Introduction
Previous studies (1–3) have
demonstrated that oxaliplatin (Ox)-based adjuvant chemotherapy is
superior to 5-furuolouracil (5-FU)-based adjuvant chemotherapy for
stage III colorectal cancer (CRC) patients, in terms of extending
the disease-free survival (DFS). For patients with stage III CRC,
recent clinical guidelines, including the National Comprehensive
Cancer Network (NCCN) (4) and
European Society for Medical Oncology (ESMO) (5), recommend Ox-based postoperative
adjuvant chemotherapy after successful surgical resection to
prevent recurrence or metastasis; however, 30% of the patients
still develop recurrence, even if postoperative adjuvant
chemotherapy has been successfully completed.
In Japan, oral 5-FU regimens have been used for
stage III CRC patients after curative resection (6). We conducted the FACOS study to verify
the efficacy and safety of Ox+5-FU/LV (FOLFOX therapy) and
Ox+capecitabine (XELOX therapy) as postoperative adjuvant
chemotherapy for Japanese patients with stage III colon cancer (CC)
(7,8). Even in stage III CC patients in whom
Ox-based adjuvant chemotherapy was successfully completed, a subset
of patients still developed tumor recurrence, regardless of
clinicopathological factors. Although there are several reports
(9–11) of prognostic factors, including the
KRAS or BRAF mutation status and proficient or
deficient mismatch repair genes in stage III CC patients receiving
Ox-based adjuvant chemotherapy, only a few reports (12,13) have
demonstrated prognostic molecular markers at the mRNA expression
level to predict recurrence in stage III CC patients receiving
Ox-based adjuvant chemotherapy. Validation studies of the
expressions of 12 genes in Oncotype DX have been reported in stage
II and III CC patients receiving adjuvant FOLFOX treatment
(12). The recurrence score,
calculated based on the gene expression levels (14), was positively correlated with the
disease stage. The SUNRISE Study (15) also reported results consistent with
previous reports (12), even in
stage II and III CC Japanese patients who did not receive any
adjuvant chemotherapy. Another report (13) showed that classification based on the
gene expression profiles is useful to predict the prognosis in
stage III CRC patients receiving adjuvant FOLFOX therapy. The DFS
and overall survival (OS) associated with these classifications
were similar to those associated with classifications based on the
consensus molecular subtypes (CMS) (16). While these classifications are
available to predict poor prognosis in CRC patients, these
molecular signatures are not specific for detecting tumor
recurrence in stage III CC patients. Furthermore, the efficacy of
Ox-based adjuvant chemotherapy following curative resection for
stage III CRC patients is not included in the molecular signature.
Therefore, we investigated the predictive molecular markers of
tumor recurrence specifically in stage III CC patients receiving
adjuvant FOLFOX or XELOX therapy.
In the present study, we performed microarray-based
gene expression profiling for detecting tumor recurrence in a half
of the patients that were registered for the FACOS study.
Microarray-based gene expression profiling has been established as
a method to identify CRC patients with relapse (17–19). In
a previous study (17), 58 genes
were found to be upregulated and 160 genes were found to be
downregulated in Dukes' C CRC patients with a poor prognosis, as
compared to the expression levels in those with a good prognosis,
even though the status of KRAS and TP53 mutations
failed to predict tumor recurrence. It would be convenient to
identify patients with a high risk of tumor recurrence using the
expression profiles of molecular markers, especially a small number
of genes, in cancer tissues derived from resected specimens. In the
present study, we identified a set of molecular markers in patients
with a high risk of tumor recurrence among stage III CC patients
receiving FOLFOX or XELOX treatment.
Patients and methods
Ethical considerations
The present study was conducted with the approval of
the local Ethics Committee of Saitama Medical Center. From every
patient registered for this study, informed consent for
registration was obtained.
Patients and tissue samples
The study was a phase II clinical study to
investigate the efficacy and safety of FOLFOX and XELOX therapy as
postoperative adjuvant chemotherapy for Japanese patients with
stage III CC. Among the 132 CC patients enrolled (7,8), gene
expression analyses using a microarray was conducted in 51
patients. The regimens of mFOLFOX6 and XELOX are described in our
previous report (7,8): Briefly, the mFOLFOX6 regimen comprises
intravenous infusions of oxaliplatin (85 mg/m2) and LV
(200 mg/m2) for 2 h, followed by rapid intravenous bolus
infusion of 5-FU (400 mg/m2) for 5 min, and continuous
intravenous infusion of 5-FU (2,400 mg/m2) for 46 h.
This regimen is repeated every 2 weeks for 12 cycles. The XELOX
regimen comprises intravenous infusion of oxaliplatin (130
mg/m2 over 2 h) on day 1 and oral administration of
capecitabine (1,000 mg/m2 twice daily) from the evening
of day 1 to the morning of day 15. This regimen is repeated every 3
weeks for 8 cycles.
