Introduction
In China, endometrial cancer is one of the most
common malignant carcinomas of the female reproductive organs, and
its incidence has steadily increased (1). The vast majority of endometrial cancer
cases are endometrioid adenocarcinoma (EA) cases (2,3). This
type of uterine cancer forms in the glandular cells of the uterine
lining (2). EA is commonly detected
early and has a high cure rate (4,5). A
number of risk factors are involved in the etiology of endometrial
cancer, including obesity, diabetes and ageing of the population
(6). It is believed that
tumorigenesis results from cell growth dysregulation and the
failure of the host to provide a sufficient antitumor immune
response (7). The immune system,
which is markedly influenced by age and certain hormones, has been
reported to be associated with the development and progression of
endometrial cancer (6). Immune
cells, including tumor-associated macrophages, cluster of
differentiation (CD)8+ cytotoxic T cells, natural killer
(NK) cells, CD4+ T helper cells and dendritic cells
(DCs) constitute an important part of the microenvironment and are
thought to be critical for endometrial cancer development and
progression (6). However, the
precise mechanisms by which the immune system is modulated in
patients with endometrial cancer remain poorly understood.
Regulatory T cells (Tregs), characterized by the
co-expression of CD4 and CD25, have been revealed to suppress T
cell-mediated host immune responses against self- and
non-self-antigens (8–10). The transcription factor Forkhead box
protein P3 (FOXP3) is considered to be one of the most specific
markers of Tregs. Evidence has confirmed that Tregs are involved in
the immune tolerance of malignant neoplasms, as they suppress
immune responses by cytokine secretion and direct contact (11). Increased levels of Tregs have been
reported at tumor sites, draining lymph nodes and in the peripheral
blood in many kinds of human cancer, including breast, colon, lung,
prostate, liver, ovarian cancer and hematological malignancies
(12–16). An increased level of Tregs in human
cancer is considered to be a poor prognostic factor. Studies have
attempted to reduce the number of Tregs in order to control cancer
in experimental models; Turk et al (17) demonstrated enhanced tumor immunity in
mouse models by depletion of Tregs.
Some researchers have reported the presence of Tregs
in the tumor tissue, peripheral blood and tumor draining lymph
nodes of patients with endometrial cancer (18–21).
High levels of intratumoral were revealed to associate with poorer
disease-free survival, increased grade of differentiation, cancer
stage, the extent of lymph node metastases and myometrial invasion
(18,20). The mechanisms underlying Treg
enrichment in patients with endometrial cancer are not clear; the
proportion and the role of Tregs in the peripheral blood of
patients with endometrial cancer is still ambiguous and
controversial, as a limited number of small studies have been
reported (18,21). The increased number of Tregs in the
peripheral blood of patients might be responsible for suppressing
anti-tumor immunity.
In the present study, to elucidate the role of Tregs
in EA, the frequencies and suppressive functions of Tregs in the
peripheral blood of patients with EA and healthy controls were
evaluated. The association between the frequency of Tregs in the
peripheral blood of patients with EA and certain clinical
prognostic parameters was also analyzed.
Materials and methods
Study participants
The present study was approved by the Ethical
Committee of Zhejiang Cancer Hospital (Hangzhou, China). Written
informed consent was obtained from all participants. The current
study included a total of 82 female patients with EA who were
admitted to the Department of Gynecologic Oncology at Zhejiang
Cancer Hospital between August 2012 and June 2015, in addition to
30 healthy women who were recruited during routine health checkups
at Zhejiang Cancer Hospital, enrolled as the controls. The age
range of the patients with EA was 30–70 years, while the age range
of the healthy controls was 31–69 years. All patients with EA were
diagnosed by endometrial biopsy prior to recruitment. At the
recruitment stage, none of the patients had received chemotherapy,
radiotherapy or any other previous medical intervention. Prior to
surgery, 20 ml heparinized peripheral venous blood was obtained,
and the patients with EA underwent subsequent staging surgery; the
surgical specimens were examined by experienced pathologists.
Clinical and pathological parameters, including age, menopausal
status, stage, grade of differentiation, lymphatic or vascular
permeation and lymph node metastatic status were evaluated for each
patient. The surgical staging of EA was performed according to the
International Federation of Gynecology and Obstetrics (FIGO)
classification (22). None of the
participants had received hormonal or immunosuppressant therapy for
≥6 months prior to staging. A total of 20 ml heparinized peripheral
venous blood was also obtained from healthy controls.
