Introduction
Astrocytoma is reportedly one of the most
vascularized types of tumor, and angiogenesis is important for the
growth and metastasis of astrocytomas (1). Anti-angiotherapy for endothelial cells
has been used successfully in ~50% of patients with astrocytoma;
however as a result of resistance to current treatments (2–5), it is
necessary to establish novel therapeutic targets.
Vasculogenic mimicry (VM) is a novel pattern of
neovascularization that differs from the classical tumor
angiogenesis pathway, and does not depend on endothelial cells
(6). A number of studies have
reported that VM exists in melanoma, inflammatory breast cancer,
colorectal cancer, liver cancer, prostate cancer and other
malignant tumors (7,8). VM is not only associated with tumor
growth and metastasis, but also with the prognosis of disease
(9), suggesting a poor prognosis for
patients with astrocytoma. A previous study confirmed that VM was
involved in the angiogenesis of astrocytomas and associated with
tumor differentiation (10).
In recent years, a number of studies have
demonstrated that twist family bHLH transcription factor 1 (Twist1)
is highly expressed in various types of cancer, including bladder,
gastric, nasopharyngeal and prostate carcinoma, in addition to
breast cancer and synovial sarcoma. The role of Twist1 in the
development of cancer involves a variety of mechanisms, including
the regulation of tumor cell growth, differentiation, inhibition of
apoptosis, increased resistance to chemotherapy and the promotion
of tumor angiogenesis (11–15). It has been demonstrated that Twist1
downregulates the expression level of epithelial E-cadherin and
upregulates that of vascular endothelial (VE)-cadherin and matrix
metalloproteinase (MMP)-9/2 to promote VM formation in hepatoma
cells (16–19). However, the number of studies
surrounding VM formation in astrocytomas remains limited.
The role of Twist1 in astrocytomas was examined in
the present study and the association between Twist1 and VM was
evaluated. It was concluded that Twist1 may induce the progression
of astrocytomas by stimulating VM.
Materials and methods
Samples
A total of 108 cases of human astrocytoma were
selected from the Surgical Department in Shengjing Hospital, China
Medical University between June 2007 and July 2013. These cases
included 57 males and 51 females, with a mean age of 48 years
(range, 24–76 years). According to the 2007 World Health
Organization (WHO) histological classification criteria (20), 31 cases were diffuse astrocytoma
(WHOII), 47 cases were anaplastic astrocytoma (WHOIII grade), and
30 cases were WHOIV grade glioblastoma. None of the patients were
given chemo- or radiotherapy prior to surgery, nor did they receive
postoperative routine radiotherapy or chemotherapy. All specimens
were fixed using 4% neutral formaldehyde at 4°C, overnight,
embedded in paraffin, sectioned at 5 µm and routinely stained with
hematoxylin and eosin for diagnosis at room temperature. The
specific steps are as follows: The slices were placed in an oven at
60°C for 2 h and then stained. After dewaxing to water, the
hematoxylin dye solution was dyed for 2–3 min, washed with tap
water, washed with 1% hydrochloric acid alcohol solution for
several seconds, washed with tap water, dyed with eosin dye
solution for 1 min, washed with tap water, and sealed with neutral
gum. The samples were examined by light microscopy (magnification,
×100 and ×400; Leica DM2500M; Leica Microsystems GmbH) in
five-randomly selected visual fields. The protocols were approved
by the Ethics Committee of General Hospital of Shengjing Hospital
(China Medical University), and all patients signed informed
consent prior to surgery.
Immunohistochemistry
The specimens were fixed in a 4% neutral formalin
solution at 4°C, overnight, embedded in paraffin and cut into 5 µm
slices. The paraffin sections were subsequently dewaxed in water,
and incubated with 3% H2O2 for 10 min at room
temperature. The sections were incubated with 3% goat serum (cat.
no. C0265; Beyotime Institude of Biotechnology, Shanghai, China) at
room temperature for 10 min, and subsequently incubated with the
following primary antibody at 4°C overnight: Primary antibodies
against Twist1 (cat. no. ab50887; 1:1,000), VE-cadherin (cat. no.
ab33168; 1:800) and MMP-9 (cat. no. ab38898; 1:1,000) purchased
from Fuzhou Maxin Biological Technology Development Co., Ltd.
Sections were incubated with streptavidin-horseradish peroxidase
(HRP) kit (Fuzhou Maxin Biological Technology Development Co.,
Ltd.) for 10 min, stained with 3,3′-diaminobenzidine (DAB; cat.
no., kit-0017; Fuzhou Maxin Biological Technology Development Co.,
Ltd.), according to the manufacturer's protocols and then
counter-stained with hematoxylin for 2–3 min at room temperature
and sealed with neutral gum. The samples were examined by light
microscopy (magnification, ×400; Leica DM2500M; Leica Microsystems
GmbH) in five-randomly selected visual fields. Immunohistochemical
results were assessed using histological score (HSCORE) (21). The intensity of staining was graded
according to the following criteria: 0, no staining; 1 weak
staining (light yellow); 2, moderate staining (yellow-brown); 3,
strong staining (brown). The staining index (SI) was calculated as
the product of staining intensity score and proportion of positive
tumor cells. This study assessed the indicated protein expression
in IHC-stained tumor sections determined by SI scores as 0, 1, 2,
3, 4, 6, and 9.
VM assessment
Immunohistochemical staining was carried out against
CD34 (ab54208, 1:1,000, Abcam) according to the method (22). Periodic Acid Schiff (PAS) (cat. no.
C0730; 1:1,000; Tianjin Bai Hao Biological Technology Co.) staining
was performed on the paraffin-embedded samples for 15 min at room
temperature, following incubation with 3%
H2O2 for 10 min at room temperature.
Double-stained tissue sections were observed with light microscopy
(magnification, ×100; Leica DM2500M; Leica Microsystems GmbH), in
five-randomly selected visual fields, and
PAS+/CD34− cells were identified as VM
vessels.
Cell culture
U251 and SHG44 cells (National Infrastructure of
Cell Line Resource) were cultured in Dulbecco's Modified Eagle's
medium (DMEM) and RPMI-1640 medium containing 10% FBS (all
Invitrogen; Thermo Fisher Scientific, Inc.), respectively; HUVECs
(National Infrastructure of Cell Line Resource) were cultured in
RPMI-1640 medium containing 15% FBS and Endothelial Cell Growth
Supplement (Invitrogen; Thermo Fisher Scientific, Inc.). All cells
were cultured at 37°C with 5% CO2.
