Introduction
Gallbladder carcinoma (GBC) is the most common
malignant tumor of the biliary tract and one of the common
malignant tumor types of the gastrointestinal tract (1). GBC is infrequent in the majority of
developed countries, but common in certain specific geographical
regions of developing countries, including northern India, South
Karachi in Pakistan and eastern Europe (2). GBC is characterized by late diagnosis,
metastasis and recurrence which occur readily, and poor prognosis
and survival rates (2–4). Surgery is considered the only curative
therapy for GBC. However, at diagnosis, <20% of patients are
suitable candidates for surgical treatment (4). GBC should be treated with systemic
chemotherapy, and drug/chemical development is important for the
prevention and treatment of GBC.
Antitumor promotion with phytochemicals is currently
regarded as an efficient and reliable strategy for cancer
chemoprevention and therapy. The genus Isodon (formerly
Rabdosia) comprising ~150 species of undershrubs,
subundershrubs and perennial herbs, is found throughout the world,
primarily in tropical and subtropical Asia (5). Several Isodon species are widely
used in popular Chinese folk medicine for the treatment of
bacterial infections, inflammation, cancer and hepatotoxicity
(5). A large number of secondary
metabolites with diverse biological activities have been isolated
from this species. These chemical constituents include
diterpenoids, triterpenoids and flavonoids (6–8). In the
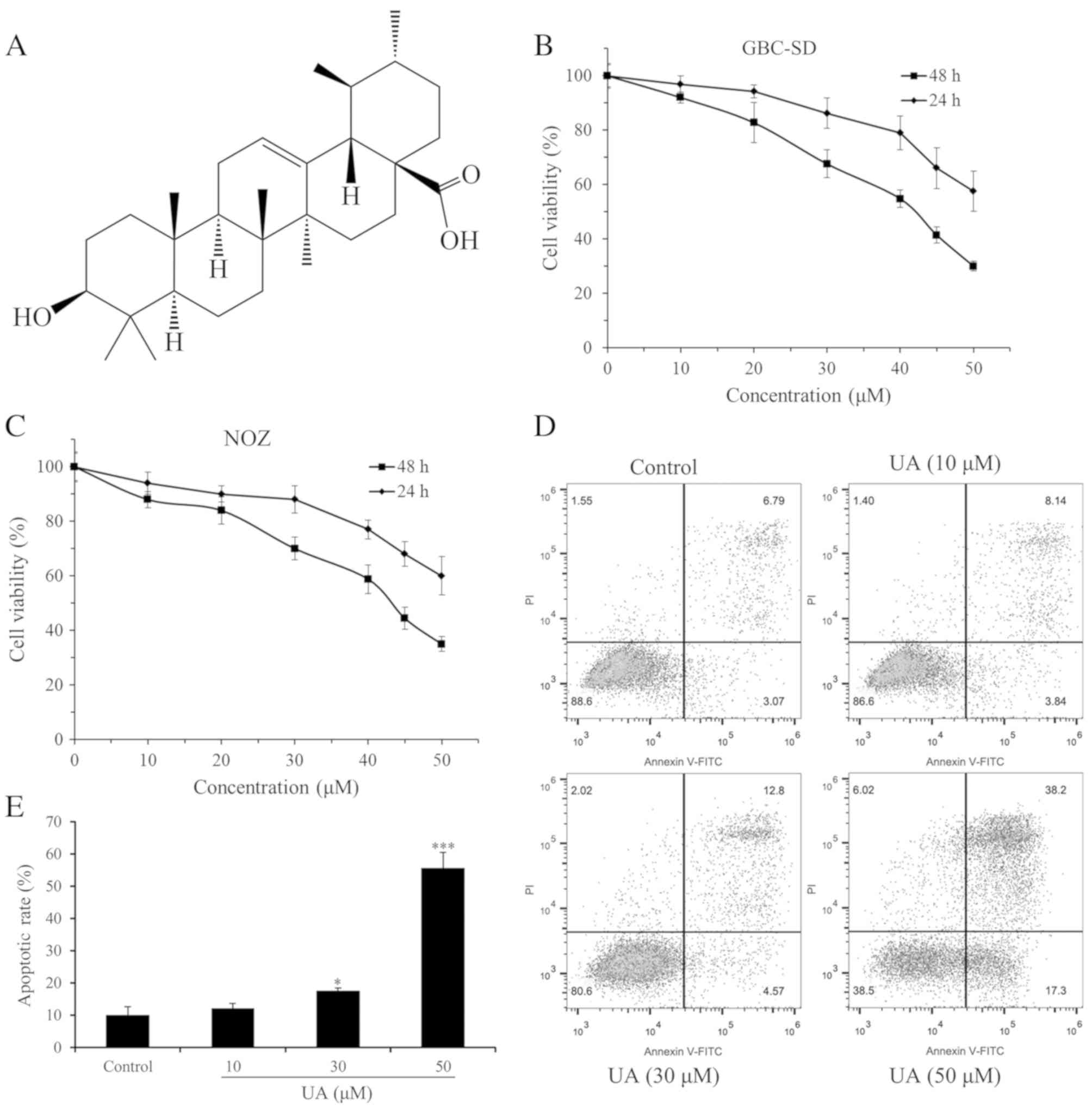
present study, ursolic acid (UA; Fig.
