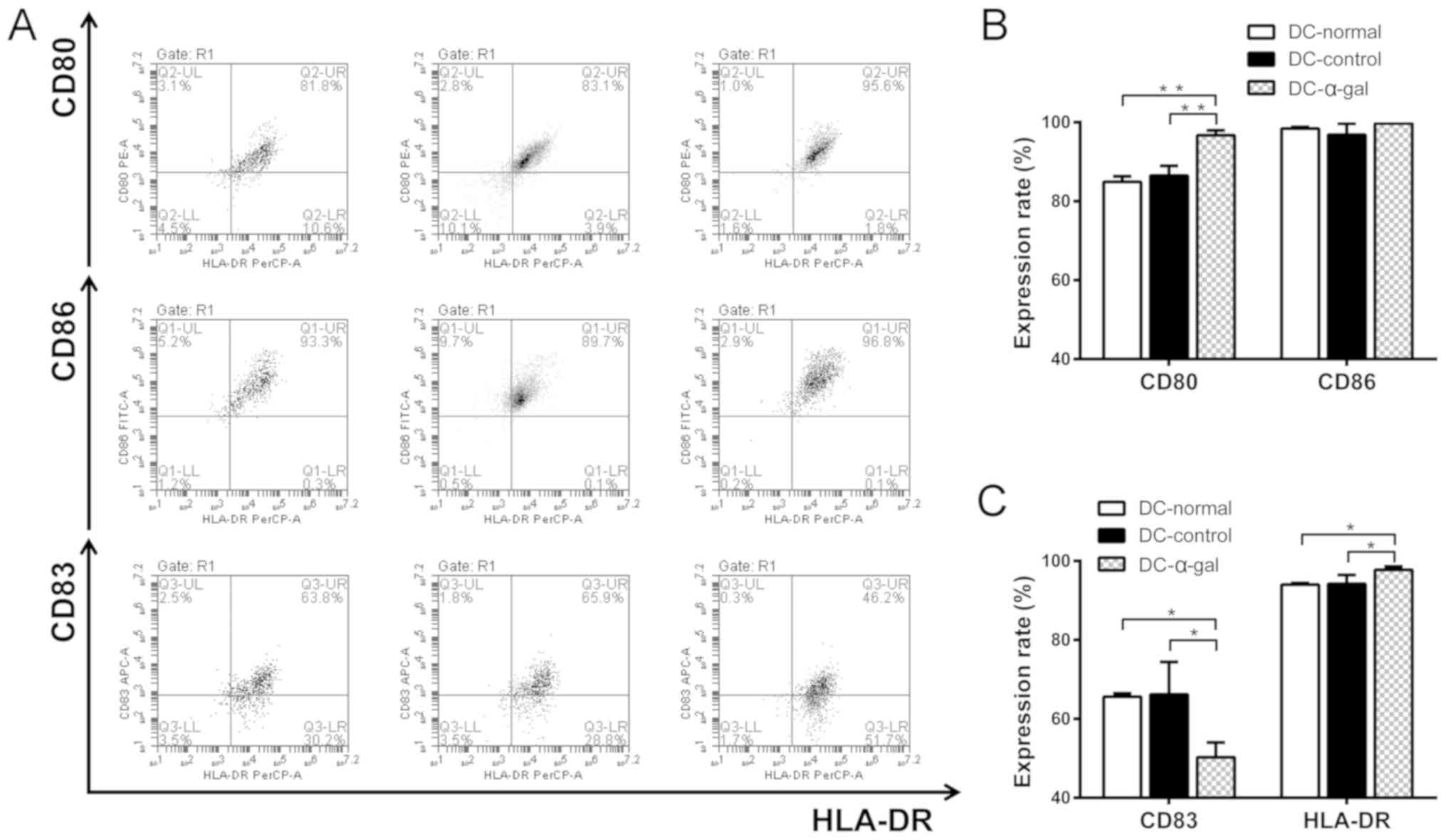
DC-control and DC-α-gal, respectively. The co-stimulatory molecules
CD80 and CD86, and the maturation markers CD83 and HLA-DR were
evaluated. As presented in Fig. 3,
the SW620 cell lysate- and SW620-control cell lysate-treated DCs
exhibited significantly lower expression levels of CD80 and HLA-DR,
and significantly higher expression levels of CD83 compared with
SW620-α-gal cell lysate-treated DCs. No significant difference in
the expression of CD86 was identified between the DCs treated with
the different cell lysates.