Three-dimensional (3D) cell culture
and the formation of an in vitro cavity structure
A total of 50 µl Matrigel was plated into each well
of a 96-well plate. After incubation for 2 h at 37°C,
5×104 cells were added into each well with 100 µl of the
appropriate medium. After 48 h of culture, five fields were
visualized using an inverted phase contrast microscope
(magnification, ×100). The formation of cell tubules was observed,
and images were captured and counted.
Transfection
The transfection was carried out by
Lipofectamine® 2000 (Beyotime Institute of
Biotechnology, Shanghai, China). Lipofectamine® 2000
were mixed with small interfering (si)RNA or plasmids at room
temperature for 20 min, added into cells and transfected for 24 h.
Si-Twist1 and the corresponding negative control RNA were purchased
from Wuhan Crystal Bio Technology Co., Ltd. The RNA interference
sequences were as follows: Si-Twist1-1,
5′-CCUGAGCAACAGCGAGGAATT-3′; si-Twist1-2,
5′-GCAAGAUUCAGACCCUCAATT-3′; si-Twist1-3′
5′-GAUGGCAAGCUGCAGCUAUTT-3′; control′ 5′-UUCUCCGAACGUGUCACGUTT-3′.
A total of 5 µg small interfering RNA or corresponding negative
control RNA was transfected into U251 and SHG44 cells. The control
group of si-Twist1 was transfected with corresponding negative
control RNA (mock group).
A total of 18 µg pcDNA3.1-Twist (Invitrogen; Thermo
Fisher Scientific, Inc.) was also transfected into U251 and SHG44
cells. The control group of pcDNA3.1-Twist was transfected with
empty vector alone (vec group). Untreated cells were also used as a
control in these experiments (con group). Follow-up studies were
done 48 h after transfection.
Western blotting
Total protein was extracted from cells by RIPA
(Beyotime Institute of Biotechnology). Bicinchoninic acid assay kit
(cat. no. 71285-3, Merck KGaA) was used to quantify protein
concentration, according to the manufacturer's protocols. A total
of 40 µg of each protein sample was separated using 10% SDS-PAGE
and transferred to a polyvinylidene difluoride membrane (70V, 1.5
h). After incubation in 5% skimmed milk for 2 h at room temperature
the membranes were incubated with the following primary antibodies,
purchased from Abcam: Anti-Twist1 (cat. no. ab50581, 1:1,000)
anti-VE-cadherin (cat. no. ab119785, 1:1,000) anti-MMP9 (cat. no.
ab73734, 1:1,000) β actin (cat. no. ab8227, 1:1,000) overnight at
4°C. The membranes were subsequently incubated with secondary
antibodies also purchased from Abcam [Goat anti-rabbit IgG (HRP,
ab7090, 1:1,000) and Goat anti-mouse IgG (HRP, ab6789, 1:1,000)]
for 2 h at room temperature. After washing three times with TBS
Tween-20, protein bands were visualized with enhanced
chemiluminescence reagents (Beyotime Institute of Biotechnology).
β-actin was used as an internal control. Densitometry analysis used
Image J 18.0 (National Institutes of Health).
Statistical analysis
All experiments were repeated at least three times.
The data were processed by SPSS for Windows 17 statistical analysis
software (SPSS, Inc., Chicago, IL, USA). Statistical comparisons
were made using one-way analysis of variance and Least Significant
Difference post hoc test, and associations between Twist1
expression and clinicopathological features, VM formation and the
expression of other related proteins were assessed using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of Twist1 in astrocytoma
tissues
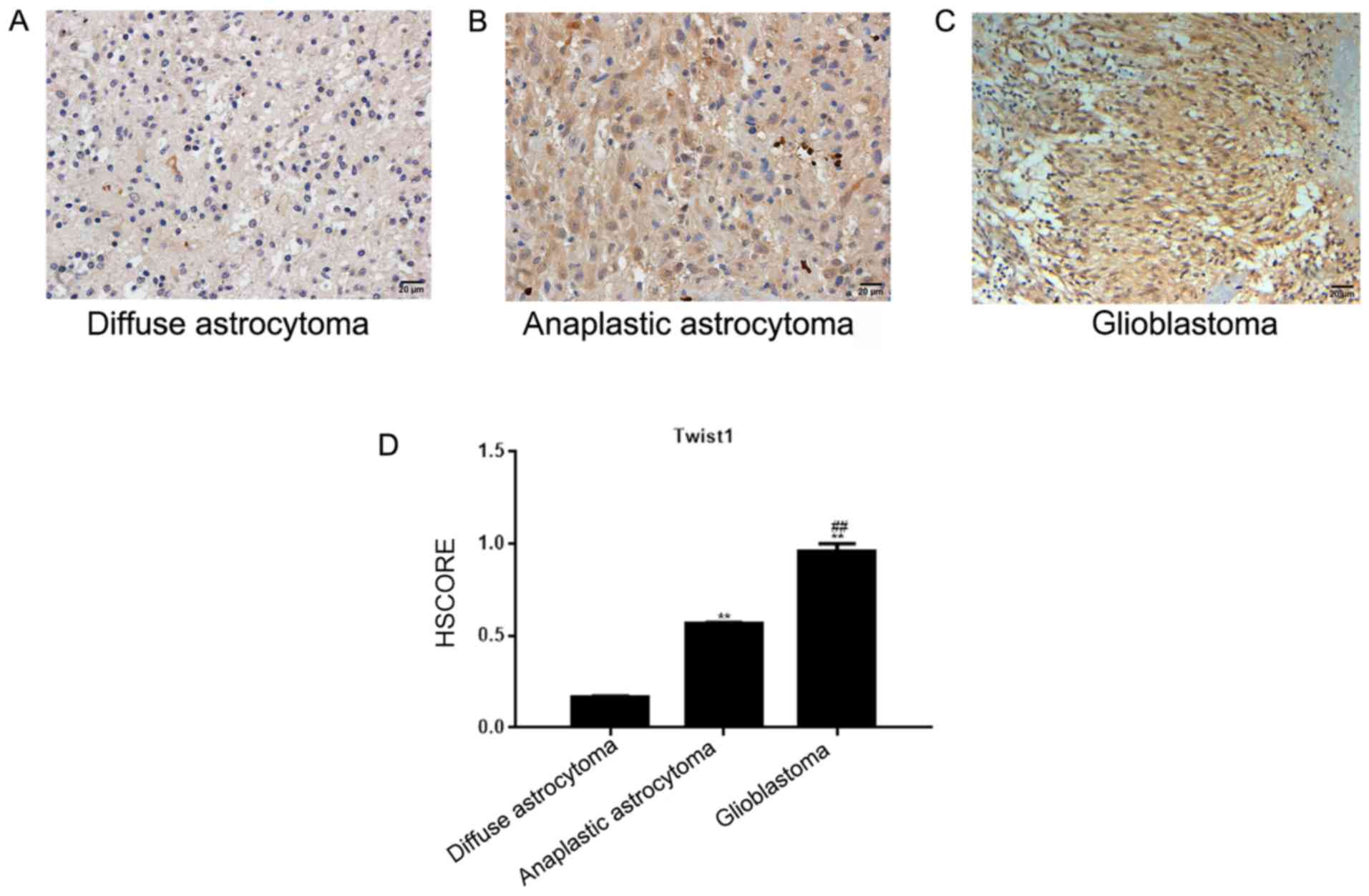
In diffuse and anaplastic astrocytoma tissues,
Twist1 was located in the nucleus, and to a lesser degree in the
cytoplasm. The number of positively-stained cells was 16.13% in
diffuse, and 57.45% in anaplastic astrocytoma tissues (Fig. 1A and B). In the glioblastoma tissue,
Twist1 was located in the nucleus and cytoplasm, and demonstrated
medium-strong positive expression (the positive rate was 93.33%;
Fig. 1C). The HSCORE is presented in
Fig. 1D. Compared with the diffuse
and anaplastic astrocytoma tissues, Twist1 was highly expressed in
glioblastoma tissues, and the expression level of Twist1 was
significantly associated with the WHO astrocytoma classification
(P<0.05), but not with the sex or age of the patients
(P>0.05; Table I).