1A), a pentacyclic triterpenoid, was isolated from Isodon
excisoides. UA has been reported to exhibit various
pharmaceutical effects, including anticancer, antiangiogenic,
antioxidant, anti-inflammatory, antibacterial, hepatoprotective,
cardioprotective, antihyperlipidemic and hypoglycemic activities,
among others (9,10). The anticancer effects of UA are
mediated by suppression of the phosphoinositide-3-kinase
(PI3K)/protein kinase B (Akt), nuclear factor (NF)-κB and signal
transducer and activator of transcription 3 pathways with their
regulated gene products, including cyclin D1, Bcl-2, Bcl-xL,
survivin, myeloid cell leukemia-1 and vascular endothelial growth
factor (11). In addition, He et
al (12) reported that there
were 611 proteins possibly interacting with UA and >49
functional clusters responding to UA. Numerous studies have
suggested that UA is a promising sensitizer for cancer therapy
(11). Previous evidence has
revealed that UA inhibits the growth of GBC cells through inducing
cell cycle arrest and apoptosis (13). However, the anti-invasive effect of
UA and the associated mechanism in GBC remain to be fully
elucidated. Therefore, the present study aimed to investigate the
antiproliferative and anti-invasive effects of purified UA in the
GBC-SD human GBC cell line in vitro. The results will assist
in expanding current understanding of the anticancer effect and
mechanism of UA. Furthermore, the results may contribute to the
development of UA as a potent anticancer agent for GBC.
Materials and methods
Plant material, cells and
reagents
The aerial parts of I. excisoides were
collected from Luanchuan County in Henan Province, China in July
2014 and authenticated by Professor Jicheng Li of Zhengzhou
University (Zhengzhou, China). A voucher specimen (no.
20140706167LY) was deposited in the herbarium of the College of
Pharmacy, Zhengzhou University. The human GBC cell lines GBC-SD
(cat. no. CC2502) and NOZ (cat. no. CC2501), which exhibit correct
short tandem repeat profiles, were originally purchased from
Guangzhou Cellcook Biotech Co., Ltd (Guangzhou, China) and stored
in the Henan Key Laboratory for Pharmacology of Liver Diseases
(Institute of Medical and Pharmaceutical Sciences, Zhengzhou
University, Zhengzhou, China). The reagents used included RPMI-1640
medium (cat. no. SH30809.01) and high-glucose Dulbecco's modified
Eagle's medium (DMEM; cat. no. SH30022.01; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA), fetal bovine serum (FBS; cat. no.
900-108; Gemini Bio Products, West Sacramento, CA, USA), Cell
Counting Kit-8 (CCK-8; cat. no. CK04; Dojindo Molecular
Technologies Inc., Shanghai, China), Annexin V-FITC/Propidium
iodide (PI) Apoptosis Detection kit (cat. no. 70-AP101-60; Multi
Sciences Biotech Co., Ltd., Hangzhou, China), Transwell chambers
with polycarbonate filters (8-µm pore size; cat. no. 3422; Corning,
Inc., Corning, NY, USA), Matrigel (cat. no. 356234; BD Biosciences,
Franklin Lakes, NJ, USA), TRIzol reagent (cat. no. 15596-026;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
PrimeScript™ RT Reagent kit (cat. no. DRR037A; Takara Biotechnology
Co., Ltd., Dalian, China), RT2 Profiler ‘human
apoptosis’ polymerase chain reaction (PCR) arrays (cat. no.
PAHS-012Z) and RT2 Profiler ‘human extracellular matrix
and adhesion molecules’ PCR arrays (cat. no. PAHS-013Z; Qiagen,
Inc., Valencia, CA, USA), EvaGreen 2X qPCR MasterMix-No Dye kit
(cat. no. MasterMix-S; Applied Biological Materials, Richmond, BC,
Canada), Dimethylsulfoxide (DMSO; cat. no. D8371), bovine serum
albumin (BSA; cat. no. A8010), RIPA lysis buffer (cat. no. R0020;
Solarbio Science and Technology, Beijing, China), phosphorylated
(phosphor)-NF-κB p65 (Ser536) antibody (cat. no. 3033), NF-κB p65
antibody (cat. no. 3034), phospho-Akt (Ser473) antibody (cat. no.
9271), Akt antibody (cat. no. 9272; Cell Signaling Technology,
Inc., Beverly, MA, USA) and GAPDH monoclonal antibody (cat. no.
60004-1-lg; ProteinTech Group, Inc., Chicago, IL, USA). Other
chemicals and reagents were of analytical grade.
Extraction, isolation and purification
of UA from I. excisoides
The dried and powdered aerial parts of I.
excisoides (3 kg) were extracted with anhydrous ether (12 L).