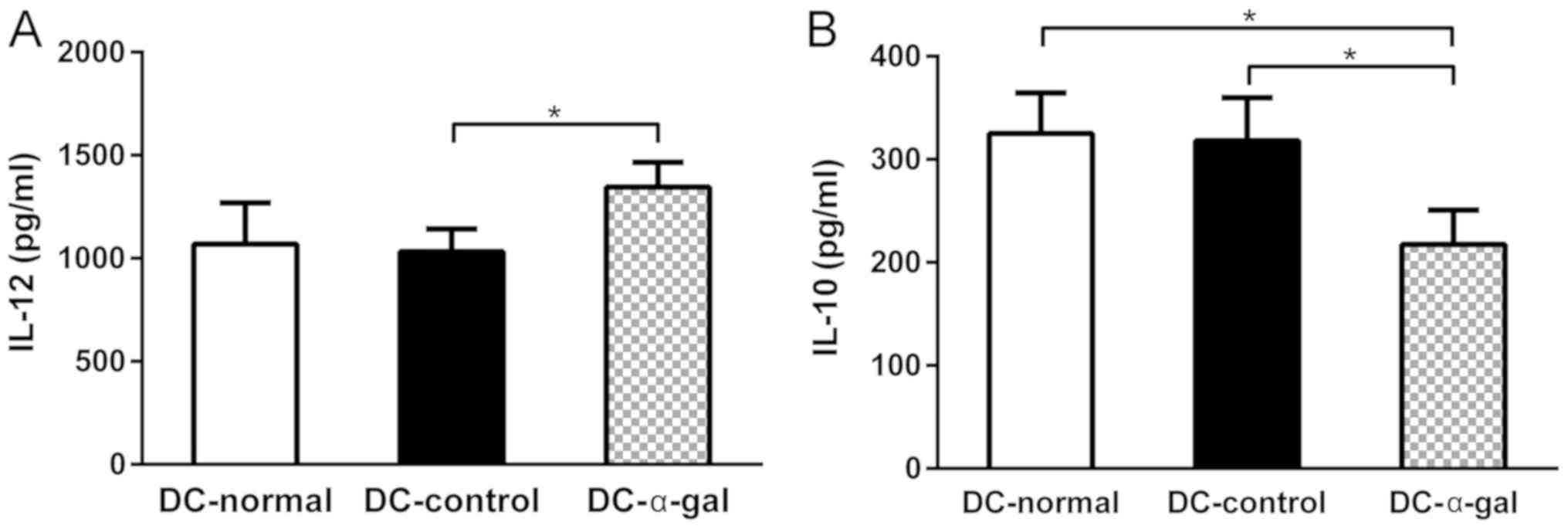
The secretion of inflammatory cytokines is another
important function of DCs. Therefore, the secretion of IL-10 and
IL-12 from DCs was detected using ELISA. The level of IL-12
secreted by DC-α-gal was significantly higher compared with that
secreted by DC-control and higher than that secreted by DC-normal,
although no statistical difference was revealed. The level of IL-10
secreted by DC-α-gal was significantly lower compared with that
secreted by DC-control and DC-normal (Fig. 4). These results demonstrate that
pulsing with α-gal expressing SW620 cell lysate may enhance the
Th1-type cytokine secretion and impair the Th2-type cytokine
secretion of DCs.
Subgroup and cytokine production
analysis of T cells
T cell subgroups and cytokine production were also
detected by flow cytometry and ELISA. The frequencies of T helper
cells (CD3+CD4+), CTLs
(CD3+CD8+), NKT cells
(CD3+CD56+) and regulatory T cells (Tregs;
CD3+CD4+CD25hiCD127lo)
were evaluated. As presented in Fig.