 | Table I.Association between Twist1 expression
and clinicopathological features in patients with astrocytoma. |
Table I.
Association between Twist1 expression
and clinicopathological features in patients with astrocytoma.
|
| Twist1
expression |
|
|
|---|
|
|
|
|
|
|---|
| Patient
characteristic | + | − |
χ2-value | P-value |
|---|
| II | 5 | 26 | 36.924 |
>0.001a |
|
III | 27 | 20 |
|
|
| IV | 28 | 2 |
|
|
| Sex |
|
|
|
|
|
Male | 31 | 26 | 0.067 | 0.796 |
|
Female | 29 | 22 |
|
|
| Age |
|
|
|
|
|
>48 | 33 | 21 | 1.350 | 0.246 |
|
≤48 | 27 | 27 |
|
|
Association between the expression of
Twist1 and the formation of VM
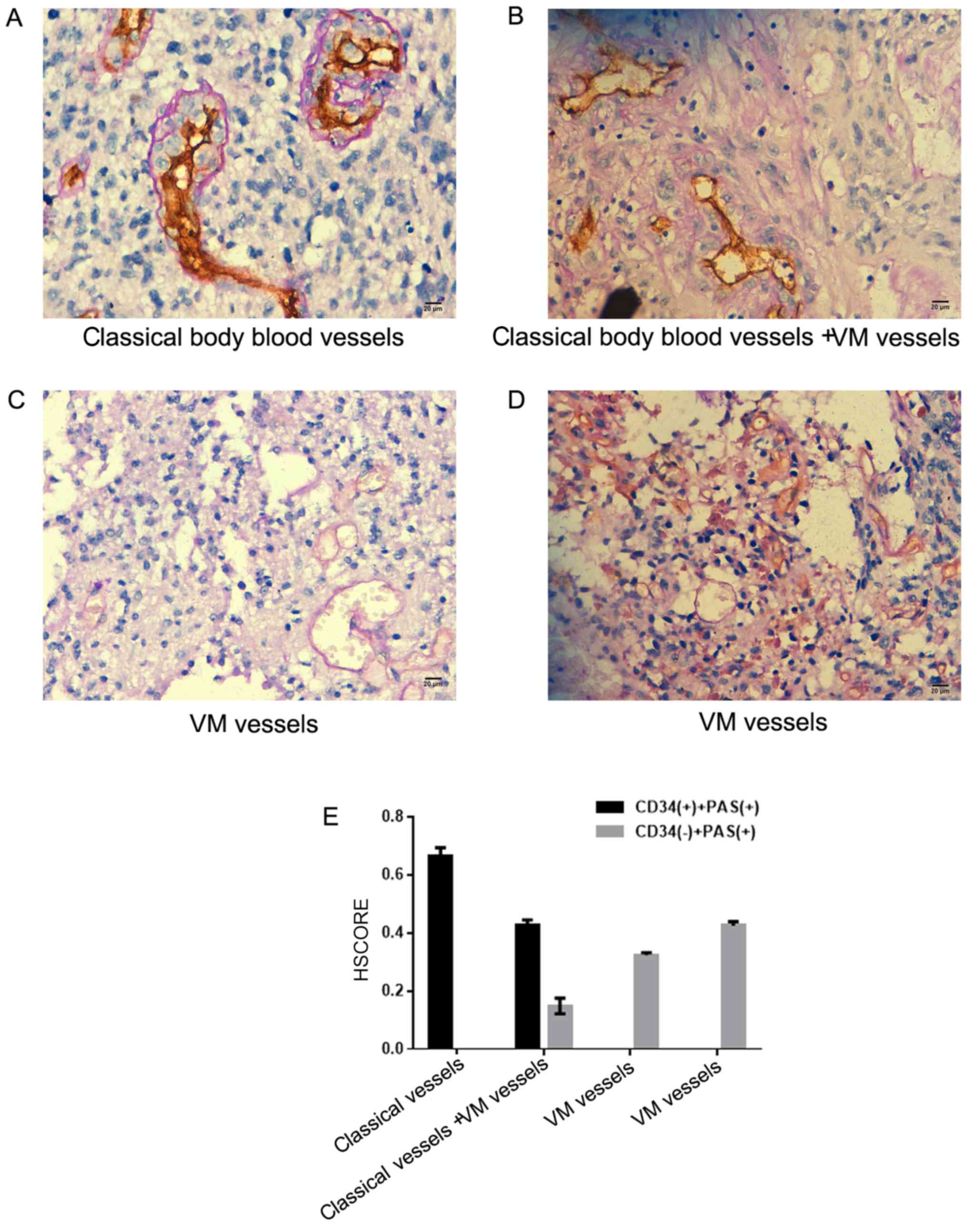
By observing the CD34/PAS double staining of
astrocytoma samples, it was demonstrated that most of the vascular
structures in the tumor tissues were typical. The blood vessels
comprised endothelial cells (CD34+) and basement
membranes (PAS+; Fig. 2A and
B). In certain tissues, the VM was composed of CD34−
cells and the basement membrane (PAS+; Fig. 2B-D). The HSCORE is presented in
Fig. 2E. It was demonstrated that
out of 108 astrocytoma tissues, 16 cases exhibited VM (11 in
glioblastoma and 5 in astrocytoma). Twist1 expression in
astrocytoma was significantly associated with VM formation
(Table II).
 | Table II.Association between Twist1 expression
and the incidence of VM in astrocytomas. |
Table II.