Of 51 patients in whom we analyzed the gene
expression profiles, tumor recurrence was observed in 6 patients
within 5 years. These patients were categorized into the recurrence
group and remaining 45 patients were categorized into the
non-recurrence group for this study.
The cancer and/or normal tissues taken from resected
specimens were immediately frozen in liquid nitrogen and stored at
−80°C until RNA extraction.
Microarray analysis
Before extraction of the total RNAs, each tissue
sample was minced and homogenized in TRIzol reagents (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) on ice. The total RNA
extraction procedure using TRIzol was performed in accordance with
the manufacturer's instructions. The quality of all of the total
RNAs was estimated using BioAnalyzer and the RNA600 nano kit
(Agilent Technologies, Inc., Santa Clara, CA, USA). After DNase I
treatment to remove contaminating DNA from each sample, the total
RNAs were purified using phenol/chloroform/isoamyl alcohol
(25:24:1, v/v) and isopropyl alcohol. The quality and quantity of
the RNAs were finally confirmed with the BioAnalyzer and NanoDrop
spectrometer (Thermo Fisher Scientific, Inc.), respectively.
For the microarray analysis, the extracted RNAs were
amplified and labeled with Cy3-streptavidin (Amersham Biosciences,
Buckinghamshire, UK), using the TargetAmp nano labeling kit for
Illumina Expression BeadChip (Epicentre Biotechnologies, Madison,
WI, USA). The human HT-12 v4 Expression BeadChip kit (Illumina
Inc., San Diego, CA, USA) was used to hybridize the labeled samples
and washed, in accordance with the manufacturer's instructions.
Scanning and data measurements were performed using BeadsStation
500GXDW and GenomeStudio software (Illumina), in accordance with
the manufacturer's standard protocol.
The microarray data were analyzed using the lumi
(2.18.0) (20) and limma (3.22.7)
(21) packages in R/Bioconductor
(version 3.1.3 (22) and 3.0
(23), respectively). Gene
annotations and related information were acquired from NCBI Entrez
Gene (https://www.ncbi.nlm.nih.gov/gene/), UCSC Genome
Browser (https://genome.ucsc.edu/), and Ensembl
(https://genome.ucsc.edu), based on human genome
GRCh38/hg38. Construction of scatter plots, hierarchical
clustering, and heat maps was also performed with R/Bioconductor.
Takeru for Sequencer IV (NABE International Corp, Tsukuba, Japan)
was used for all the calculation of the microarray analysis and
other relevant analyses. All expression data are represented by
their logarithmically transformed (base; 2) values.
Statistical analysis
Associations between categorical variables were
evaluated by the χ2 test, Fisher's exact test or
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference. A survival analysis was
conducted using the Kaplan-Meier method, and the log-rank test was
used to determine the significance of differences between the
survival curves. The period of OS was calculated from the time of
surgery to the date of death from any cause, and was censored at
the time of the last visit to our hospital or March 2018, whichever
came first. All statistical analyses were performed using the SPSS
v.11.0 software (SPSS Inc., Chicago, IL, USA).
Results
Comparison of the clinicopathological
characteristics between the recurrence and non-recurrence
groups
Comparison of the clinicopathological
characteristics between the recurrence and non-recurrence groups is
shown in Table I. Deeper invasion
was significantly associated with recurrence (p=0.0075). There was
no significant difference between the recurrence and non-recurrence
groups in terms of the age, gender, PS, treatment regimen, primary
tumor site, histology, lymph node metastasis, stage classification,
or presence/absence of lymphatic/venous invasion.
 | Table I.Patient characteristics in recurrent
and non-recurrent patients with stage III colon cancer. |
Table I.
Patient characteristics in recurrent
and non-recurrent patients with stage III colon cancer.
|
Characteristics | Recurrent group
(n=6) | Non-recurrent group
(n=45) | P-value |
|---|
| Agea | 68.5 (55–75) | 67 (34–75) | 0.44 |
| Sex
(male:female) | 5:1 | 33:12 | 0.98 |
| PS
(0:1)b | 6:0 | 42:3 | 0.51 |
| Treatment |
|
| 0.17 |
|
mFOLFOX6 | 1 | 25 |
|
|
XELOX | 5 | 20 |
|
| Primary tumor
site |
|
| 0.8 |
|
Cecum | 1 | 1 | (C,S,T vs.
D,S,R) |
|
Ascending colon | 0 | 8 |
|
|
Transverse colon | 0 | 5 |
|
|
Descending colon | 0 | 3 |
|
| Sigmoid
colon | 3 | 15 |
|
|
Rectosigmoid | 2 | 13 |
|
| Depth of
invasionc |
|
| 0.0075 |
| T1 | 0 | 1 | (T1-3 vs.