5, the frequency of CD3+CD8+ T cells and
NKT cells was significantly higher among the CTLs activated by
DC-α-gal (CTL-α-gal) compared with the CTLs activated by DC-normal
(CTL-normal) and DC-control (CTL-control). The frequency of
CD3+CD4+ T cells and Tregs was significantly
lower among CTL-α-gal cells. The cytokine production analyses
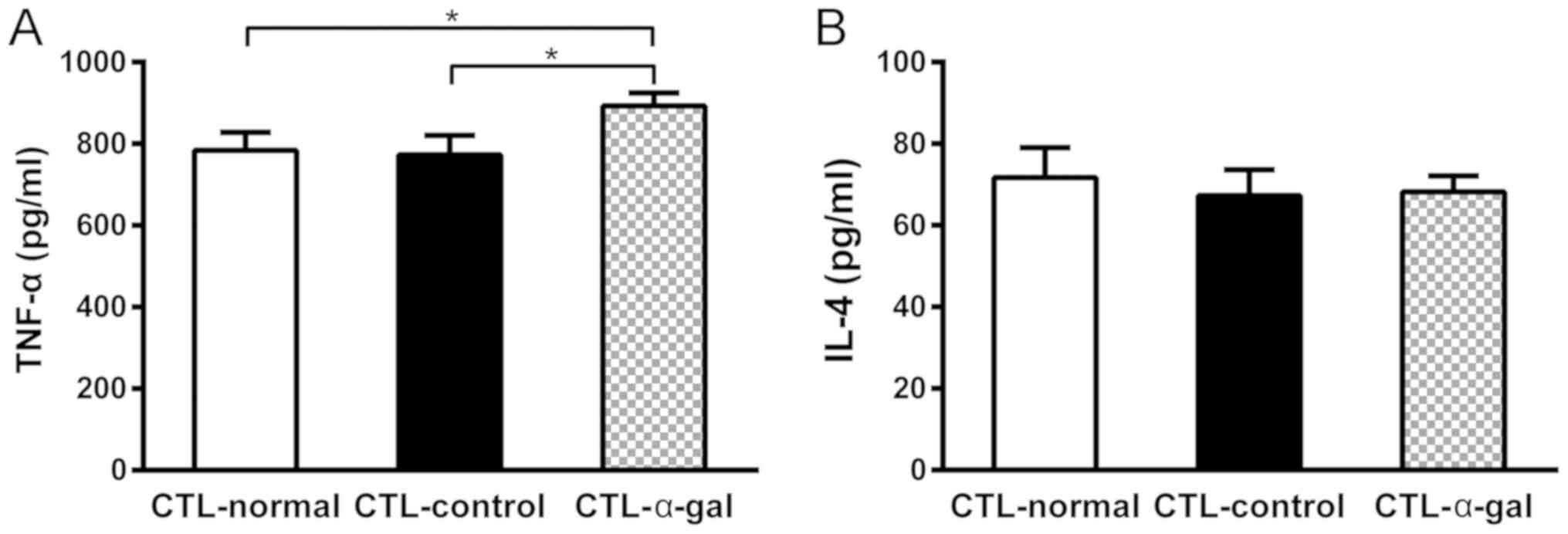
demonstrated that the level of TNF-α secreted by CTL-α-gal cells
was significantly higher compared with that secreted by CTL-normal
and CTL-control cells, whereas the level of IL-4 secreted by
different CTLs was comparable (Fig.
6).