Association between Twist1 expression
and the incidence of VM in astrocytomas.
|
| Twist1 expression
(n) |
|
|
|---|
|
|
|
|
|
|---|
| VM | + | − |
χ2-value | P-value |
|---|
| + | 15 | 1 | 11.097 | 0.001a |
| − | 45 | 47 |
|
|
Expression of VE-cadherin and MMP-9 in
astrocytomas
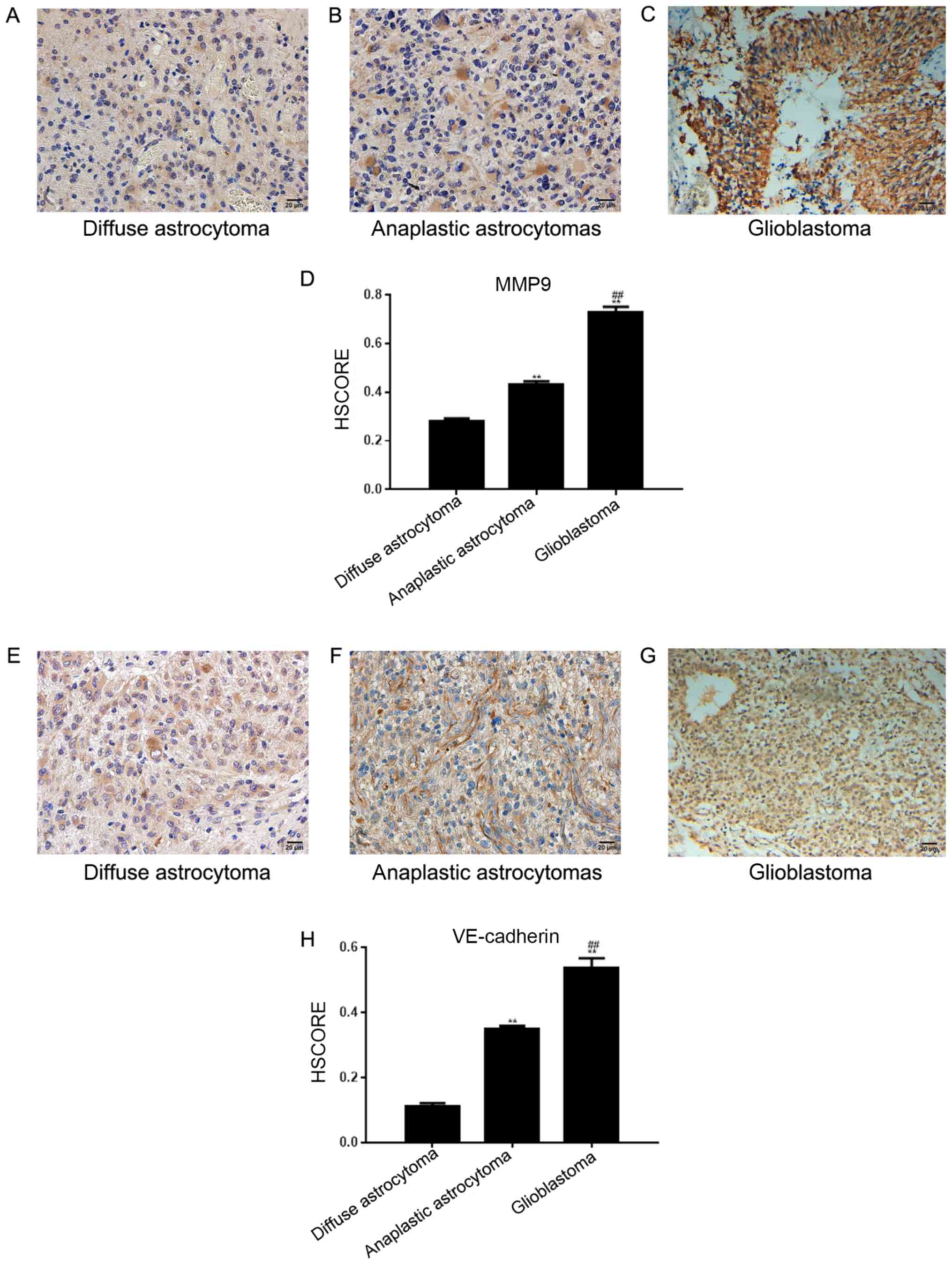
In 108 astrocytoma tissues across the 3 different
tissue groups, the positive expression rate of MMP-9 was 67.59% (73
cases; Fig. 3A-C) and the positive
rate of VE-cadherin expression was 39.81% (43 cases; Fig. 3E-G). The HSCORE is presented in
Fig. 3D and H. The two proteins were
detected in the cytoplasm of tumor cells; VE-cadherin was expressed
in vascular endothelial cells. The expression of Twist1 in
astrocytomas was associated with that of MMP-9 and VE-cadherin
(Table III). Furthermore, the
formation of VM was related to that of VE-cadherin and MMP-9
(Table IV).
 | Table III.Association between Twist1 expression
and MMP-9/VE-cadherin. |
Table III.
Association between Twist1 expression
and MMP-9/VE-cadherin.
|
| Twist1
expression |
|
|
|---|
|
|
|
|
|
|---|
| Protein | + | − |
χ2-value | P-value |
|---|
| MMP-9 |
|
|
|
|
| + | 46 | 27 |
5.075 | 0.024a |
| − | 14 | 21 |
|
|
| VE-cadherin |
|
|
|
|
| + | 34 | 9 | 15.999 |
6.337×10−05a |
| − | 26 | 39 |
|
|
 | Table IV.Association between VM and
MMP-9/VE-cadherin in astrocytoma. |
Table IV.
