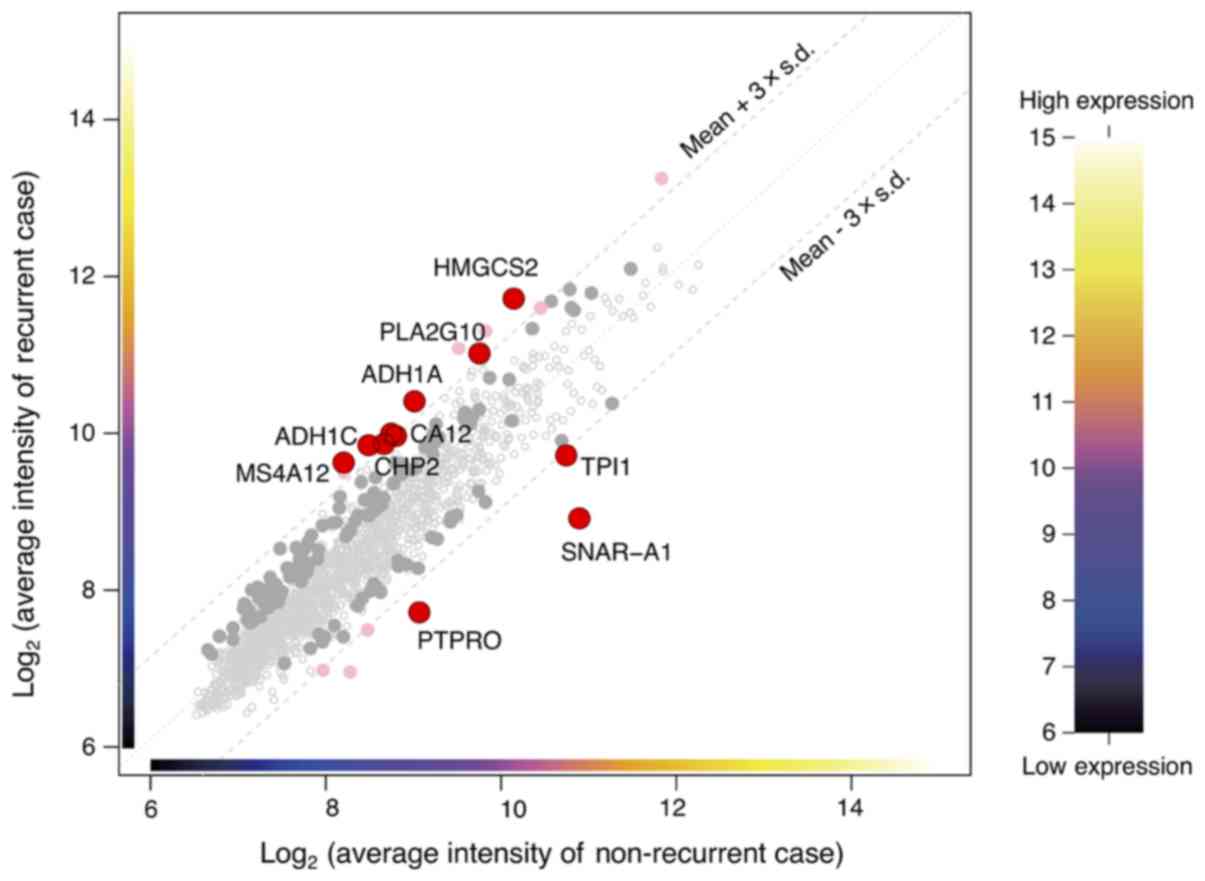
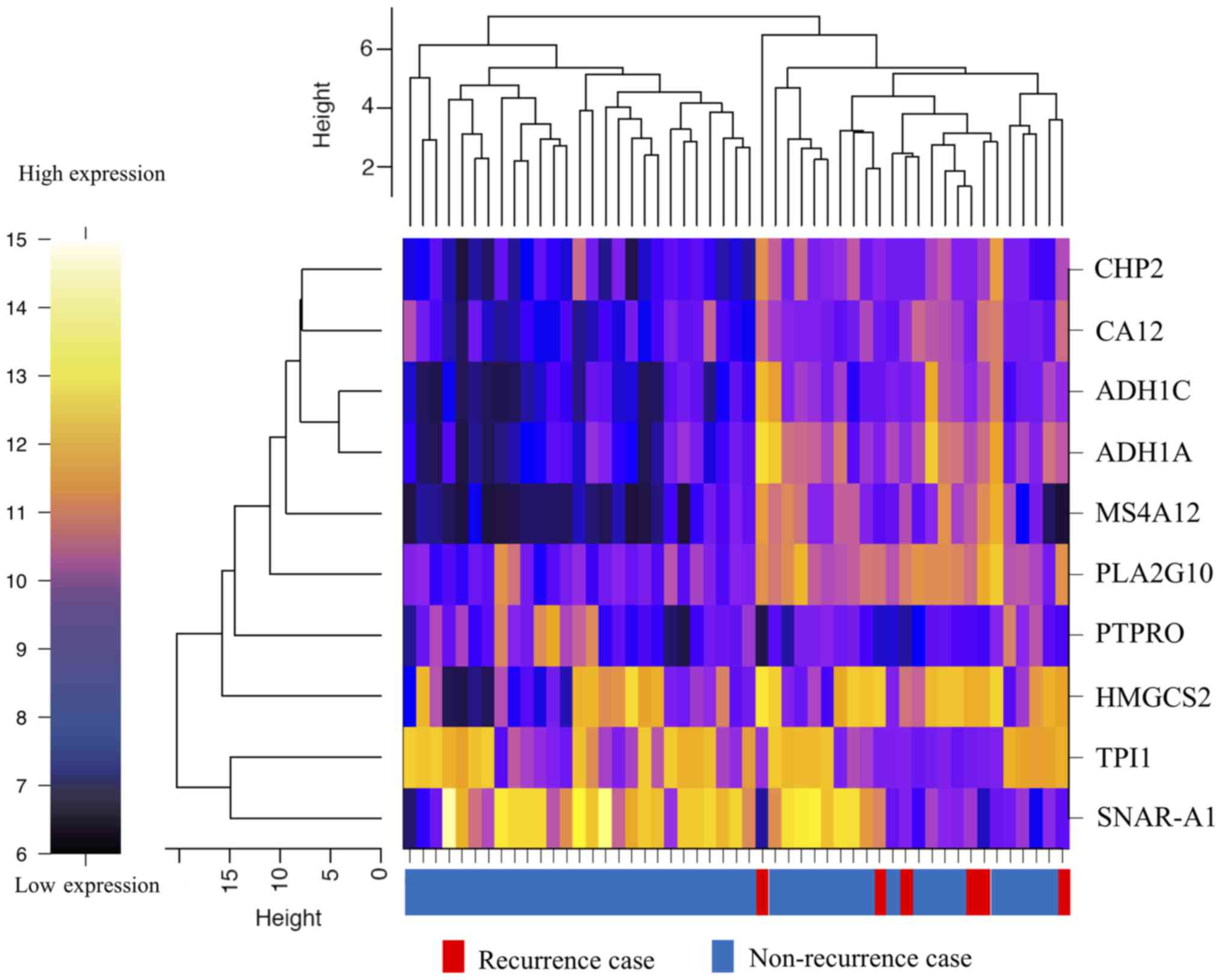
|
1
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmoll HJ, Tabernero J, Maroun J, de
Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K and
Haller DG: Capecitabine plus oxaliplatin compared with
fluorouracil/folinic acid as adjuvant therapy for stage III colon
cancer: Final results of the NO16968 randomized controlled phase
III trial. J Clin Oncol. 33:3733–3740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN Guidelines Version 2. 2018, . Colon
cancer. National comprehensive cancer network. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
|
|
5
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamaguchi T, Shirao K, Moriya Y, Yoshida
S, Kodaira S and Ohashi Y; NSAS-CC Group, : Final results of
randomized trials by the national surgical adjuvant study of
colorectal cancer (NSAS-CC). Cancer Chemother Pharmacol.