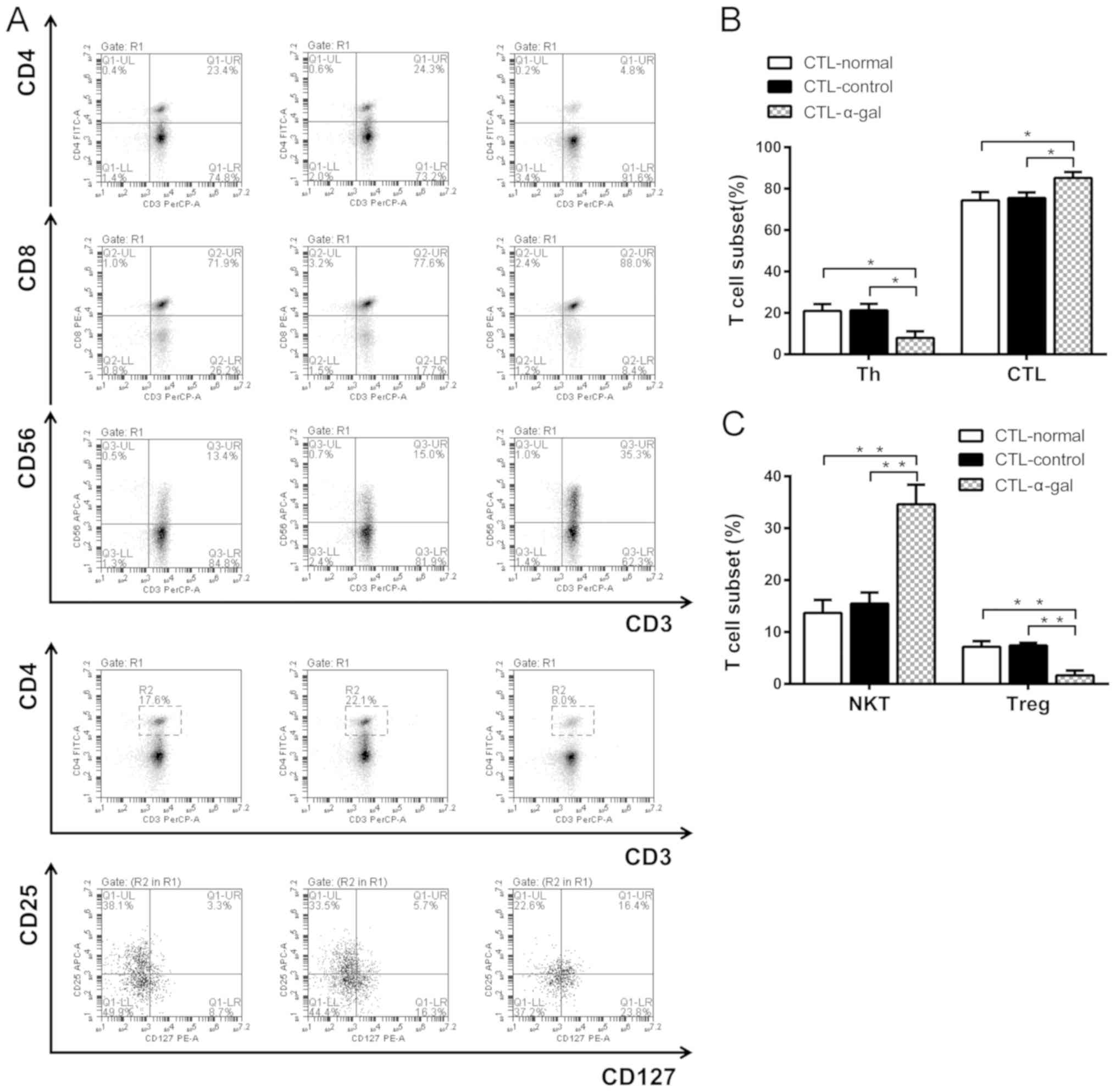 | Figure 5.Frequency of T cell subsets. (A)
Percentage of T cell subsets, including T helper cells
(CD3+CD4+), CTLs
(CD3+CD8+), NKT cells
(CD3+CD56+) and Tregs
(CD3+CD4+CD25hiCD127lo),
was measured using flow cytometry. The results revealed (B) a lower
proportion of CD3+CD4+ T cells, a higher
proportion of CD3+CD8+ CTLs, (C) a lower
proportion of Tregs and a higher proportion of NKT cells among
CTL-α-gal cells compared with CTL-control and CTL-normal cells.
*P<0.05, **P<0.01. CTL, cytotoxic T lymphocyte; NKT, natural
killer T cell; Treg, regulatory T cell. |
Cytotoxic effect of CTLs on the target
cells SW620-normal, SW-620-control and SW620-α-gal
The cytotoxic activity against SW620-normal,
SW-620-control and SW620-α-gal cells was assessed using CTLs
induced by different DCs. The effector target ratio was 10:1. As
presented in Table I, the strongest
killing effect was observed when CTL-α-gal cells were applied to
kill SW620-α-gal cells. At the same effector target ratio, the
killing effect of CTL-α-gal cells on SW620-normal cells was
significantly greater compared with that of CTL-normal and
CTL-control cells. Furthermore, when SW620-control was applied as
the target cells, the killing effect of CTL-control was
significantly greater compared with that of CTL-normal and
CTL-α-gal cells. These results indicate that more effective
tumor-specific CTLs can be induced by DCs pulsed with α-gal
expressing tumor lysate.
 | Table I.CTLs activated by dendritic cells
pulsed with α-gal expressing tumor lysate elicited significant
cytotoxic responses against SW620 and SW620-α-gal cells as
determined by the cytolytic assay. |
Table I.