Association between VM and
MMP-9/VE-cadherin in astrocytoma.
|
| VM |
|
|
|---|
|
|
|
|
|
|---|
| Protein | + | − |
χ2-value | P-value |
|---|
| MMP-9 |
|
|
|
|
| + | 15 | 58 | 5.867 | 0.015a |
| − | 1 | 34 |
|
|
| VE-cadherin |
|
|
|
|
| + | 12 | 31 | 9.704 | 0.001a |
| − | 4 | 61 |
|
|
Formation of an in vitro cavity
structure
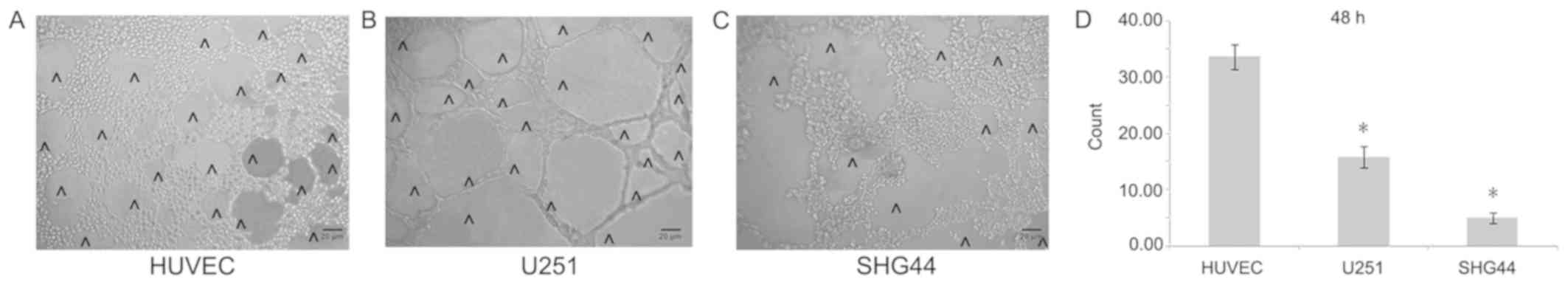
In order to better detect the angiogenesis of
different tumor cells, this study carried out a 3D cell culture.
In vitro 3D culture demonstrated that the number of cavity
structures in U251 cells notably increased compared with that in
SHG44 cells, but was significantly lower compared with HUVECs
(Fig. 4A-D).
Twist1, VE-cadherin and MMP-9
expression in U251 and SHG44 cells
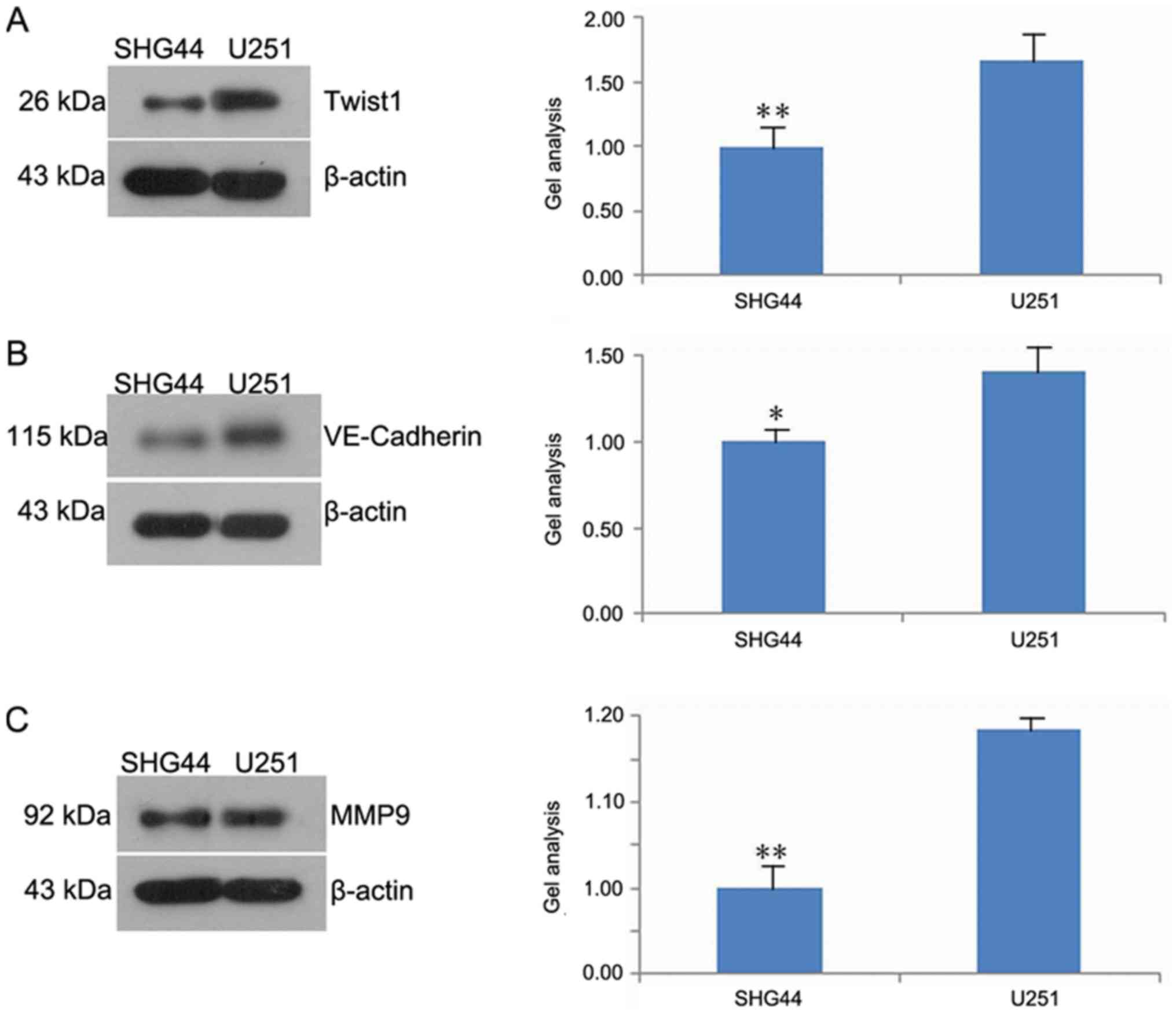
The results of western blot analysis demonstrated
that the expression of Twist1, VE-cadherin and MMP-9 in the SHG44
was significantly downregulated compared with the U251 cell line
(Fig. 5A-C).