The extract was then filtered and evaporated in a rotatory
evaporator under reduced pressure. The concentrated residue (89 g)
was dissolved in methanol (3.6 L) and activated carbon (108 g) was
added. The mixture was heated under reflux and further filtered and
evaporated to produce a crude product (71 g). The crude product was
then successively separated by silica gel chromatographic column
and Sephadex LH-20 column chromatography, giving a compound (56
mg). This compound was identified as UA on the basis of its mass
and nuclear magnetic resonance spectra. UA (ursolic acid,
C30H48O3): HR-EIMS m/z 456.3608
(456.3603 calcd. for C30H48O3);
1H-NMR (C5D5N, 400 MHz): δ 5.52
(1H, t, J=3.5 Hz, H-12), 3.48 (1H, dd, J=9.9, 5.4 Hz,
H-3α), 2.68 (1H, d, J=11.4 Hz, H-18), 1.27, 1.05, 0.91, 1.08
and 1.25 (each 3H, s, H-23, H-24, H-25, H-26 and H-27), 0.97 (3H,
d, J=6.0 Hz, H-29) and 1.03 (3H, d, J=6.3 Hz, H-30);
13C-NMR (C5D5N, 100 MHz): δ 39.1
(C-1), 28.1 (C-2), 78.1 (C-3), 39.4 (C-4), 55.8 (C-5), 18.8 (C-6),
33.6 (C-7), 40.0 (C-8), 48.1 (C-9), 37.3 (C-10), 25.0 (C-11), 125.6
(C-12), 139.4 (C-13), 42.5 (C-14), 28.7 (C-15), 23.9 (C-16), 48.1
(C-17), 53.6 (C-18), 39.5 (C-19), 39.5 (C-20), 31.1 (C-21), 37.3
(C-22), 28.8 (C-23), 16.6 (C-24), 15.7 (C-25), 17.6 (C-26), 23.7
(C-27), 180.2 (C-28), 17.5 (C-29) and 21.5 (C-30).
Cell culture and UA treatment
The GBC-SD and NOZ cells were cultured in RPMI-1640
or high-glucose DMEM supplemented with 10% FBS at 37°C in a 5%
CO2 humidified atmosphere, and routinely passaged at
2–3-day intervals. UA was dissolved in DMSO to a 50 mM stock
concentration. The final DMSO concentration was accounted for ≤0.1%
(v/v). DMSO (0.05%)-treated cells were used as a vehicle
control.
Cell viability assay
A CCK-8 assay was performed to evaluate the effect
of UA on cell viability. A total of 1.2×104 cells/well
were seeded in 96-well plates overnight and then treated with
varying concentrations of UA (0, 10, 20, 30, 40, 45 and 50 µM). The
cells were incubated for either 24 or 48 h at 37°C in a humidified
incubator, following which 10 µl CCK-8 solution was added into each
well and incubated for a further 1 h. The absorbance was measured
at 450 nm in each well using a microplate spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA). The results are presented as
the mean values of three independent experiments performed over
multiple days. Cell viability was calculated using the following
formula: Cell viability (%)=[optical density (OD) of the experiment
samples/OD of the control] × 100%. The half maximal inhibitory
concentration (IC50) value of UA against the GBC-SD or
NOZ cells was calculated using GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA).
Apoptosis assays
Cell apoptosis was detected using an Annexin
V-FITC/PI Apoptosis Detection kit. In brief, the cells from the
UA-treated (10, 30 and 50 µM) and untreated groups were seeded in
6-well plates (1×106 cells/well) and cultured for 24 h.
The cells were collected, washed with ice-cold PBS, and resuspended
in binding buffer at a cell density of 1×106 cells/ml.
The cells were stained with 5 µl Annexin V-FITC and 10 µl PI (20
µg/ml) and then incubated in the dark at 25°C for 15 min. Apoptotic
cells were analyzed by flow cytometry (CytoFlex; Beckman Coulter
Inc., Brea, CA, USA). The percentage of apoptotic cells was
analyzed using FlowJo software (version 9.8.3, FlowJo LLC, Ashland,
OR, USA). The experiments were repeated three times.
Cell invasion assay
A cell invasion assay was performed using 8-µm pore
size Transwell chambers. The upper side of the Transwell filter
inserts was coated with 80 µl diluted (1:8 in serum-free medium)
Matrigel in 24-well plates. The GBC-SD cells at a density of
1×105 cells/well were suspended in serum-free RPMI-1640
medium and added to the upper chambers containing various
concentrations of UA (10, 30 and 50 µM). The lower chambers were
filled with 500 µl RPMI-1640 medium containing 20% FBS. After 24 h,
the non-invaded cells were removed, and the invasive cells were
fixed with 95% ethanol, stained with 0.1% crystal violet and images
were captured (magnification, ×100) with a light microscope (XDS-1B
inverted biological microscope, Chongqing Optical & Electrical
Instrument Co., Ltd., Chongqing, China). The assay was repeated in
three independent experiments.
RT2 profiler PCR arrays for
apoptosis and invasion
In brief, cells from the UA-treated (50 µM) and
untreated groups were seeded in 6-well plates (1×106
cells/well) and cultured for 24 h. Total RNA was extracted from
each experimental group using TRIzol reagent and quantified by
spectrophotometry. Subsequently, 1 µg of total RNA was reverse
transcribed with the PrimeScript™ RT Reagent kit, according to the
manufacturer's protocol. RT2 Profiler ‘human apoptosis’
PCR arrays and RT2 Profiler ‘human extracellular matrix
and adhesion molecules’ PCR arrays were performed in duplicate
according to the manufacturer's protocol. The PCR was performed as
follows: 10 min at 95°C and 40 cycles (15 sec at 95°C, 1 min at
60°C). The specificity of the SYBR Green assay was confirmed by
melting curve analysis. Relative fold changes in mRNA levels were
calculated following normalization to housekeeping control gene
targets using the comparative Cq method (14).
Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analysis
In order to investigate the signaling pathways that
may be involved in the effects of UA on GBC-SD cells, KEGG pathway
analysis of differentially expressed genes was performed using the
Database for Annotation, Visualization and Integrated Discovery
(https://david.ncifcrf.gov/) (15,16).
Pathway terms with P<0.05 were considered statistically
significant.