CTLs activated by dendritic cells
pulsed with α-gal expressing tumor lysate elicited significant
cytotoxic responses against SW620 and SW620-α-gal cells as
determined by the cytolytic assay.
| SW620 cell | CTL-normal | CTL-control | CTL-α-gal |
|---|
| SW620-normal | 46.1±3.52 | 48.4±4.00 |
57.2±5.27a,b |
| SW620-control | 44.6±2.81 |
49.6±1.56a |
42.6±1.75b |
| SW620-α-gal | 46.7±2.33 | 46.8±2.17 |
64.8±2.74a,b |
Discussion
Previous immunotherapy strategies for the treatment
of CRC have demonstrated promising results; however, the clinical
benefits were limited (22–25). There may be several possible reasons
for the insufficient antitumor effect of immune cells. The first
and possibly most important reason is the low immunogenicity of
TAAs, which is insufficient to induce tumor-specific immune
responses (26). The second reason
may be due to the impaired T cell function of patients with CRC.
The tumor-induced suppression of T cell function is recognized as a
main factor that contributes to tumor growth and metastasis
(27,28). The idea to overcome these problems is
to increase the immunogenicity of TAAs and use these highly
immunogenic TAAs to pulse DCs, which would subsequently activate
tumor-specific CTLs with strong cytotoxicity. The approach
described in the present study fulfills the aforementioned
requirements. First, α-gal epitopes were synthesized on the surface
of tumor cells to increase the immunogenicity of TAAs.
Subsequently, the adhesive cells from PBMCs were induced to
differentiate into DCs, which were pulsed with α-gal expressing
tumor lysate. Finally, those DCs were co-cultured with naïve T
cells to produce specific CTLs.
In the present study, α-gal epitopes were
synthesized on the surface of tumor cells to increase tumor
antigenicity. Using this approach, α-gal expressing tumor lysate
could contain foreign antigen α-gal and TAAs. The α-Gal antibodies
bind to α-Gal epitopes to form immune complexes and the recognition
of immune complexes by activating Fcγ receptors expressed on DCs
results in the engulfment of the complexes by endocytosis and the
maturation of DCs. This maturation includes upregulation of the
expression of the co-stimulatory molecule CD80 and the MHC class II
molecule HLA-DR (29); therefore,
the presentation of TAAs was increased. Induction of a
CD8+ effector T cell response by DCs requires the
following three signals: MHC class II molecules, including HLA-DR,
co-stimulatory molecules, including CD80, and signal
pro-inflammatory cytokines, including IL-12 (30). The α-Gal epitopes increased CD80 and
HLA-DR expression of DCs; therefore, two signals may be more
effectively activated and more CD8+ effector T cells may
be induced. TNF-α was predominantly secreted by CD8+ T
cells and a higher frequency of CD8+ T cells led to a
higher level of TNF-α. The results of the present study
demonstrated that CTLs activated by DCs pulsed with α-gal
expressing tumor lysate elicit significant cytotoxic responses
against SW620 and SW620-α-gal cells, as determined by a cytolytic
assay.
There are several possible reasons for this stronger
cytotoxicity. The level of TNF-α secreted by CTL-α-gal cells was
higher compared with that secreted by CTL-normal cells. TNF-α can
bind to tumor necrosis factor receptor 1 to directly induce tumor
cells apoptosis (31); therefore, a
higher level of TNF-α can decrease tumor proliferation
significantly. Additionally, the frequency of CD8+ CTLs
among the T cells activated by DCs pulsed with α-gal expressing
tumor lysates was higher compared with that among the T cells
activated by DCs pulsed with normal tumor lysates. CD8+
CTLs can recognize tumor cells by an interaction between their
T-cell receptor and the MHC class I peptide complex on the surface
of tumor cells, and can eventually kill target cells by the
delivery of toxic granule contents that induce apoptosis of tumor
cells to which they attach (32,33).