Effects of Twist1 on in vitro cavity
structure
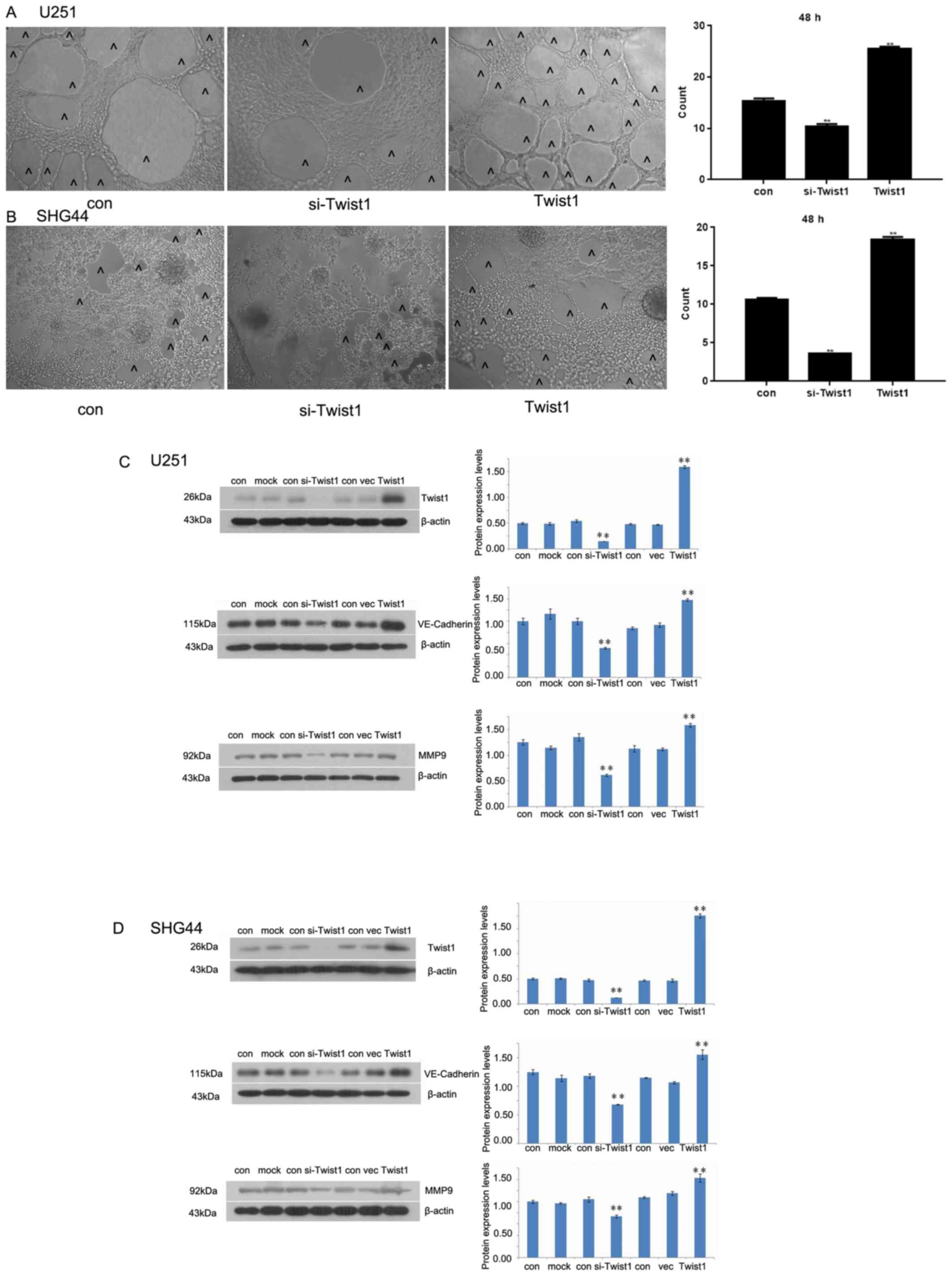
In vitro 3D culture illustrated that the
number of cavity structures in cultured cells decreased as a result
of Twist1 silencing. Following Twist1 overexpression, the number of
cavity structures increased significantly compared with the control
(Fig. 6A and B). Western blotting
demonstrated that si-Twist1 was able to inhibit the expression of
Twist1, VE-cadherin and MMP-9 in U251 and SHG44 cells.
Overexpression of Twist1 promoted a significant increase in the
expression levels of these proteins in both cell lines compared
with the corresponding control (Fig. 6C
and D).
Discussion
Micro-vessel proliferation is an important factor to
consider when grading astrocytomas. Angiogenesis is also a
prominent feature of astrocytoma, and is associated with its
malignant progression (23,24). Glioblastoma is the highest grade of
astrocytoma (WHOIV) and is the most vasculogenic malignancy
(25,26). In order to increase the effectiveness
of clinical treatment, further investigation into the molecular
mechanism of astrocytoma angiogenesis is required.
Twist1 can promote tumor progression by regulating
tumor cell growth, differentiation, drug resistance, angiogenesis
and metastasis (27,28). The exogenous overexpression of Twist1
increased the invasive and metastatic capacity of cancer cells by
promoting the downregulation of E-cadherin, and the induction of
epithelial-mesenchymal transition (EMT) (29). A previous study has indicated that
other transcription factors involved in EMT, including
transcription factor 7 and lymphoid enhancer-binding factor 1, are
also involved in astrocytoma progression (30). The results of the present study
indicated that the expression of Twist1 in glioblastoma was
increased compared with that in anaplastic and diffuse
astrocytomas, and that the expression level of Twist1 was
associated with the degree of tumor heterogeneity. Furthermore,
tumor types with high malignancy and invasiveness frequently
exhibit upregulated Twist1 expression (31). The results of the present
demonstrated that Twist1 was important in the occurrence and
development of astrocytoma.
VM has been identified in a number of malignant
tumors, including uveal melanoma and ovarian cancer (32). VM is not only associated with tumor
growth, metastasis and other biological functions, but also with
the prognosis of patients with tumors (33).
Studies have also demonstrated that in
hepatocellular carcinoma cells, Twist1 can promote VM by
upregulating the expression levels of VE-cadherin and MMP-2/9,
whilst downregulating that of E-cadherin (8,34). The
present study revealed that Twist1 was associated with the
formation of VM in astrocytomas. Out of the 16 cases with VM, 15
exhibited positive expression of MMP-9. Therefore, it was concluded
that MMP-9 may be involved in the formation of VM in astrocytomas.
It is reported that Twist1 is able to promote tumor progression by
increasing the expression of MMP-9 (35). Concurrently, it was also demonstrated
that the expression of Twist1 was associated with that of MMP-9;
therefore Twist1 may indirectly regulate VM via the regulation of
MMP-9.