Flow cytometry
A total of 20 ml peripheral venous blood was
collected from each participant into sterile heparinized container.
Peripheral blood mononuclear cells (PBMCs) were isolated by
Ficoll-Hypaque (GE Healthcare Life Sciences, Uppsala, Sweden)
density gradient separation for 30 min at 800 × g at 20°C. The
PBMCs were washed twice in PBS and then resuspended in PBS at
1×106 cells/ml for further analysis. PBMCs were analyzed
by flow cytometry for the phenotypic characterization of Tregs. In
brief, PBMCs were incubated with specific antibodies (1:10
dilution; mouse anti-human, monoclonal; Beckman Coulter, Brea, CA,
USA) against phycoerythrin-cyanine 5-conjugated anti-CD4 (cat. no.
A07752), fluorescein isothiocyanate-conjugated anti-CD25 (cat. no.
IM0478U) and phycoerythrin-conjugated anti-CD127 (cat. no. IM1980
U), or isotype controls (1:10 dilution; IgG1-FITC, cat. no. A07795;
and IgG1-PE, cat. no. A07796; Beckman Coulter), in the dark for 30
min at 20°C. Subsequently, a washing step was performed using PBS.
For blocking, cells were suspended in 0.2% (w/v) BSA (cat. no.
554657; BD Biosciences, San Jose, CA, USA) with Human BD Fc Block™
(cat. no. 564220; BD Biosciences) for 10 min at 20°C. Data from
1×105 cells/sample were acquired using the FC500 flow
cytometer (Beckman Coulter, Brea, CA, USA) and analyzed with Win 7
CXP version 2.2 software (Beckman Coulter). To determine the
relative ratio of Tregs, the CD4+ population was gated,
followed by the CD25+CD127− population. FOXP3
is widely accepted as the most reliable marker for Tregs, thus
validation experiments were performed by intracellular FOXP3
staining with the Anti-Human Foxp3 Staining Set APC kit (cat. no.
77-5776-40; eBioscience; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) according to the manufacturer's protocol;
CD4+CD25+CD127− cells with high
expression of FOXP3 (>90%) were confirmed.
Cell culture, cytokine and
proliferation assays
PBMCs from the peripheral venous blood of patients
with EA and healthy controls were isolated by Ficoll-Hypaque
density gradient separation.
CD4+CD25+CD127− Tregs and
CD4+CD25− T cells were purified with the MACS
CD4 Multisort kit (cat. no. 130-055-101), CD25 (cat. no.
130-092-983) and CD127 (cat. no. 130-094-945) Microbeads (Miltenyi
Biotec, Auburn, CA, USA) using magnetic separation columns,
according to the manufacturer's protocol. The purities of the
enriched cells were >92% (determined by flow cytometry).
Purified CD4+CD25+CD127− Tregs and
CD4+CD25− T cells (1×105 cells)
were placed on anti-CD3 mAb (10 ng/ml; cat. no. M725429-2; DAKO;
Agilent Technologies, Inc., Santa Clara, CA, USA)-coated 96-well
flat-bottomed plates and cultured in 200 µl AIM-V medium (Thermo
Fisher Scientific, Inc.) at 37°C for 24 h.
CD4+CD25+CD127− Tregs and
CD4+CD25− T cells were stimulated with
immobilized anti-CD3 mAbs. The supernatants were harvested and the
level of IFN-γ and IL-10 production was detected using the Human
IFN-γ Quantikine ELISA kit (cat. no. DIF50; R&D Systems, Inc.,
Minneapolis MN, USA) and the Human IL-10 Quantikine ELISA kit (cat.
no. D1000B; R&D Systems, Inc.), according to the manufacturer's
protocols. Subsequently, the anti-proliferative function of
CD4+CD25+CD127− Tregs was assessed
by evaluating the proliferative activity of
CD4+CD25− T cells in response to anti-CD3
plus anti-CD28 antibodies, in the presence of autologous
CD4+CD25+CD127− cells. Briefly,
purified CD4+CD25+CD127− Tregs
were incubated with autologous CD4+CD25− T
cells (1×105 cells each) on anti-CD3 mAb (10
ng/ml)-coated 96-well round-bottomed plates in the presence of an
anti-CD28 mAb (10 µg/ml; cat. no. 555726; BD Biosciences) for 72 h.