Western blot analysis
Following treatment with UA (0, 10, 30 and 50 µM)
for 6 h, the GBC-SD cells were washed with PBS and lysed in RIPA
lysis buffer. Based on total protein concentrations calculated from
the BCA assays, the total cell lysates (15 µg total protein) were
separated via SDS-PAGE (8% gel) and then transferred onto
polyvinylidene difluoride membranes in a standard transfer buffer.
Following blocking with 1% BSA (blocking solution) for 1.5 h at
room temperature, the membranes were incubated with primary
antibodies to phospho-NF-κB p65 (Ser536; 1:1,000), NF-κB p65
(1:1,000), phospho-Akt (Ser473; 1:1,000), Akt (1:1,000) or GAPDH
(1:5,000) overnight at 4°C. The membranes were washed three times
with TBST and then incubated with HRP-conjugated secondary
antibodies (1:5,000; cat. no. SA00001-1/SA00001-2; ProteinTech
Group, Inc., Chicago, IL, USA) for 2 h at room temperature.
Following extensive washing in TBST, the protein signals were
visualized using enhanced chemiluminescence (ECL) western blotting
substrate (cat. no. B500014; ProteinTech Group, Inc., Chicago, IL,
USA) and an ECL system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Equal protein loading was assessed by normalizing against the
expression of GAPDH. Quantification of protein bands densitometry
was carried out using ImageJ software (version 1.8.0_112; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 22.0 (IBM
Corp., Armonk, NY, USA). Groups were compared using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Structural determination of UA
isolated from I. excisoides and cell viability assay
Consistent with previous results (17,18), UA
was isolated from an Isodon species (I. excisoides).
UA was identified by comparison of its HR-EIMS, 1H-NMR
and 13C-NMR spectra data with those previously reported
for UA (19,20), and to an authentic sample
(Sigma-Aldrich; Merck KGaA, Darmstadt Germany). The proliferation
inhibition effect of UA on GBC-SD and NOZ cells was determined
using a CCK-8 assay. Within the dose range and time period
measured, UA was able to inhibit cell proliferation in a dose- and
time-dependent manner (Fig. 1B and
C). Furthermore, the IC50 values of UA at the same
exposure time for GBC-SD cells were marginally lower compared with
those for NOZ cells. The IC50 values for GBC-SD cells at
24 and 48 h were 57.44 and 39.12 µM, respectively, and the values
for NOZ cells were 61.58 and 41.81 µM, respectively. Therefore,
GBC-SD cells were selected to further investigate the effect of UA
on the invasive capacity of GBC cells. In addition, the
IC50 value for GBC-SD cells at 48 h was lower than that
reported in the literature (for example, the IC50 value
for GBC-SD cells at 48 h was previously reported to be 47.6 µM)
(13), which may derive from
differences in experimental design and operation.
UA induces apoptosis of GBC-SD cells
in a dose-dependent manner
The UA-induced apoptosis of GBC-SD cells was
determined using an Annexin V-FITC/PI assay. As indicated in
Fig. 1D and E, UA treatment
increased the apoptosis of GBC-SD cells in a dose-dependent manner.
The apoptotic rates of the cells were 11.98, 17.37 and 55.50%
following treatment with 10, 30 and 50 µM UA, respectively, which
were all increased compared with the apoptotic rate of GBC-SD cells
cultured under normal conditions (9.96%). The apoptotic rates of
GBC-SD cells in the middle (30 µM) and high (50 µM) dose groups
were statistically significant (P<0.05). UA (10, 30 or 50 µM)
treatment had no significant effect on the distribution of GBC-SD
cells in the cell cycle (data not shown). These results indicate
that UA inhibits GBC-SD cell proliferation by inducing
apoptosis.
UA inhibits the invasion of GBC-SD
cells in a dose-dependent manner
Cell invasion is a driving force in the process of
tumor metastasis formation. Therefore, the effects of UA on
invasion in GBC-SD cells was evaluated (Fig. 2A and B). The in vitro invasion
assay indicated that UA at concentrations of 10–50 µM significantly
reduced the rate of GBC-SD cell invasion when compared with the
control group following cell treatment for 24 h (P<0.01).
Furthermore, UA at concentrations of 10 and 30 µM did not
significantly reduce the viability of GBC-SD cells following cell
treatment for 24 h (Fig. 1B). These
results suggested that the inhibition of GBC-SD cell invasion by UA
did not result from a reduction of cell viability. These
observations suggested that UA was able to regulate the invasive
capacity of GBC-SD cells in a dose-dependent manner.