Additional studies have indicated that VE-cadherin
may be a ‘switch’ for the induction of VM (8,36). The
present study showed that the expression of VE-cadherin was
significantly associated with the expression of Twist1 and the
formation of VM in astrocytomas. Therefore, it was concluded that
Twist1 and VE-cadherin may serve a synergistic role in regulating
the formation of VM in astrocytomas.
Furthermore, the association between Twist1 and the
formation of cavity structures was investigated in U251 and SHG44
cells. It was revealed that both U251 and SHG44 cells formed cavity
structures, and that the number of cavity structures formed by U251
cells was greater than that of SHG44 cells. In addition, it was
also highlighted that the expression level of Twist1 was higher in
U251 cells than in SHG44 cells, indicating that Twist1 promoted the
formation of cavity structures in U251 and SHG44 cells.
The present study also revealed that the expression
levels of VE-cadherin and MMP-9 in U251 cells were increased
compared with those in SHG44 cells. Furthermore, the formation of
VM may be associated with VE-cadherin and MMP-9. Twist1 was also
shown to promote the expression of VE-cadherin and MMP-9; however,
the inhibition of Twist1 decreased the expression of VE-cadherin
and MMP-9. It was therefore concluded that Twist1, VE-cadherin and
MMP-9 all served roles in the formation of VM in astrocytoma, and
that MMP-9 and VE-cadherin are important downstream constituents,
possibly targeted by Twist1 in this process.
In the present study it was determined that Twist1
regulated the formation of VM in astrocytomas potentially through
the Twist1/VE-cadherin/MMP-9 signaling axis. This conclusion
provides a novel theoretical basis for Twist1 as a therapeutic
target, presenting new insights for the targeted therapy of
astrocytoma.
Acknowledgements
We would like to acknowledge the helpful comments
received from our reviewers.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
WC provided the conception and design of the study,
participated in all experimentation, drafted and critically revised
the manuscript. CX cultured the cell lines and performed
experiments. XL participated in the design of the study and
experimentation. XY participated in the experiment design and the
subject establishment of this article, in addition they wrote and
critically revised the manuscript and gave final approval of the
submitted version.
Ethics approval and consent to
participate
All methods were carried out in accordance with
relevant guidelines and regulations. The study protocol was
approved by the Ethics Committee of the Shengjing Hospital, China
Medical University and Human Clinical Trials Committee. The
procedures were approved by the ethics committee according to the
Chinese Community guide-lines, and written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mahzouni P, Mohammadizadeh F, Mougouei K,
Moghaddam NA, Chehrei A and Mesbah A: Determining the relationship
between ‘microvessel density’ and different grades of astrocytoma
based on immunohistochemistry for ‘factor VIII-related antigen’
(von Willebrand factor) expression in tumor microvessels. Indian J
Pathol Microbiol. 53:605–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang JX, Chen ZH, Chen DL, Tian XP, Wang
CY, Zhou ZW, Gao Y, Xu Y, Chen C, Zheng ZS, et al:
LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer
angiogenesis and metastasis. Oncogene. 37:2660–2675. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huttala O, Palmroth M, Hemminki P, Toimela
T, Heinonen T, Ylikomi T and Sarkanen JR: Development of versatile
human in vitro vascularized adipose tissue model with serum-free
angiogenesis and natural adipogenesis induction. Basic Clin
Pharmacol Toxicol1. 23 (Suppl):S62–S71. 2018. View Article : Google Scholar
|
|
4
|
Bao MH, Li GY, Huang XS, Tang L, Dong LP
and Li JM: Long noncoding RNA LINC00657 acting as a miR-590-3p
sponge to facilitate low concentration oxidized low-density
lipoprotein-induced angiogenesis. Mol Pharmacol. 93:368–375. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee TJ, Shim MS, Yu T, Choi K, Kim DI, Lee
SH and Bhang SH: Bioreducible polymer micelles based on
acid-degradable poly(ethylene glycol)-poly(amino ketal) enhance the
stromal cell-derived factor-1alpha gene transfection efficacy and
therapeutic angiogenesis of human adipose-derived stem cells. Int J
Mol Sci. 19:2018.
|
|
6
|
Yeo C, Lee HJ and Lee EO: Serum promotes
vasculogenic mimicry through the EphA2/VE-cadherin/AKT pathway in
PC-3 human prostate cancer cells. Life Sci. 221:267–273. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo J, Cai H, Liu X, Zheng J, Liu Y, Gong
W, Chen J, Xi Z and Xue Y: Long non-coding RNA LINC00339 stimulates
glioma vasculogenic mimicry formation by regulating the
miR-539-5p/TWIST1/MMPs Axis. Mol Ther Nucleic Acids. 10:170–186.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Zhu DM, Zhou XG, Yin N, Zhang Y,
Zhang ZX, Li DC and Zhou J: HIF-2alpha promotes the formation of
vasculogenic mimicry in pancreatic cancer by regulating the binding
of Twist1 to the VE-cadherin promoter. Oncotarget. 8:47801–47815.
2017.PubMed/NCBI
|
|
9
|
Liu K, Sun B, Zhao X, Wang X, Li Y, Qiu Z,
Gu Q, Dong X, Zhang Y, Wang Y and Zhao N: Hypoxia induced
epithelial-mesenchymal transition and vasculogenic mimicry
formation by promoting Bcl-2/Twist1 cooperation. Exp Mol Pathol.
99:383–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yue WY and Chen ZP: Does vasculogenic
mimicry exist in astrocytoma? J Histochemi Cytochem. 53:997–1002.
2005. View Article : Google Scholar
|
|
11
|
Chang Z, Cui J and Song Y: Long noncoding
RNA PVT1 promotes EMT via mediating microRNA-186 targeting of
Twist1 in prostate cancer. Gene. 654:36–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gou W, Zhou X, Liu Z, Wang L, Shen J, Xu
X, Li Z, Zhai X, Zuo D and Wu Y: CD74-ROS1 G2032R mutation
transcriptionally up-regulates Twist1 in non-small cell lung cancer
cells leading to increased migration, invasion, and resistance to
crizotinib. Cancer Lett. 422:19–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grither WR, Divine LM, Meller EH, Wilke
DJ, Desai RA, Loza AJ, Zhao P, Lohrey A, Longmore GD and Fuh KC:
TWIST1 induces expression of discoidin domain receptor 2 to promote
ovarian cancer metastasis. Oncogene. 37:1714–1729. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu J, Tian J, Zhu S, Sun L, Yu J, Tian H,
Dong Q, Luo Q, Jiang N, Niu Y and Shang Z: Sox5 contributes to
prostate cancer metastasis and is a master regulator of
TGF-beta-induced epithelial mesenchymal transition through
controlling Twist1 expression. British J Cancer. 118:88–97. 2018.