Furthermore, the frequency of Tregs among the T cells activated by
DCs pulsed with α-gal expressing tumor lysates was lower compared
with that among T cells activated by DCs pulsed with normal tumor
lysate. Tregs are potent immunosuppressive cells that can impair
the function of CD8+ CTLs (34,35). A
lower frequency of Tregs indicates a lower immunosuppressive
effect, which can contribute to the cytotoxicity of CD8+
CTLs. It has been reported that IL-12 can paralyze Treg activity
and inhibit their proliferation (36). The present study indicated that the
level of IL-12 secreted by DCs pulsed with α-gal expressing tumor
lysate was higher; when T cells are co-cultured with those DCs,
IL-12 could inhibit the proliferation of Tregs. This may explain
the lower frequency of Tregs among T cells. NKT cells comprise a
heterogeneous lymphoid population that exhibit the characteristics
of both the innate and adaptive immune system. NKT cells can
directly kill tumor cells or indirectly combat cancer via the
activation of additional immune cells, thus enhancing tumor
immunity (37–39). A higher frequency of NKT cells could
be another reason for the significant cytotoxic response observed
in the present study.
Although recent studies have reported that α-gal
epitopes can improve immunogenicity in several types of cancer, the
efficacy of α-gal epitopes remains controversial (40,41). The
application of α-gal epitopes to induce a stronger immune response
in CRC required further investigation. The present study provides
detailed information for the clinical application of α-gal epitopes
in CRC immunotherapy. However, all the experiments conducted in the
present study were in vitro. Although the results are
promising, verification of these in future in vivo studies
is required. In addition, the signaling mechanisms underlying the
enhanced antitumor effect caused by α-gal epitopes have not been
fully elucidated and require further research.
In summary, the new therapeutic immunotherapy
approach evaluated in the present study was demonstrated to
significantly increase the cytotoxic responses of CTLs against CRC
cells in vitro. The mechanism was further clarified by
assessing different T cell subgroups. An increased level of TNF-α,
higher frequencies of CD8+ CTLs and NKT cells, and a
decreased frequency of Tregs may contribute to the enhanced
cytotoxicity of the CTLs. Further clinical studies are required to
test this approach in patients with CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XD designed the experiments. XX and ZZ interpreted
the data and wrote the manuscript. CH, ZH and YW performed data
analysis. KL and WL performed the cellular and biochemical
experiments. KL and WL also critically revised the manuscript. All
authors contributed to discussions regarding the results and the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Chinese People's Liberation Army General Hospital
(Beijing, China). All procedures were performed in accordance with
the Ethical Standards of the Institutional and/or National Research
Committee. Written informed consent was obtained from all
volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ACT
|
adoptive cellular therapy
|
|
DC
|
dendritic cell
|
|
CTL
|
cytotoxic T lymphocyte
|
|
TAA
|
tumor-associated antigen
|
|
NKT
|
natural killer T cell
|
|
Treg
|
regulatory T cell
|
|
HLA
|
human leukocyte antigen
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
References
|
1
|
Adam R and Vinet E: Regional treatment of
metastasis: Surgery of colorectal liver metastases. Ann Oncol. 15
(Suppl 4):iv103–iv106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramos A and Hemann MT: Drugs, bugs, and
cancer: Fusobacterium nucleatum promotes chemoresistance in
colorectal cancer. Cell. 170:411–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan K, Wale A, Brown G and Chau I:
Colorectal cancer with liver metastases: Neoadjuvant chemotherapy,
surgical resection first or palliation alone? World J
Gastroenterol. 20:12391–12406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castro JE and Kipps TJ: Adoptive cellular
therapy for chronic lymphocytic leukemia and B cell malignancies.
CARs and more. Best Pract Res Clin Haematol. 29:15–29. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schubert ML, Hückelhoven A, Hoffmann JM,
Schmitt A, Wuchter P, Sellner L, Hofmann S, Ho AD, Dreger P and
Schmitt M: Chimeric antigen receptor T cell therapy targeting
CD19-positive leukemia andlymphoma in the context of stem cell
transplantation. Hum Gene Ther. 27:758–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reissfelder C, Stamova S, Gossmann C,
Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M,
Saadati M, et al: Tumor-specific cytotoxic T lymphocyte activity
determines colorectal cancer patient prognosis. J Clin Invest.