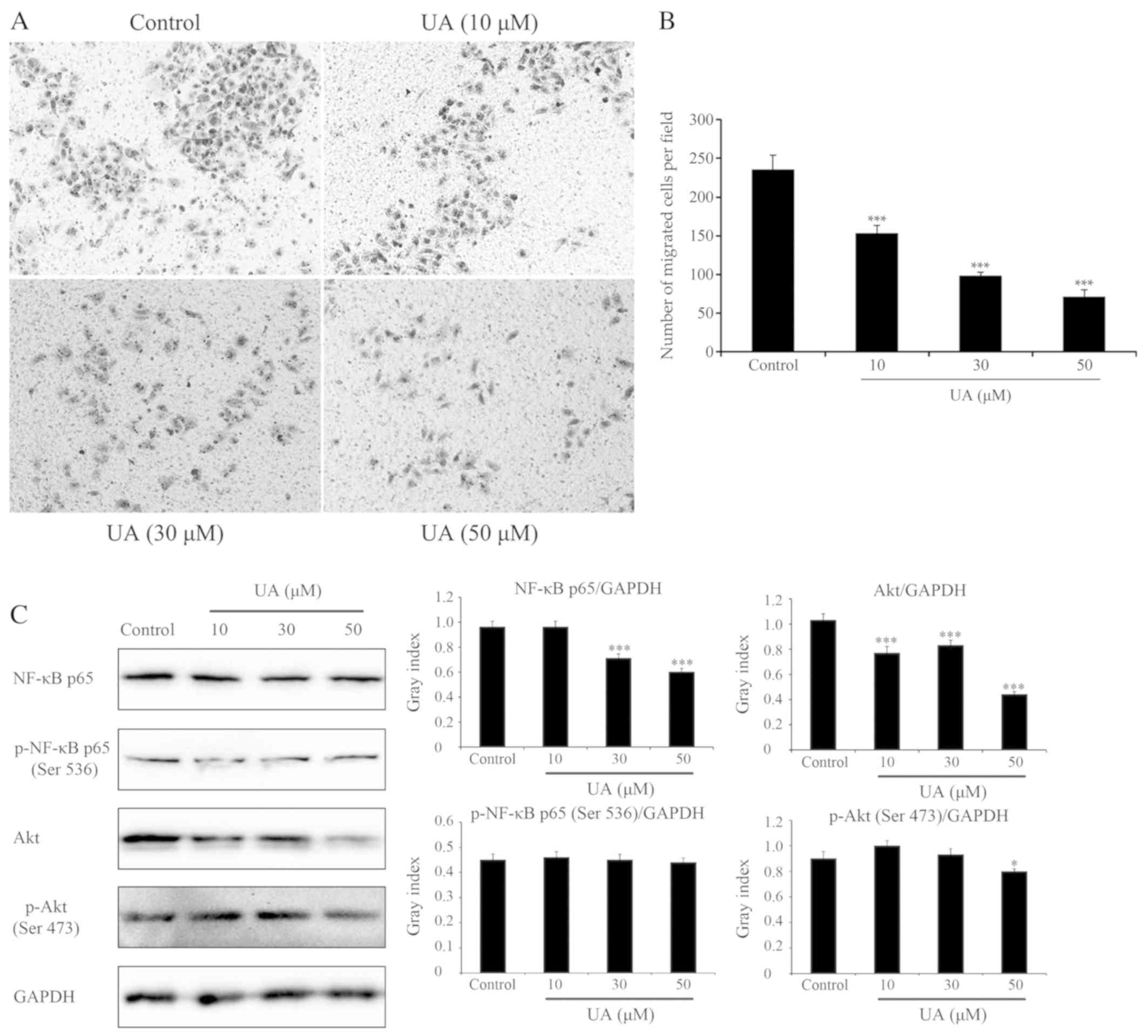 | Figure 2.Effects of UA on cell migration and
signaling pathways (NF-κB and Akt). Effects of UA on GBC-SD cell
migration, evaluated using a Transwell assay. Cells suspended in
serum-free RPMI-1640 were overlaid in the upper chamber of each
Transwell. Following incubation with different concentrations of UA
for 24 h, penetrating cells were stained with crystal violet and
recorded under a microscope mounted with a CCD camera. (A) Images
depicting migration of GBC-SD cells. (B) Quantified data are
expressed as the mean ± standard deviation from three independent
experiments. ***P<0.001, vs. control group (0 µM). (C) Effect of
UA treatment on the NF-κB and Akt signaling pathways. In GBC-SD
cells treated with various UA concentrations for 6 h, expression of
NF-κB p65, p-NF-κB p65 (Ser536), Akt and p-Akt (Ser473) was
analysed by western blotting. GAPDH was used as the sample loading
control. Quantification of protein bands densitometry was carried
out using ImageJ software. Data are presented as the mean ±
standard deviation from three independent experiments. *P<0.05
and ***P<0.001, vs. control group (0 µM). UA, ursolic acid;
NF-κB, nuclear factor κB; Akt, protein kinase B; p-,
phosphorylated. |
Effects of UA on GBC-SD cells are at
least in part via NF-κB and Akt signaling pathways
As UA induced apoptosis and inhibited invasion in
GBC-SD cells, the expression of 168 key genes involved in these two
processes was subsequently evaluated with a PCR array. The cells
were incubated with UA (50 µM) for 24 h, and 168 related genes (84
apoptosis-related genes and 84 adhesion/invasion-related genes)
were analyzed, compared with untreated GBC-SD cells. Of these 168
genes, there were 24 with log2 fold change values of
either >1.5 or ≥1.5, which were considered differentially
expressed (Table I). Of these 24
genes, eight apoptosis-related genes were screened. Of these eight
genes, three genes encoding pro-apoptotic members of the tumor
necrosis factor (TNF) family, including TNF (log2 fold
change: 3.17), lymphotoxin-α (log2 fold change: 2.19)
and CD27 (log2 fold change: 3.56) were enhanced in the
UA-treated GBC-SD cells compared with the control groups (Table I). Therefore, these results suggested
that UA induces apoptosis in GBC-SD cells through activation of the
cell extrinsic pathway, which is initiated by members of the TNF
superfamily (21,22). TNF is able to trigger either the
formation of complex-I, driving the activation of NF-κB and an
inflammatory response, or the formation of complex-II, which can
trigger apoptosis (23). Of the 24
differentially expressed genes, a further 16
adhesion/invasion-related genes were screened out, including nine
upregulated and seven downregulated genes. These upregulated and
downregulated genes may have multiple effects on the invasion of
GBC-SD cells. KEGG pathway analysis of the 24 differentially
expressed genes was performed using the Database for Annotation,
Visualization and Integrated Discovery (15,16).