T4a,b) |
| T2 | 0 | 2 |
|
| T3 | 0 | 27 |
|
|
T4a | 4 | 14 |
|
|
T4b | 2 | 1 |
|
| Type of
histology |
|
| 0.6 |
|
tub1 | 0 | 0 |
|
|
tub2 | 6 | 37 |
|
|
por | 0 | 5 |
|
|
muc | 0 | 3 |
|
| Lymph node
metastasisc |
|
| 0.3 |
| N1 | 3 | 32 |
|
| N2 | 3 | 13 |
|
| Stagec |
|
| 0.15 |
|
IIIA | 0 | 3 |
|
|
IIIB | 2 | 32 |
|
|
IIIC | 4 | 10 |
|
| No. of lymph node
dissectiona | 19.5 (9–28) | 20 (5–67) | 0.35 |
| Lymphatic
invasion | 5 | 38 | 0.6 |
| Venous
invasion | 6 | 38 | 0.68 |
| Preoperative
complications |
|
|
|
|
Perforation | 1 | 0 |
|
| Colon
obstruction | 0 | 1 | 0.55 |
| Lymph node
dissectiond |
|
|
|
| D2 | 0 | 4 |
|
| D3 | 6 | 41 | 0.45 |
Candidate genes for prediction of
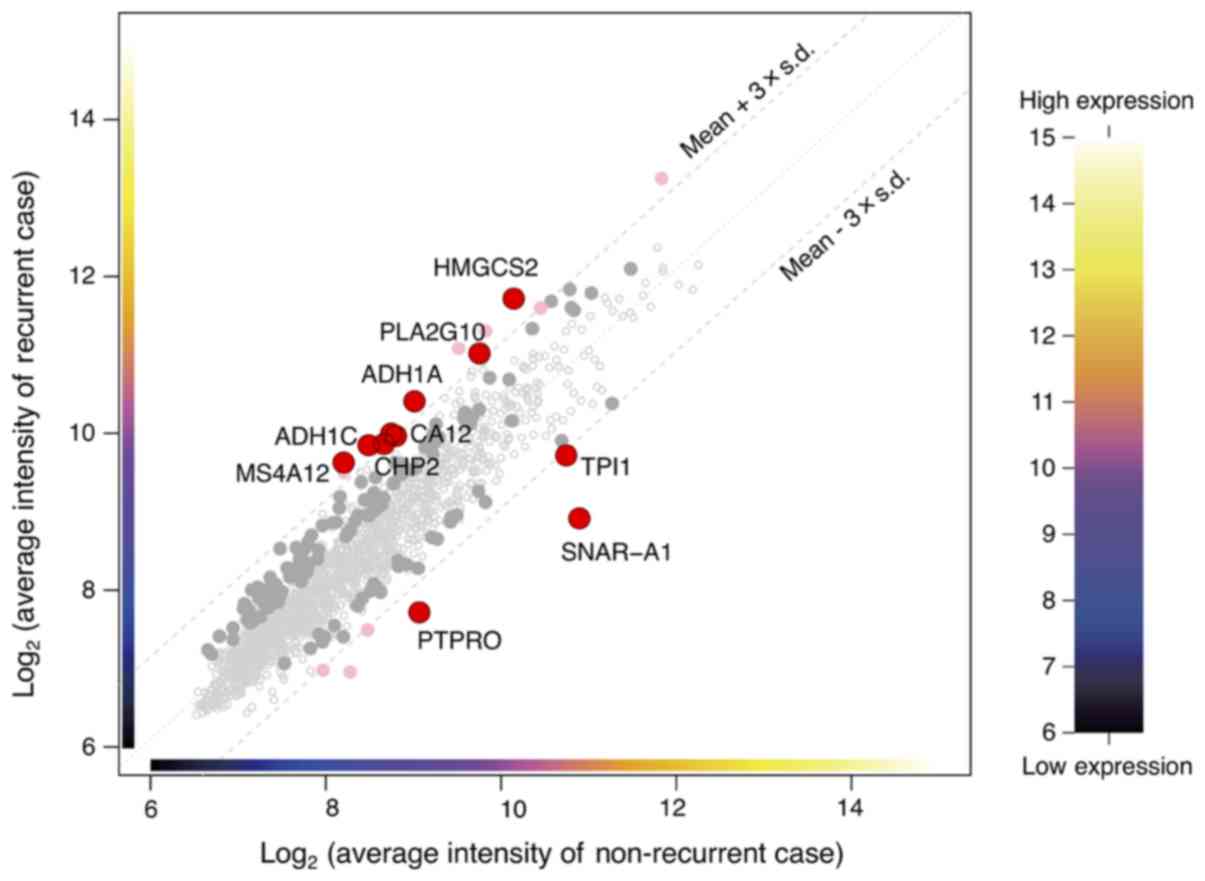
tumor recurrence
From the microarray (which contained a total of
34,694 genes), initially, the analysis dataset of 1,734 genes was
selected as a collection of genes showing the top 5% values of the
coefficient of variation among the 51 samples. Then, the
differentially expressed genes (10 genes) between the two groups
were detected using two criteria to filter the dataset. One of the
criteria was a P-value of the difference of less than 0.05, and the
other was a difference in the expression levels of the genes in