67:587–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimatsu K, Ishibashi K, Koda K,
Yokomizo H, Oda N, Oshiro M, Kato H, Oya M, Nakajima H, Ooki S, et
al: A Japanese multicenter phase II study of adjuvant chemotherapy
with mFOLFOX6/CAPOX for stage III colon cancer treatment after
D2/D3 lymphadenectomy. Surg Today. 49:498–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kosugi C, Koda K, Ishibashi K, Yoshimatsu
K, Tanaka S, Kato R, Kato H, Oya M, Narushima K, Mori M, et al:
Safety of mFOLFOX6/XELOX as adjuvant chemotherapy after curative
resection of stage III colon cancer: Phase II clinical study (The
FACOS study). Int J Colorectal Dis. 33:809–817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinicrope FA, Shi Q, Smyrk TC, Thibodeau
SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M,
Goldberg RM, et al: Molecular markers identify subtypes of stage
III colon cancer associated with patient outcomes.
Gastroenterology. 148:88–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaanan A, Fléjou JF, Emile JF, Des GG,
Cuilliere-Dartigues P, Malka D, Lecaille C, Validire P, Louvet C,
Rougier P, et al: Defective mismatch repair status as a prognostic
biomarker of disease-free survival in stage III colon cancer
patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer
Res. 17:7470–7478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinicrope FA, Mahoney MR, Smyrk TC,
Thibodeau SN, Warren RS, Bertagnolli MM, Nelson GD, Goldberg RM,
Sargent DJ and Alberts SR: Prognostic impact of deficient DNA
mismatch repair in patients with stage III colon cancer from a
randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin
Oncol. 31:3664–3672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yothers G, O'Connell MJ, Lee M, Lopatin M,
Clark-Langone KM, Millward C, Paik S, Sharif S, Shak S and Wolmark
N: Validation of the 12-gene colon cancer recurrence score in NSABP
C-07 as a predictor of recurrence in patients with stage II and III
colon cancer treated with fluorouracil and leucovorin (FU/LV) and
FU/LV plus oxaliplatin. J Clin Oncol. 31:4512–4519. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon Y, Park M, Jang M, Yun S, Kim WK, Kim
S, Paik S, Lee HJ, Hong S, Kim TI, et al: Prognosis of stage III
colorectal carcinomas with FOLFOX adjuvant chemotherapy can be
predicted by molecular subtype. Oncotarget. 8:39367–39381. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gray RG, Quirke P, Handley K, Lopatin M,
Magill L, Baehner FL, Beaumont C, Clark-Langone KM, Yoshizawa CN,
Lee M, et al: Validation study of a quantitative multigene reverse
transcriptase-polymerase chain reaction assay for assessment of
recurrence risk in patients with stage II colon cancer. J Clin
Oncol. 29:4611–4619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamanaka T, Oki E, Yamazaki K, Yamaguchi
K, Muro K, Uetake H, Sato T, Nishina T, Ikeda M, Kato T, et al:
12-Gene recurrence score assay stratifies the recurrence risk in
stage II/III colon cancer with surgery alone: The SUNRISE study. J
Clin Oncol. 34:2906–2913. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arango D, Laiho P, Kokko A, Alhopuro P,
Sammalkorpi H, Salovaara R, Nicorici D, Hautaniemi S, Alazzouzi H,
Mecklin JP, et al: Gene-expression profiling predicts recurrence in
Dukes' C colorectal cancer. Gastroenterology. 129:874–884. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Jatkoe T, Zhang Y, Mutch MG,
Talantov D, Jiang J, McLeod HL and Atkins D: Gene expression
profiles and molecular markers to predict recurrence of Dukes' B
colon cancer. J Clin Oncol. 22:1564–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bandrés E, Malumbres R, Cubedo E, Honorato
B, Zarate R, Labarga A, Gabisu U, Sola JJ and García-Foncillas J: A
gene signature of 8 genes could identify the risk of recurrence and
progression in Dukes' B colon cancer patients. Oncol Rep.