According to KEGG pathway enrichment analysis, the differentially
expressed genes were significantly associated with NF-κB, TNF,
PI3K-Akt and other signaling pathways (Table II). To determine whether UA
regulates the NF-κB and Akt signaling pathways, the effect of UA on
the expression and activation of proteins in these signaling
pathways was detected by western blotting. As shown in Fig. 2C, the GBC-SD cells treated with UA
exhibited a significant and dose-dependent reduction in total NF-κB
p65 and Akt, and a marginal decrease in total p-NF-κB p65 (Ser536)
and p-Akt (Ser473). Therefore, it was demonstrated that the effects
of UA on GBC-SD cells were, at least in part, mediated through the
NF-κB and Akt signaling pathways.
 | Table I.Genes with >1.5-fold change in
expression between GBC-SD cells treated with UA and the control
group. |
Table I.
Genes with >1.5-fold change in
expression between GBC-SD cells treated with UA and the control
group.
| RT2
profiler PCR arrays | Gene symbol | GenBank accession
no. | Description | Log2
fold changea |
|---|
| Human
apoptosis | NOL3 | NM_003946 | Nucleolar protein 3
(apoptosis repressor with CARD domain) | −2.56 |
|
| LTA | NM_000595 | Lymphotoxin α (TNF
superfamily, member 1) | 2.19 |
|
| TNFSF8 | NM_001244 | Tumor necrosis
factor (ligand) superfamily, member 8 | −1.87 |
|
| BCL2L2 | NM_004050 | BCL2-like 2 | −1.80 |
|
| BCL2A1 | NM_004049 | BCL2-related
protein A1 | 2.50 |
|
| BCL2L10 | NM_020396 | BCL2-like 10
(apoptosis facilitator) | 2.60 |
|
| CD27 | NM_001242 | CD27 molecule | 3.56 |
|
| TNF | NM_000594 | Tumor necrosis
factor | 3.17 |
| Human | COL11A1 | NM_080629 | Collagen, type XI,
α1 | −2.90 |
| extracellular
matrix | SELL | NM_000655 | Selectin L | −2.58 |
| and adhesion
molecules | MMP11 | NM_005940 | Matrix
metallopeptidase 11 | −2.38 |
|
| MMP9 | NM_004994 | Matrix
metallopeptidase 9 | −2.03 |
|
| MMP2 | NM_004530 | Matrix
metallopeptidase 2 | −1.81 |
|
| SPARC | NM_003118 | Secreted protein,
acidic, cysteine-rich (osteonectin) | −1.78 |
|
| VCAN | NM_004385 | Versican | −1.76 |
|
| FN1 | NM_002026 | Fibronectin 1 | 1.56 |
|
| ICAM1 | NM_000201 | Intercellular
adhesion molecule 1 | 1.66 |
|
| ITGA2 | NM_002203 | Integrin, α2 | 1.68 |
|
| ITGA1 | NM_181501 | Integrin, α1 | 1.75 |
|
| PECAM1 | NM_000442 |
Platelet/endothelial cell adhesion
molecule | 2.16 |
|
| HAS1 | NM_001523 | Hyaluronan synthase
1 | 2.22 |
|
| KAL1 | NM_000216 | Kallmann syndrome 1
sequence | 2.26 |
|
| MMP10 | NM_002425 | Matrix
metallopeptidase 10 | 4.41 |
|
| MMP1 | NM_002421 | Matrix
metallopeptidase 1 | 4.79 |
 | Table II.Kyoto Encyclopedia of Genes and
Genomes pathway analysis of differentially expressed genes. |
Table II.
Kyoto Encyclopedia of Genes and
Genomes pathway analysis of differentially expressed genes.
| Terma | Genes | P-value |
|---|
| hsa04064: NF-κB
signaling pathway | ICAM1, TNF, BCL2A1,
LTA | 0.001 |
| hsa05205:
Proteoglycans in cancer | TNF, MMP9, ITGA2,
MMP2, FN1 | 0.001 |
| hsa04512:
ECM-receptor interaction | ITGA1, ITGA2,
COL11A1, FN1 | 0.001 |
| hsa04668: TNF
signaling pathway | ICAM1, TNF, MMP9,
LTA | 0.002 |
| hsa04670: Leukocyte
transendothelial migration | ICAM1, MMP9,
PECAM1, MMP2 | 0.002 |
| hsa04514: Cell
adhesion molecules (CAMs) | ICAM1, SELL,
PECAM1, VCAN | 0.004 |
| hsa05219: Bladder
cancer | MMP9, MMP2,
MMP1 | 0.004 |
| hsa05144:
Malaria | ICAM1, TNF,
PECAM1 | 0.006 |
| hsa05200: Pathways
in cancer | MMP9, ITGA2, MMP2,
MMP1, FN1 | 0.011 |
| hsa04510: Focal
adhesion | ITGA1, ITGA2,
COL11A1, FN1 | 0.011 |
| hsa05410:
Hypertrophic cardiomyopathy (HCM) | TNF, ITGA1,
ITGA2 | 0.014 |
| hsa04060:
Cytokine-cytokine receptor interaction | TNF, CD27, LTA,
TNFSF8 | 0.015 |
| hsa05414: Dilated
cardiomyopathy | TNF, ITGA1,
ITGA2 | 0.016 |
| hsa04640:
Hematopoietic cell lineage | TNF, ITGA1,
ITGA2 | 0.016 |
| hsa05323:
Rheumatoid arthritis | ICAM1, TNF,
MMP1 | 0.017 |
| hsa05146:
Amoebiasis | TNF, COL11A1,
FN1 | 0.024 |
| hsa04151: PI3K-Akt
signaling pathway | ITGA1, ITGA2,
COL11A1, FN1 | 0.043 |
Discussion
UA is a pentacyclic triterpenoid widely found in the
plant kingdom. It has attracted attention in recent years due to it
numerous activities and low toxicity (10). It exerts anticancer effects in
various cancer cell lines (24), and
inhibits the growth of GBC cells through inducing cell cycle arrest
and apoptosis (13). The present
study identified that low doses of UA (10, 30 and 50 µM) did not
affect cell cycle but induced cell apoptosis in GBC-SD cells. In
addition, previous studies have reported that UA can cause cell
death by autophagy or necrosis (25,26).