125:739–751. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu H, Yang X, Li J, Ren Y, Zhang T, Zhang
C, Zhang J, Li J and Pang Y: Immune response, safety, and survival
and quality of life outcomes for advanced colorectal cancer
patients treated with dendritic cell vaccine and cytokine-induced
killer cell therapy. Biomed Res Int. 2014:6038712014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chou J, Voong LN, Mortales CL, Towlerton
AM, Pollack SM, Chen X, Yee C, Robbins PF and Warren EH: Epigenetic
modulation to enable antigen-specific T-cell therapy of colorectal
cancer. J Immunother. 35:131–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao D, Li C, Xie X, Zhao P, Wei X, Sun W,
Liu HC, Alexandrou AT, Jones J, Zhao R and Li JJ: Autologous tumor
lysate-pulsed dendritic cell immunotherapy with cytokine-induced
killer cells improves survival in gastric and colorectal cancer
patients. PLoS One. 9:e938862014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baratin M, Kayibanda M, Ziol M, Romieu R,
Briand JP, Guiller JG and Viguier M: Amino acid modifications in
the wild type sequence p53 232–240 overcome the poor immunogenicity
of this self tumour epitope. J Pept Sci. 8:327–334. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalinski P: Dendritic cells in
immunotherapy of established cancer: Roles of signals 1, 2, 3 and
4. Curr Opin Investig Drugs. 10:526–535. 2009.PubMed/NCBI
|
|
12
|
Iorgulescu JB, Braun D, Oliveira G, Keskin
DB and Wu CJ: Acquired mechanisms of immune escape in cancer
following immunotherapy. Genome Med. 10:872018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galili U: Autologous tumor vaccines
processed to express alpha-gal epitopes: A practical approach to
immunotherapy in cancer. Cancer Immunol Immunother. 53:935–945.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galili U, Clark MR, Shohet SB, Buehler J
and Macher BA: Evolutionary relationship between the natural
anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates.
Proc Natl Acad Sci USA. 84:1369–1373. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galili U: Anti-Gal: An abundant human
natural antibody of multiple pathogeneses and clinical benefits.
Immunology. 140:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdel-Motal UM, Wigglesworth K and Galili
U: Mechanism for increased immunogenicity of vaccines that form in
vivo immune complexes with the natural anti-Gal antibody. Vaccine.
27:3072–3082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huai G, Qi P, Yang H and Wang Y:
Characteristics of α-Gal epitope, anti-Gal antibody, α1,3
galactosyltransferase and its clinical exploitation (Review). Int J
Mol Med. 37:11–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galili U: Evolution of alpha
1,3galactosyltransferase and of the alpha-Gal epitope. Subcell
Biochem. 32:1–23. 1999.PubMed/NCBI
|
|
19
|
Yao X, Dong Z, Zhang Q, Wang Q and Lai D:
Epithelial ovarian cancer stem-like cells expressing α-gal epitopes
increase the immunogenicity of tumor associated antigens. BMC
Cancer. 15:9562015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Y, Li S, Jia T, Du X, Xu Y, Zhao Y, Li
L, Liang K, Liang W, Sun H and Li R: Combined therapy with CTL
cells and oncolytic adenovirus expressing IL-15-induced enhanced
antitumor activity. Tumour Biol. 36:4535–4543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carluccio S, Delbue S, Signorini L, Setola
E, Bagliani A, Della Valle A, Galli A, Ferrante P and Bregni M:
Generation of tumor-specific cytotoxic T-lymphocytes from the
peripheral blood of colorectal cancer patients for adoptive T-cell
transfer. J Cell Physiol. 230:1457–1465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunyadi J, András C, Szabó I, Szántó J,
Szluha K, Sipka S, Kovács P, Kiss A, Szegedi G, Altorjay I, et al:
Autologous dendritic cell based adoptive immunotherapy of patients
with colorectal cancer-A phase I–II study. Pathol Oncol Res.