dataset by at least threefold of the standard deviation (±) from
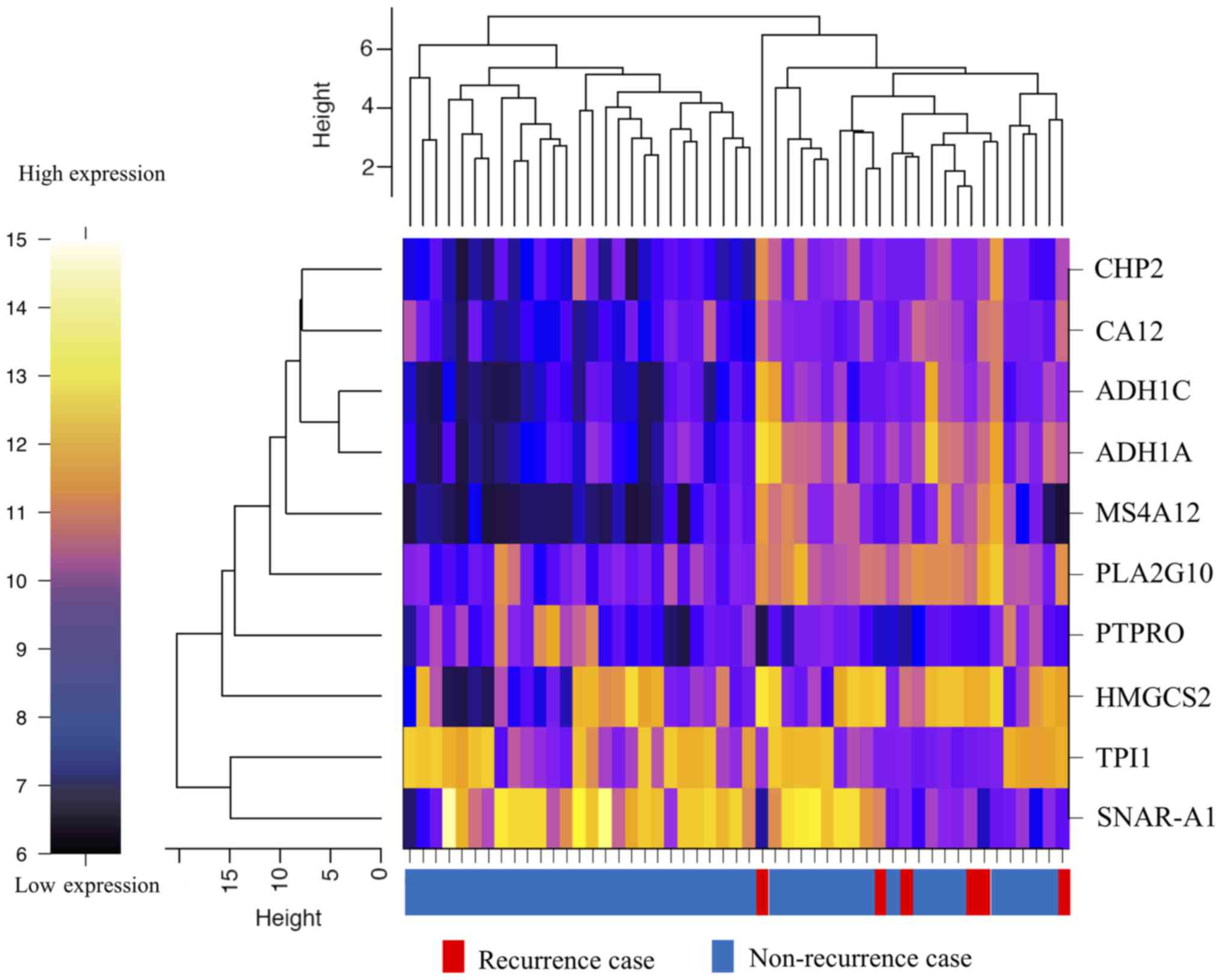
the mean (Fig. 1). The heat map
showed that determination of the expressions of 10 genes (alcohol
dehydrogenase 1A (ADH1A), alcohol dehydrogenase 1C
(ADH1C), carbonic anhydrase XII (CA12),
calcineurin-like EF-hand protein 2 (CHP2), mitochondrial
3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS2), small NF90
(ILF3)-associated RNA A1 (SNAR-A1), triosephosphate
isomerase 1 (TPI1), membrane-spanning 4-domain A12
(MS4A12), phospholipase A2 group X (PLA2G10), protein
tyrosine phosphatase, receptor type O (PTPRO)) allowed
classification of the specimens into the recurrence and
non-recurrence groups (Fig. 2). The
expression level of each of these genes was significantly different
between the recurrence and non-recurrence groups (Table II); while the expression levels of 7
of the genes (ADH1A, ADH1C, CA12, CHP2, HMGCS2, MS4A12, and
PLA2G10) were significantly higher in the recurrence group,
those of 3 of the remaining genes (SNAR-A1, TPI1, and
PTPRO) were significantly lower in the recurrence group as
compared to the non-recurrence group (Table II).
 | Table II.The differental expression of 10
genes for prediction of tumor recurrence. |
Table II.