These results indicate that UA inhibits GBC-SD cell proliferation
primarily by inducing cell death via apoptosis, and possibly also
via autophagy or necrosis. To the best of our knowledge, no
previous studies have reported the anti-invasive effect of UA on
GBC. It was identified in the present study that UA significantly
reduced the rate of GBC-SD cell invasion. Furthermore, the effects
of UA on GBC-SD cells were at least partly mediated via the
suppression of NF-κB and Akt signaling pathways.
The anticancer mechanism of UA is complex and
multifaceted. The results of the present study suggested that UA
induced the apoptosis of GBC-SD cells through activation of the
cell extrinsic pathway. However, a previous study demonstrated that
activation of the mitochondrial-mediated apoptotic pathway is also
involved in UA-induced GBC-SD cell apoptosis (13). Another study on gastrointestinal
cancer demonstrated that UA modulates the expression of executioner
caspase (C-3, C-8 and C-9) proteins involved in the intrinsic and
extrinsic pathways of apoptosis (27). UA-induced apoptosis can be mediated
by an increase in activated extracellular signal regulated kinase
1/2, Janus kinase and p38 mitogen-activated protein kinase
(28). UA-induced GBC-SD cell
apoptosis may also be involved in the intrinsic and extrinsic
pathways of apoptosis. In addition, inhibition of the NF-κB and Akt
signaling pathways by UA has been reported in other cell types
(29,30), which is consistent with the present
study. The pro-survival Akt and NF-κB signaling pathways are
constitutively activated in several types of cancer, and contribute
to cancer development and progression. The anticancer effects of
certain dietary natural compounds are mediated by targeting the Akt
and NF-κB pathways (31,32). The NF-κB pathway also has the ability
to cross-talk with Akt pathways in various cancer types. The
suppression of Akt may contribute to inhibiting downstream targets,
including NF-κB p-65 and the mRNA levels of matrix
metalloproteinase 2 (MMP2) and MMP9 in GBC-SD cells (33). These two genes were downregulated in
the present study (Table I) and are
critical to tumor invasion. Certain clinical studies with a small
number of patients have demonstrated the safety and efficacy of UA
in cancer therapy (11). Future
investigations are required to analyze the precise mechanisms of UA
and to exploit its full potential for GBC chemotherapy.
In conclusion, UA was isolated from I.
excisoides. The antiproliferative and anti-invasive effects of
UA in GBC-SD cells were investigated and validated. Furthermore, UA
was demonstrated to induce apoptosis and inhibit invasion in GBC-SD
cells, which may be associated with the suppression of NF-κB and
Akt signaling pathways. The effects of UA in GBC-SD cells suggest
that UA may be a candidate agent for the chemoprevention and/or
treatment of GBC progression.
Acknowledgements
The authors would like to thank Professor Jicheng Li
(Zhengzhou University, Zhengzhou, China) for assistance with
authenticating the plant material.
Funding
The present study was financially supported by the
Medical Science and Technology Planning Project of Henan Province,
China (grant no. 201702299), the Major Special Science and
Technology Projects of Henan Province, China (grant no.
161100311400) and the Natural Science Foundation of Henan Province,
China (grant no. 182300410343).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD and JZ designed the experiments and wrote the
manuscript. HC and XW performed the majority of the experiments.
DZ, MZ and ZZ were involved in chemical research and analyzed the
data. XZ and XY were involved in data interpretation and assisted
in drafting the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu AX, Hong TS, Hezel AF and Kooby DA:
Current management of gallbladder carcinoma. Oncologist.
15:168–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aloia TA, Járufe N, Javle M, Maithel SK,
Roa JC, Adsay V, Coimbra FJ and Jarnagin WR: Gallbladder cancer:
Expert consensus statement. HPB; Oxford: 17. pp. 681–690. 2015,
PubMed/NCBI
|
|
4
|
Kanthan R, Senger JL, Ahmed S and Kanthan
SC: Gallbladder cancer in the 21st century. J Oncol 2015.
9674722015.
|
|
5
|
Sun HD, Huang SX and Han QB: Diterpenoids
from Isodon species and their biological activities. Nat
Prod Rep. 23:673–698. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, Li XM and Liu JK: New terpenoids
from Isodon sculponeata. Chem Pharm Bull; Tokyo: 57:pp.