Proliferation was measured by incorporation of
3H-thymidine (10 uCi/ml). Cells were harvested after 16
h and thymidine incorporation was measured using a scintillation
counter and expressed as counts per minutes.
Statistical analysis
All data were expressed as the mean ± standard
deviation. All experiments were performed at least three times.
Comparisons between two groups were analyzed using the Student's
t-test for normally distributed variables. ANOVA followed by
Bonferroni correction was performed for more than two groups of
data. Spearman's rank correlation coefficient test was used to
analyze the association between clinical prognostic parameters and
Treg frequency. P<0.05 was considered to indicate a
statistically significant difference. Analyses were performed using
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics
All endometrial cancer cases were classified as EA.
The mean age of the patients with EA was 53.65±7.06 years, whilst
the mean age of healthy controls was 51.58±6.83 years; though this
difference was not significant. A total of 28 patients with EA were
<50 years old, while the other 54 patients were ≥50 years old.
Among the 82 patients, 22 were premenopausal and 60 were
postmenopausal.
Increased Treg/CD4+ ratio
in the peripheral blood of patients with EA
The CD4+CD25+CD127−
Treg/CD4+ ratio in the peripheral blood of patients with
EA was 4.89±1.42%, significantly higher compared with that in
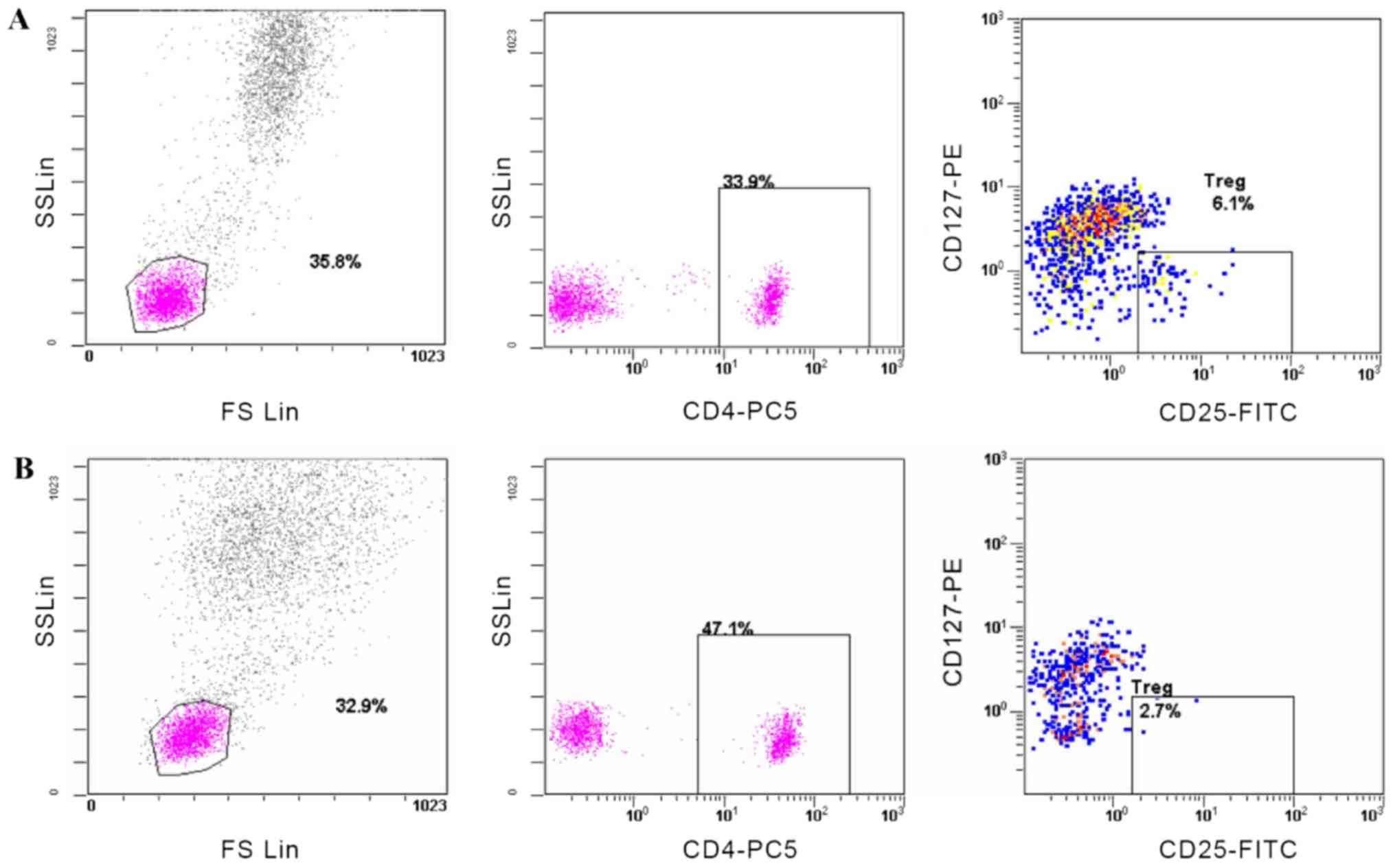
healthy controls (3.34±0.84; P=0.019; Table I). Representative flow cytometry
plots illustrate the percentage of Tregs in both groups (Fig. 1). As presented in Table II, no significant difference in
CD4+CD25+CD127− Treg frequency was
observed among patients with EA in association with tumor stage
(P=0.753) or differentiation grade (P=0.686). As endometrial cancer
occurs predominantly in postmenopausal women (23), 82 patients with EA were divided into
postmenopausal (n=60) and premenopausal (n=22) groups; no
significant difference in
CD4+CD25+CD127− Treg frequency was
observed in association with menopausal status (P=0.581). As the
majority of women reach the menopause at ~50 years of age (1),
CD4+CD25+CD127− Treg frequency
between EA patients <50 years-old and those ≥50 years-old was
compared; no significant difference was observed (P=0.667). No
association between Treg frequency and tumor stage, grade of
differentiation, menopausal status or age was observed.
 | Table I.Percentage of
CD4+CD25+CD127− Tregs within the
CD4+ cell population in the peripheral blood. |
Table I.
Percentage of
CD4+CD25+CD127− Tregs within the
CD4+ cell population in the peripheral blood.