The differental expression of 10
genes for prediction of tumor recurrence.
| Gene symbol | Log2
ratio (RG/NRG) | P-value | Gene name | Accession no. |
|---|
| ADH1A | 1.412 | 0.026 | Alcohol
dehydrogenase 1A | NM_000667 |
| ADH1C | 1.377 | 0.015 | Alcohol
dehydrogenase 1C | NM_000669 |
| CA12 | 1.182 | 0.009 | Carbonic anhydrase
XII | NM_001218 |
| CHP2 | 1.217 | 0.011 | Calcineurin-like
EF-hand protein 2 | NM_022097 |
| HMGCS2 | 1.589 | 0.029 | Mitochondrial
3-hydroxy-3-methylglutaryl-CoA synthase 2 | NM_005518,
NM_001166107 |
| SNAR-A1 | −1.962 | 0.009 | Small
ILF3/NF90-associated RNA A1 (non-coding RNA) | BU536065,
NR_004435 |
| TPI1 | −1.008 | 0.043 | Triosephosphate
isomerase 1 | XM_001725700,
NM_000365 |
| MS4A12 | 1.441 | 0.036 | Membrane spanning
4-domains A12 | NM_017716 |
| PLA2G10 | 1.282 | 0.005 | Phospholipase A2
group X | NM_003561 |
| PTPRO | −1.335 | 0.007 | Protein tyrosine
phosphatase, receptor type O | NM_030667,
NM_002848 |
Discussion
In the present study, conducting using the database
of the FACOS study, we found novel predictive molecular markers for
recurrence in stage III CC patients receiving postoperative
adjuvant oxaliplatin-based chemotherapy (8). The recurrence group could be
definitively identified according to the expression levels of 10
genes, including ADH1A, ADH1C, CA12, CHP2, HMGCS2, SNAR-A1,
TPI1, MS4A12, PLA2G10, and PTPRO; high expression levels
of 7 genes (ADH1A, ADH1C, CA12, CHP2, HMGCS2, MS4A12, and
PLA2G10) and low expression levels of 3 genes (SNAR-A1,
TPI1, and PTPRO) were associated with tumor recurrence.
In the FACOS study, tumor recurrence developed in 27 (20.5%) of the
132 CC patients. In this study, 6 patients (11.8%) with tumor
recurrence included for the mRNA expression analysis in 51 CC
patients. Of the 6 patients with relapse, 2 patients were stage
IIIB [among the 34 stage IIIB patients (5.9%)] and the remaining 4
patients were stage IIIC [among the 14 stage IIIC patients
(28.6%)]. In regard to the depth of invasion, all the 6 patients
with tumor recurrence had T4 disease [among the 21 CC patients with
T4 disease (28.6%)]. Although tumor recurrence was statistically
associated with deep invasion, it may be difficult to predict
recurrence strictly based on the clinicopathological
characteristics alone, since patients with recurrence constituted
only a small part of the clinicopathological categories. Along with
deep invasion as one of the predictive clinicopathological
characteristics, our 10-gene signature is expected to be useful to
predict patients with tumor recurrence in stage III CC patients
receiving Ox-based adjuvant chemotherapy.
Previous studied have identified KRAS or
BRAF mutation is one of the prognostic biomarkers in stage
III CC patients receiving adjuvant FOLFOX therapy (9,11,24).
Defective mismatch repair gene (dMMR) is also reported as an
independent prognostic factor in stage III CC patients receiving
adjuvant FOLFOX therapy (9–11,24); in
particular, right-sided colon patients with dMMR had good outcomes
(11,24). Consequently, patients with both
proficient MMR (pMMR)-tumors and KRAS or BRAF
mutations are at risk for poor outcomes. These evidences may be
relevant to clinical practice, since they are consistent with the
results of randomized clinical trials, including the N0470
(9,11), conducted in stage III CC patients.
However, the KRAS and/or BRAF mutation status alone
selects approximately a half of stage III CC patients since these
mutation types account for 40 and 10% of CC patients, respectively.