525–527. 2009, PubMed/NCBI
|
|
7
|
Jiao K, Li HY, Zhang P, Pi HF, Ruan HL and
Wu JZ: Three new ursane-type triterpenoids from the aerial parts of
Isodon excisoides. J Asian Nat Prod Res. 15:962–968. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang H, Sun H and Zhao S: Flavonoids from
Isodon oresbius. Phytochemistry. 42:1247–1248. 1996.
View Article : Google Scholar
|
|
9
|
Kashyap D, Tuli HS and Sharma AK: Ursolic
acid (UA): A metabolite with promising therapeutic potential. Life
Sci. 146:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
López-Hortas L, Pérez-Larrán P,
González-Muñoz MJ, Falqué E and Domínguez H: Recent developments on
the extraction and application of ursolic acid. A review. Food Res
Int. 103:130–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasad S, Tyagi AK and Aggarwal BB:
Chemosensitization by ursolic acid: A new avenue for cancer
therapy. Role of nutraceuticals in cancer chemosensitization.
Bharti AC and Aggarwal BB: Academic Press; Cambridge,
Massachusetts: pp. 99–109. 2018, View Article : Google Scholar
|
|
12
|
He W, Shi F, Zhou ZW, Li B, Zhang K, Zhang
X, Ouyang C, Zhou SF and Zhu X: A bioinformatic and mechanistic
study elicits the antifibrotic effect of ursolic acid through the
attenuation of oxidative stress with the involvement of ERK,
PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate
cells and rat liver. Drug Des Devel Ther. 9:3989–4104.
2015.PubMed/NCBI
|
|
13
|
Weng H, Tan ZJ, Hu YP, Shu YJ, Bao RF,
Jiang L, Wu XS, Li ML, Ding Q, Wang XA, et al: Ursolic acid induces
cell cycle arrest and apoptosis of gallbladder carcinoma cells.
Cancer Cell Int. 14:962014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nature Protoc. 4:44–57. 2009. View Article : Google Scholar
|
|
17
|
Yang YC, Wei MC and Huang TC: Optimisation
of an ultrasound-assisted extraction followed by RP-HPLC separation
for the simultaneous determination of oleanolic acid, ursolic acid
and oridonin content in Rabdosia rubescens. Phytochem Anal.
23:627–636. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang B, Hou AJ, Li ML, Li SH, Han QB,
Wang SJ, Lin ZW and Sun HD: Cytotoxic ent-kaurane diterpenoids from
Isodon sculponeata. Planta Med. 68:921–925. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali MS, Ibrahim SA, Jalil S and Choudhary
MI: Ursolic acid: A potent inhibitor of superoxides produced in the
cellular system. Phytother Res. 21:558–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seebacher W, Simic N, Weis R, Saf R and
Kunert O: Complete assignments of 1H and 13C
NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid
and their 11-oxo derivatives. Magn Reson Chem. 41:636–638. 2003.
View Article : Google Scholar
|
|
21
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savva CG, Totokotsopoulos S, Nicolaou KC,
Neophytou CM and Constantinou AI: Selective activation of TNFR1 and
NF-κB inhibition by a novel biyouyanagin analogue promotes
apoptosis in acute leukemia cells. BMC Cancer. 16:2792016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Annibaldi A and Meier P: Checkpoints in
TNF-induced cell death: Implications in inflammation and cancer.
Trends Mol Med. 24:49–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woźniak Ł, Skąpska S and Marszałek K:
Ursolic acid-A pentacyclic triterpenoid with a wide spectrum of
pharmacological activities. Molecules. 20:20614–20641. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung J, Seo J, Kim J and Kim JH: Ursolic
acid causes cell death in PC-12 cells by inducing apoptosis and
impairing autophagy. Anticancer Res. 38:847–853. 2018.PubMed/NCBI
|
|
26
|
Lu CC, Huang BB, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Zhang F, Yang L, Mei Y, Long H,
Zhang X, Zhang J, Qimuge-Suyila and Su X: Ursolic acid inhibits
proliferation and induces apoptosis of cancer cells in vitro and in
vivo. J Biomed Biotechnol 2011. 4193432011.
|
|
28
|
Wu CC, Cheng CH, Lee YH, Chang IL, Chen
HY, Hsieh CP and Chueh PJ: Ursolic acid triggers apoptosis in human
osteosarcoma cells via caspase activation and the ERK1/2 MAPK
pathway. J Agric Food Chem. 64:4220–4226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gai L, Cai N, Wang L, Xu X and Kong X:
Ursolic acid induces apoptosis via Akt/NF-κB signaling suppression
in T24 human bladder cancer cells. Mol Med Rep. 7:1673–1677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Wen X, Li M, Li S and Zhao H:
Targeting cancer stem cells and signaling pathways by resveratrol
and pterostilbene. Biofactors. 44:61–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Yue Z, Guo M, Fang L, Bai L, Li X,
Tao Y, Wang S, Liu Q, Zhi D and Zhao H: Dietary flavonoid
hyperoside induces apoptosis of activated human LX-2 hepatic
stellate cell by suppressing canonical NF-κB signaling. Biomed Res
Int 2016. 10685282016.
|
|
33
|
Cui H, Yuan J, Du X, Wang M, Yue L and Liu
J: Ethyl gallate suppresses proliferation and invasion in human
breast cancer cells via Akt-NF-κB signaling. Oncol Rep.
33:1284–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|