17:1089–1094. 2007.PubMed/NCBI
|
|
20
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du P, Kibbe WA and Lin SM: lumi: A
pipeline for processing Illumina microarray. Bioinformatics.
24:1547–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Team RC: R: A language and environment for
statistical computing. R Foundation for statistical computing;
Vienna, Austria: 2015, http://www.R-project.org/PubMed/NCBI
|
|
23
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: Open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinicrope FA, Shi Q, Allegra CJ, Smyrk TC,
Thibodeau SN, Goldberg RM, Meyers JP, Pogue-Geile KL, Yothers G,
Sargent DJ and Alberts SR: Association of DNA mismatch repair and
mutations in BRAF and KRAS with survival after recurrence in stage
III colon cancers: A secondary analysis of 2 randomized clinical
trials. JAMA Oncol. 3:472–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You YN, Rustin RB and Sullivan JD:
Oncotype DX(®) colon cancer assay for prediction of
recurrence risk in patients with stage II and III colon cancer: A
review of the evidence. Surg Oncol. 24:61–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Viikilä P, Kivelä AJ, Mustonen H,
Koskensalo S, Waheed A, Sly WS, Pastorek J, Pastorekova S, Parkkila
S and Haglund C: Carbonic anhydrase enzymes II, VII, IX and XII in
colorectal carcinomas. World J Gastroenterol. 22:8168–8177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kopecka J, Campia I, Jacobs A, Frei AP,
Ghigo D, Wollscheid B and Riganti C: Carbonic anhydrase XII is a
new therapeutic target to overcome chemoresistance in cancer cells.
Oncotarget. 6:6776–6793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen SW, Chou CT, Chang CC, Li YJ, Chen
ST, Lin IC, Kok SH, Cheng SJ, Lee JJ, Wu TS, et al: HMGCS2 enhances
invasion and metastasis via direct interaction with PPARα to
activate Src signaling in colorectal cancer and oral cancer.
Oncotarget. 8:22460–22476. 2017.PubMed/NCBI
|
|
29
|
Lee YE, He HL, Shiue YL, Lee SW, Lin LC,
Wu TF, Chang IW, Lee HH and Li CF: The prognostic impact of lipid
biosynthesis-associated markers, HSD17B2 and HMGCS2, in rectal
cancer treated with neoadjuvant concurrent chemoradiotherapy.
Tumour Biol. 36:7675–7683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koslowski M, Sahin U, Dhaene K, Huber C
and Türeci O: MS4A12 is a colon-selective store-operated calcium
channel promoting malignant cell processes. Cancer Res.
68:3458–3466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asbagh LA, Vazquez I, Vecchione L,
Budinska E, De Vriendt V, Baietti MF, Steklov M, Jacobs B, Hoe N
and Singh S: The tyrosine phosphatase PTPRO sensitizes colon cancer
cells to anti-EGFR therapy through activation of SRC-mediated EGFR
signaling. Oncotarget. 5:10070–10083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi S, Hiwasa T, Arasawa T, Kagaya
A, Ishii S, Shimada H, Ito M, Suzuki M, Kano M, Rahmutulla B, et
al: Identification of specific and common diagnostic antibody
markers for gastrointestinal cancers by SEREX screening using
testis cDNA phage library. Oncotarget. 9:18559–18569. 2018.
View Article : Google Scholar : PubMed/NCBI
|