Moreover, the frequency of CC patients with dMMR is 15% at the
most, while that of CC patients with proficient MMR (pMMR) accounts
for the remaining 85%. The range of prediction for patients with
tumor recurrence is still wide. In this study, 3 of the patients
with recurrence were found to have wild-type KRAS, when they
were examined as having KRAS after the detection of tumor
recurrence. Therefore, the KRAS and/or BRAF status
alone is not sufficient to identify patients having the potential
for tumor recurrence.
A 12-gene CC recurrence score in the Oncotype DX
colon cancer assay has been advocated for the prediction of tumor
recurrence in patients with stage II and III CC (12,15,25). The
12 genes include 7 recurrence genes (FAP, INHBA, BGN, Ki-67,
C-MYC, MYBL2, GADD45B) and 5 reference genes (ATP5E, GPX1,
PGK1, UBB, VDAC2), and the recurrence score is calculated using
reference-normalized expression measurements (25). This 12-gene CC recurrence score has
been reported to be correlated with the risk of tumor recurrence in
a large number of stage II and III CC patients (12,15).
However, this score does not bear a direct relationship to the use
of Ox-based adjuvant chemotherapy. We analyzed the expression
profile of the 12 genes in our samples (data not shown), and the 6
patients with tumor recurrence could not be classified into same
cluster. Although we did not calculate the recurrence score
according to the formula (25), we
consider that the molecular set of the 10 genes that we identified
in the present study is more reliable to classify patients with
tumor recurrence.
Recently, CC has been classified under 4 CMSs
according to the genetic changes, including in MMR, RAS,
BRAF, methylation pattern, and the gene related to the WNT
signaling pathway (16). Of these 4
subtypes, CMS4 tumor (mesenchymal type), which is characterized by
stromal infiltration, activation of transforming growth factor-beta
(TGF-β), and angiogenesis, and is associated with a worse
relapse-free survival and OS. CMS1 tumor (microsatellite
instability immune type), which shows microsatellite instability,
hypermethylation, and immune activation, is associated with worse
survival after relapse. Kwon et al (13), found molecular subtypes in stage III
CRC patients given FOLFOX adjuvant chemotherapy. These subtypes
were similar to the CMS classification in terms of the relapse-free
survival and OS. Although 10 up-regulated genes were identified in
each subtype, the 10 genes that were identified in this study did
not include those genes. Classification according to the CMS and
other molecular subtypes are promising classifications for
prediction of the prognosis in CRC patients. However, we have tried
to identify patients with tumor recurrence in stage III CC patients
according to expression profiling of a small number of specific
genes.
None of the 10 genes identified in this study,
including ADH1A, ADH1C, CA12, CHP2, HMGCS2, SNAR-A1, TPI1,
MS4A12, PLA2G10 and PTPRO, have been documented in
previous reports (17–19) in which microarray-based gene
expression profiling has been performed for prediction of tumor
recurrence in stage II/III CRC patients not receiving Ox-based
adjuvant chemotherapy. Among the 10 genes, overexpression of
CA12 and HMGCS2 seem to be the most likely to be
associated with chemoresistance and poor prognosis in patients with
CRC. CA12 is one of the carbonic anhydrase isoforms which catalyzes
reversible hydration of CO2 to bicarbonate for
maintenance of pH homeostasis in the human body (26). A previous report (27) demonstrated that CA12 expression was
correlated with the expression of P-glycoprotein, resulting in the
acquisition of chemoresistance in CRC cancer cells. In regard to
the clinical significance of CA12 expression, increased intensity
of immunohistochemical staining for CA12 was reported to be
significantly associated with poor survival in CRC patients
(26). HMGCS2 is the rate-limiting
enzyme that catalyzes acetyl-CoA to ketone bodies (28). HMGCS2 expression has been reported to
be enhanced in the tumor tissue in rectal cancer patients
administered preoperative chemoradiotherapy, and HMGCS2
overexpression to be associated with a poor disease-free survival,
local recurrence-free survival, and metastasis-free survival
(29). Furthermore, a recent report
(28) has indicated that HMGCS2
enhances cellular invasion and metastasis in CRC. Both CA12 and
HMGCS2 are considered to be potential targets for cancer treatment.
Of the remaining 8 genes, MS4A12 (30) and PTPRO (31) have been documented to be involved in
the epidermal growth factor signaling pathway in CRC. TPI1 has been
detected as an auto-antibody in the sera of CRC patients (32). No associations between the
expressions of other genes and the clinical outcomes in CRC
patients have been reported. Although tumor recurrence was
statistically associated with deep invasion in this clinical
information, the genes associated with deep invasion may be
missing. In this study, we found a novel 10 gene-expression
signature. Since the biological functions of several of these genes
have been established, our 10 gene-expression signature may be a
promising biomarker for the prediction of tumor recurrence in stage
III CC patients receiving Ox-based adjuvant chemotherapy.