| Group |
CD4+CD25+CD127−
Tregs/CD4+ T cells (%) |
|---|
| Endometrioid
adenocarcinoma (n=82) |
4.89±1.42a |
| Healthy controls
(n=30) | 3.34±0.84 |
 | Table II.Association between Treg frequency
and clinicopathological factors. |
Table II.
Association between Treg frequency
and clinicopathological factors.
| Clinicopathological
factor |
CD4+CD25+CD127−
Tregs/CD4+T cells (%) | P-value |
|---|
| Stag |
| 0.753 |
| I
(n=59) | 4.90±1.45 |
|
| II
(n=8) | 4.88±1.47 |
|
| III
(n=15) | 4.88±1.45 |
|
| Grade |
| 0.686 |
| G1
(n=17) | 4.89±1.46 |
|
| G2
(n=42) | 4.89±1.45 |
|
| G3
(n=23) | 4.91±1.41 |
|
| Menopausal
status |
| 0.581 |
|
Premenopausal (n=22) | 4.87±1.43 |
|
|
Postmenopausal (n=60) | 4.90±1.46 |
|
| Age, years |
| 0.667 |
| <50
(n=28) | 4.86±1.43 |
|
| ≥50
(n=54) | 4.89±1.43 |
|
Cytokines produced by
CD4+CD25+CD127− Tregs
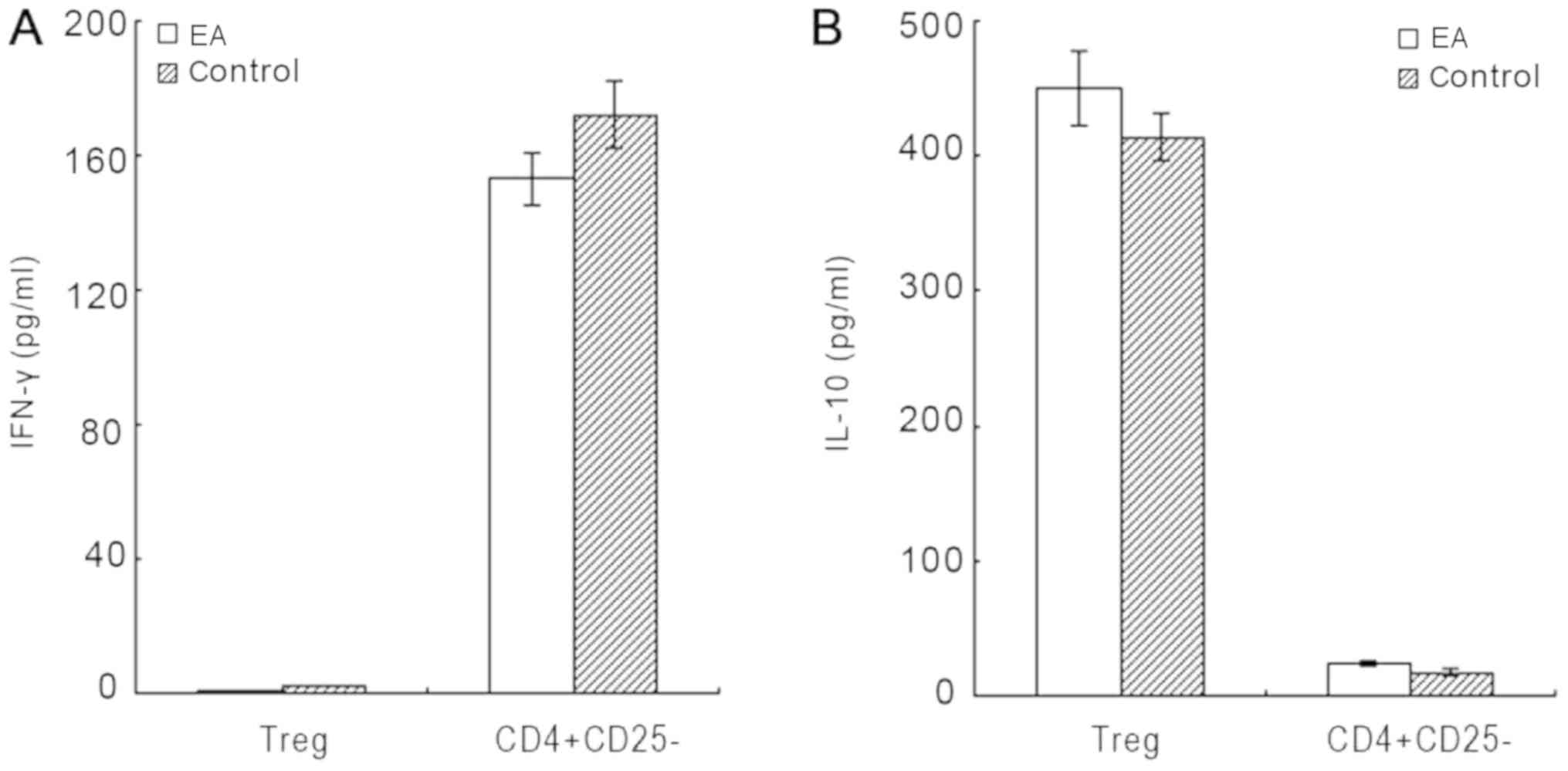
As illustrated in Fig.
2, CD4+CD25+CD127− Tregs
derived from patients with EA produced little IFN-γ, but large
amounts of IL-10. CD4+CD25− T cells derived
from both groups secreted large amounts of IFN-γ but notably less
IL-10. Similarly, CD4+CD25+CD127−
Tregs derived from healthy controls secreted little IFN-γ, but
large amounts of IL-10. There was no significant difference in
IL-10 secretion by Tregs between the two groups (P=0.274).