View Article : Google Scholar
|
|
15
|
Jianwei Z, Qi L, Quanquan X, Tianen W and
Qingwei W: TMPRSS4 upregulates TWIST1 expression through STAT3
activation to induce prostate cancer cell migration. Pathol Oncol
Res. 24:251–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu K, Sun B, Zhao X, Wang X, Li Y, Qiu Z,
Liu T, Gu Q, Dong X, Zhang Y, et al: Hypoxia promotes vasculogenic
mimicry formation by the Twist1-Bmi1 connection in hepatocellular
carcinoma. Int J Mol Med. 36:783–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J, Sun B, Sun R, Zhu D, Zhao X, Zhang
Y, Dong X, Che N, Li J, Liu F, et al: HMGA2 promotes vasculogenic
mimicry and tumor aggressiveness by upregulating Twist1 in gastric
carcinoma. Sci Rep. 7:22292017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun T, Sun BC, Zhao XL, Zhao N, Dong XY,
Che N, Yao Z, Ma YM, Gu Q, Zong WK and Liu ZY: Promotion of tumor
cell metastasis and vasculogenic mimicry by way of transcription
coactivation by Bcl-2 and Twist1: A study of hepatocellular
carcinoma. Hepatology. 54:1690–1706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang D, Zheng J, Liu X, Xue Y, Liu L, Ma
J, He Q, Li Z, Cai H and Liu Y: Knockdown of USF1 inhibits the
vasculogenic mimicry of glioma cells via stimulating
SNHG16/miR-212-3p and linc00667/miR-429 Axis. Mol Ther Nucleic
Acids. 14:465–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berchuck A, Soisson AP, Clarke-Pearson DL,
Soper JT, Boyer CM, Kinney RB, McCarty KS Jr and Bast RC Jr:
Immunohistochemical expression of CA 125 in endometrial
adenocarcinoma: Correlation of antigen expression with metastatic
potential. Cancer Res. 49:2091–2095. 1989.PubMed/NCBI
|
|
22
|
Liu Y, Li F, Yang YT, Xu XD, Chen JS, Chen
TL, Chen HJ, Zhu YB, Lin JY and Li Y: IGFBP2 promotes vasculogenic
mimicry formation via regulating CD144 and MMP2 expression in
glioma. Oncogene. 38:1815–1831. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhogal P, Yeo LL, Henkes H, Krings T and
Soderman M: The role of angiogenesis in dural arteriovenous
fistulae: The story so far. Interv Neuroradiol. 24:450–454. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brennan MA, Renaud A, Guilloton F, Mebarki
M, Trichet V, Sensebé L, Deschaseaux F, Chevallier N and Layrolle
P: Inferior in vivo osteogenesis and superior angiogenesis of human
adipose-derived stem cells compared with bone marrow-derived stem
cells cultured in xeno-free conditions. Stem Cells Transl Med.
7:3152018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carbone C, Piro G, Merz V, Simionato F,
Santoro R, Zecchetto C, Tortora G and Melisi D: Angiopoietin-like
proteins in angiogenesis, inflammation and cancer. Int J Mol Sci.
19:2018. View Article : Google Scholar
|
|
26
|
Casas BS, Vitoria G, do Costa MN, Madeiro
da Costa R, Trindade P, Maciel R, Navarrete N, Rehen SK and Palma
V: hiPSC-derived neural stem cells from patients with schizophrenia
induce an impaired angiogenesis. Transl Psychiatry. 8:482018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahme GJ and Israel MA: Id4 suppresses
MMP2-mediated invasion of glioblastoma-derived cells by direct
inactivation of Twist1 function. Oncogene. 34:53–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mikheeva SA, Mikheev AM, Petit A, Beyer R,
Oxford RG, Khorasani L, Maxwell JP, Glackin CA, Wakimoto H,
González-Herrero I, et al: TWIST1 promotes invasion through
mesenchymal change in human glioblastoma. Mol Cancer. 9:1942010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon NA, Jo HG, Lee UH, Park JH, Yoon JE,
Ryu J, Kang SS, Min YJ, Ju SA, Seo EH, et al: Tristetraprolin
suppresses the EMT through the down-regulation of Twist1 and Snail1
in cancer cells. Oncotarget. 7:8931–8943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pecina-Slaus N, Kafka A, Tomas D, Marković
L, Okštajner PK, Sukser V and Krušlin B: Wnt signaling
transcription factors TCF-1 and LEF-1 are upregulated in malignant
astrocytic brain tumors. Histol Histopathol. 29:1557–1564.
2014.PubMed/NCBI
|
|
31
|
Wang L, Lin L, Chen X, Sun L, Liao Y,
Huang N and Liao W: Metastasis-associated in colon cancer-1
promotes vasculogenic mimicry in gastric cancer by upregulating
TWIST1/2. Oncotarget. 6:11492–11506. 2015.PubMed/NCBI
|
|
32
|
Zhang D, Sun B, Zhao X, Ma Y, Ji R, Gu Q,
Dong X, Li J, Liu F, Jia X, et al: Twist1 expression induced by
sunitinib accelerates tumor cell vasculogenic mimicry by increasing
the population of CD133+ cells in triple-negative breast cancer.
Mol Cancer. 13:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian W, Li YS, Zhang JH, Li JJ and Gao JH:
Comprehensive analysis of DNA methylation and gene expression
datasets identified MMP9 and TWIST1 as important pathogenic genes
of lung adenocarcinoma. DNA Cell Biol. 37:336–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han H, Du L, Cao Z, Zhang B and Zhou Q:
Triptonide potently suppresses pancreatic cancer cell-mediated
vasculogenic mimicry by inhibiting expression of VE-cadherin and
chemokine ligand 2 genes. Eur J Pharmacol. 818:593–603. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu W, Lv C, Zhang B, Zhou Q and Cao Z:
MicroRNA-27b functions as a new inhibitor of ovarian
cancer-mediated vasculogenic mimicry through suppression of
VE-cadherin expression. RNA. 23:1019–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delgado-Bellido D, Serrano-Saenz S,
Fernandez-Cortes M and Oliver FJ: Vasculogenic mimicry signaling
revisited: Focus on non-vascular VE-cadherin. Mol Cancer.
16:652017. View Article : Google Scholar : PubMed/NCBI
|