20:357–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida Y, Naito M, Yamada T, Aisu N,
Daibo K, Mera T, Tanaka T, Naito K, Yasumoto K, Kamigaki T, et al:
Adoptive chemoimmunotherapy using activated αβ T cells for stage IV
colorectal cancer. Anticancer Res. 36:3741–3746. 2016.PubMed/NCBI
|
|
25
|
Yoshida Y, Naito M, Yamada T, Aisu N,
Kojima D, Mera T, Tanaka T, Naito K, Yasumoto K, Kamigaki T, et al:
Clinical study on the medical value of combination therapy
involving adoptive immunotherapy and chemotherapy for stage IV
colorectal cancer (COMVI study). Anticancer Res. 37:3941–3946.
2017.PubMed/NCBI
|
|
26
|
Wang GZ, Tang XD, Lü MH, Gao JH, Liang GP,
Li N, Li CZ, Wu YY, Chen L, Cao YL, et al: Multiple antigenic
peptides of human heparanase elicit a much more potent immune
response against tumors. Cancer Prev Res (Phila). 4:1285–1295.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimizu H, Ito H, Kimura F, Togawa A,
Yoshidome H, Ohtsuka M, Kato A, Nukui Y and Miyazaki M: Decreased
cell-mediated immune status in colorectal cancer patients with
hepatic metastasis. Hepatogastroenterology. 52:1106–1109.
2005.PubMed/NCBI
|
|
28
|
Kwak Y, Koh J, Kim DW, Kang SB, Kim WH and
Lee HS: Immunoscore encompassing CD3+ and CD8+ T cell densities in
distant metastasis is a robust prognostic marker for advanced
colorectal cancer. Oncotarget. 7:81778–81790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho NI, Huis In't Veld LGM, Raaijmakers TK
and Adema GJ: Adjuvants enhancing cross-presentation by dendritic
cells: The key to more effective vaccines? Front Immunol.
9:28742018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ugur M and Mueller SN: T cell and
dendritic cell interactions in lymphoid organs: More than just
being in the right place at the right time. Immunol Rev.
289:115–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu WM: Tumor necrosis factor. Cancer
Lett. 328:222–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Foged C, Hansen J and Agger EM: License to
kill: Formulation requirements for optimal priming of CD8(+) CTL
responses with particulate vaccine delivery systems. Eur J Pharm
Sci. 45:482–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gagnon SJ, Wang Z, Turner R, Damirjian M
and Biddison WE: MHC recognition by hapten-specific
HLA-A2-restricted CD8+ CTL. J Immunol. 171:2233–2241. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guzmán-Flores JM and Portales-Pérez DP:
Mechanisms of suppression of regulatory T-cells (Treg). Gac Med
Mex. 149:630–638. 2013.(In Spanish). PubMed/NCBI
|
|
35
|
Schmidt A, Oberle N and Krammer PH:
Molecular mechanisms of treg-mediated T cell suppression. Front
Immunol. 3:512012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian Y, Yuan C, Ma D, Zhang Y, Liu Y,
Zhang W, Hou F and Cui B: IL-21 and IL-12 inhibit differentiation
of Treg and TH17 cells and enhance cytotoxicity of peripheral blood
mononuclear cells in patients with cervical cancer. Int J Gynecol
Cancer. 21:1672–1678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujii S, Shimizu K, Okamoto Y, Kunii N,
Nakayama T, Motohashi S and Taniguchi M: NKT cells as an ideal
anti-tumor immunotherapeutic. Front Immunol. 4:4092013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robertson FC, Berzofsky JA and Terabe M:
NKT cell networks in the regulation of tumor immunity. Front
Immunol. 5:5432014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taniguchi M, Harada M, Dashtsoodol N and
Kojo S: Discovery of NKT cells and development of NKT cell-targeted
anti-tumor immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci.
91:292–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Furukawa K, Tanemura M, Miyoshi E, Eguchi
H, Nagano H, Matsunami K, Nagaoka S, Yamada D, Asaoka T, Noda T, et
al: A practical approach to pancreatic cancer immunotherapy using
resected tumor lysate vaccines processed to express α-gal epitopes.
PLoS One. 12:e01849012017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xue D, Liang Y, Duan S, He J, Su J, Zhu J,
Hu N, Liu J, Zhao Y and Lu X: Enhanced anti-tumor immunity against
breast cancer induced by whole tumor cell vaccines genetically
modified expressing α-Gal epitopes. Oncol Rep. 36:2843–2851. 2016.
View Article : Google Scholar : PubMed/NCBI
|