The main limitations of this study were the small
study population and the fact that there were only six recurrence
events. The cut-off value of the 10 genes could not be determined
using a realtime PCR method due to insufficient samples. However,
we would still like to emphasize the potential usefulness of
determining the 10-gene expression status in Japanese patients with
stage III CC receiving Ox-based adjuvant chemotherapy for
prediction of the risk of tumor recurrence. We will plan to perform
prospective study to confirm the relationship between the
expression levels of these 10 genes and tumor recurrence in stage
III CC patients.
In summary, determination of the expression levels
of a novel set of 10 genes that we found in the present study may
be a useful means to predict the risk of recurrence in stage III CC
patients receiving an Ox-based adjuvant chemotherapy, although
several prediction models of recurrence have reported for CC. Based
on the presence of the 10 gene-expression signature, high-risk CC
patients should be carefully observed to detect tumor recurrence
during the follow-up period.
Acknowledgements
Not applicable.
Funding
The present study was conducted in collaboration
with Yakult Honsha Co., Ltd., and was sponsored by Yakult Honsha
Co., Ltd. (Tokyo, Japan). (grant no. Kyodo23021). The involvement
of our collaborators had no impact or bearing on study design or
analysis.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KKu and YN drafted the manuscript. YN, YM, HE and YO
performed microarray experiments and contributed to the
bioinformatics analysis. MY, KI, CK, KKo, MK, KT and TM provided
the tissue samples and clinical information, and made substantial
contributions to conception, analysis and interpretation of data.
KKu, YO and HI conceived and designed the study, and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted with the approval of the
local Ethics Committee of Saitama Medical Center (approval no.
305-IV). Patients were required to provide written informed consent
prior to enrollment.
Patient consent for publication
All the patients have written informed consent for
the publication of any associated data.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmoll HJ, Tabernero J, Maroun J, de
Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K and
Haller DG: Capecitabine plus oxaliplatin compared with
fluorouracil/folinic acid as adjuvant therapy for stage III colon
cancer: Final results of the NO16968 randomized controlled phase
III trial. J Clin Oncol. 33:3733–3740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN Guidelines Version 2. 2018, . Colon
cancer. National comprehensive cancer network. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
|
|
5
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamaguchi T, Shirao K, Moriya Y, Yoshida
S, Kodaira S and Ohashi Y; NSAS-CC Group, : Final results of
randomized trials by the national surgical adjuvant study of
colorectal cancer (NSAS-CC). Cancer Chemother Pharmacol.
67:587–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimatsu K, Ishibashi K, Koda K,
Yokomizo H, Oda N, Oshiro M, Kato H, Oya M, Nakajima H, Ooki S, et
al: A Japanese multicenter phase II study of adjuvant chemotherapy
with mFOLFOX6/CAPOX for stage III colon cancer treatment after
D2/D3 lymphadenectomy. Surg Today. 49:498–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kosugi C, Koda K, Ishibashi K, Yoshimatsu
K, Tanaka S, Kato R, Kato H, Oya M, Narushima K, Mori M, et al:
Safety of mFOLFOX6/XELOX as adjuvant chemotherapy after curative
resection of stage III colon cancer: Phase II clinical study (The
FACOS study). Int J Colorectal Dis. 33:809–817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinicrope FA, Shi Q, Smyrk TC, Thibodeau
SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M,
Goldberg RM, et al: Molecular markers identify subtypes of stage
III colon cancer associated with patient outcomes.
Gastroenterology. 148:88–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaanan A, Fléjou JF, Emile JF, Des GG,
Cuilliere-Dartigues P, Malka D, Lecaille C, Validire P, Louvet C,
Rougier P, et al: Defective mismatch repair status as a prognostic
biomarker of disease-free survival in stage III colon cancer
patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer
Res. 17:7470–7478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinicrope FA, Mahoney MR, Smyrk TC,
Thibodeau SN, Warren RS, Bertagnolli MM, Nelson GD, Goldberg RM,
Sargent DJ and Alberts SR: Prognostic impact of deficient DNA
mismatch repair in patients with stage III colon cancer from a
randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin
Oncol. 31:3664–3672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yothers G, O'Connell MJ, Lee M, Lopatin M,
Clark-Langone KM, Millward C, Paik S, Sharif S, Shak S and Wolmark
N: Validation of the 12-gene colon cancer recurrence score in NSABP
C-07 as a predictor of recurrence in patients with stage II and III
colon cancer treated with fluorouracil and leucovorin (FU/LV) and
FU/LV plus oxaliplatin. J Clin Oncol. 31:4512–4519. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon Y, Park M, Jang M, Yun S, Kim WK, Kim
S, Paik S, Lee HJ, Hong S, Kim TI, et al: Prognosis of stage III
colorectal carcinomas with FOLFOX adjuvant chemotherapy can be
predicted by molecular subtype. Oncotarget. 8:39367–39381. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gray RG, Quirke P, Handley K, Lopatin M,
Magill L, Baehner FL, Beaumont C, Clark-Langone KM, Yoshizawa CN,
Lee M, et al: Validation study of a quantitative multigene reverse
transcriptase-polymerase chain reaction assay for assessment of
recurrence risk in patients with stage II colon cancer. J Clin
Oncol. 29:4611–4619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamanaka T, Oki E, Yamazaki K, Yamaguchi
K, Muro K, Uetake H, Sato T, Nishina T, Ikeda M, Kato T, et al:
12-Gene recurrence score assay stratifies the recurrence risk in
stage II/III colon cancer with surgery alone: The SUNRISE study. J
Clin Oncol. 34:2906–2913. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arango D, Laiho P, Kokko A, Alhopuro P,
Sammalkorpi H, Salovaara R, Nicorici D, Hautaniemi S, Alazzouzi H,
Mecklin JP, et al: Gene-expression profiling predicts recurrence in
Dukes' C colorectal cancer. Gastroenterology. 129:874–884. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Jatkoe T, Zhang Y, Mutch MG,
Talantov D, Jiang J, McLeod HL and Atkins D: Gene expression
profiles and molecular markers to predict recurrence of Dukes' B
colon cancer. J Clin Oncol. 22:1564–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bandrés E, Malumbres R, Cubedo E, Honorato
B, Zarate R, Labarga A, Gabisu U, Sola JJ and García-Foncillas J: A
gene signature of 8 genes could identify the risk of recurrence and
progression in Dukes' B colon cancer patients. Oncol Rep.
17:1089–1094. 2007.PubMed/NCBI
|
|
20
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du P, Kibbe WA and Lin SM: lumi: A
pipeline for processing Illumina microarray. Bioinformatics.
24:1547–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Team RC: R: A language and environment for
statistical computing. R Foundation for statistical computing;
Vienna, Austria: 2015, http://www.R-project.org/PubMed/NCBI
|
|
23
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: Open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinicrope FA, Shi Q, Allegra CJ, Smyrk TC,
Thibodeau SN, Goldberg RM, Meyers JP, Pogue-Geile KL, Yothers G,
Sargent DJ and Alberts SR: Association of DNA mismatch repair and
mutations in BRAF and KRAS with survival after recurrence in stage
III colon cancers: A secondary analysis of 2 randomized clinical
trials. JAMA Oncol. 3:472–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You YN, Rustin RB and Sullivan JD:
Oncotype DX(®) colon cancer assay for prediction of
recurrence risk in patients with stage II and III colon cancer: A
review of the evidence. Surg Oncol. 24:61–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Viikilä P, Kivelä AJ, Mustonen H,
Koskensalo S, Waheed A, Sly WS, Pastorek J, Pastorekova S, Parkkila
S and Haglund C: Carbonic anhydrase enzymes II, VII, IX and XII in
colorectal carcinomas. World J Gastroenterol. 22:8168–8177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kopecka J, Campia I, Jacobs A, Frei AP,
Ghigo D, Wollscheid B and Riganti C: Carbonic anhydrase XII is a
new therapeutic target to overcome chemoresistance in cancer cells.
Oncotarget. 6:6776–6793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen SW, Chou CT, Chang CC, Li YJ, Chen
ST, Lin IC, Kok SH, Cheng SJ, Lee JJ, Wu TS, et al: HMGCS2 enhances
invasion and metastasis via direct interaction with PPARα to
activate Src signaling in colorectal cancer and oral cancer.
Oncotarget. 8:22460–22476. 2017.PubMed/NCBI
|
|
29
|
Lee YE, He HL, Shiue YL, Lee SW, Lin LC,
Wu TF, Chang IW, Lee HH and Li CF: The prognostic impact of lipid
biosynthesis-associated markers, HSD17B2 and HMGCS2, in rectal
cancer treated with neoadjuvant concurrent chemoradiotherapy.
Tumour Biol. 36:7675–7683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koslowski M, Sahin U, Dhaene K, Huber C
and Türeci O: MS4A12 is a colon-selective store-operated calcium
channel promoting malignant cell processes. Cancer Res.
68:3458–3466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asbagh LA, Vazquez I, Vecchione L,
Budinska E, De Vriendt V, Baietti MF, Steklov M, Jacobs B, Hoe N
and Singh S: The tyrosine phosphatase PTPRO sensitizes colon cancer
cells to anti-EGFR therapy through activation of SRC-mediated EGFR
signaling. Oncotarget. 5:10070–10083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi S, Hiwasa T, Arasawa T, Kagaya
A, Ishii S, Shimada H, Ito M, Suzuki M, Kano M, Rahmutulla B, et
al: Identification of specific and common diagnostic antibody
markers for gastrointestinal cancers by SEREX screening using
testis cDNA phage library. Oncotarget. 9:18559–18569. 2018.
View Article : Google Scholar : PubMed/NCBI
|