Treg suppression assays
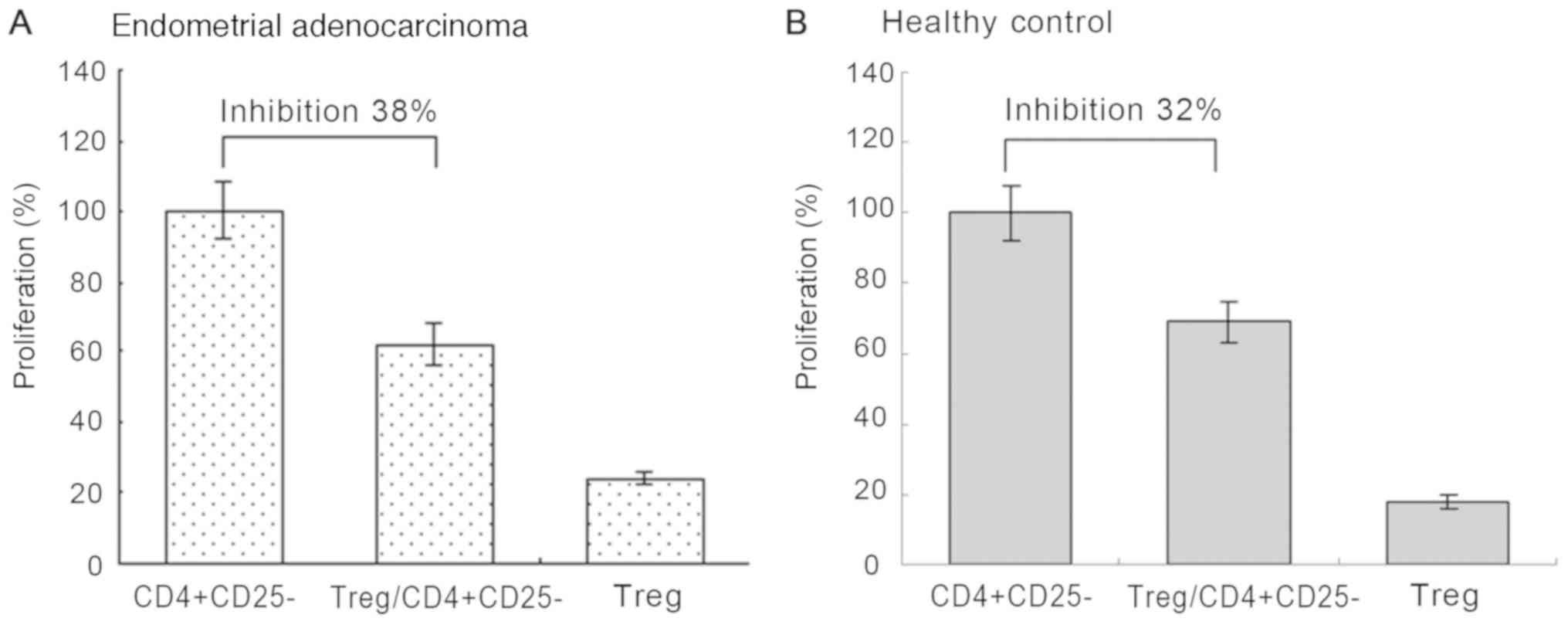
As presented in Fig.
3, it was confirmed that
CD4+CD25+CD127− Tregs markedly
suppress the proliferation of CD4+CD25− T
cells. However, no significant difference was observed in the
suppressive activity of Tregs between patients with EA (suppressive
efficacy, 38%; Fig. 3A) and healthy
controls (suppressive efficacy, 32%; Fig. 3B; P=0.196).
Discussion
The present study indicated that patients with EA
have increased Treg frequencies in their peripheral blood compared
with healthy controls. In line with a previous study (15), a significantly increased
CD4+CD25+CD127−
Treg/CD4+ ratio in the peripheral blood of patients with
EA with a suppressive function was revealed. These
CD4+CD25+CD127− Tregs produced
high levels of IL-10 and suppressed the proliferation of
CD4+CD25− T cells. However, there was no
correlation with Treg frequency in association with stage,
histological grading, menopausal status and age, which was in
contrast to other studies (18,21).
FOXP3 is considered as the most specific marker for
Tregs and is co-expressed with CD4 and CD25 (24). However, another study has reported
that intracellular FOXP3 staining may cause cell damage (25), thus the
CD4+CD25+CD127− phenotype has been
proposed as an alternative to identify Tregs in clinical samples.
In the present study, it was confirmed that
CD4+CD25+CD127− cells may be
recognized as a highly purified population of FOXP3+
Tregs.
However, in the present study, no correlation
between Treg frequency and stage, histological grade, menopausal
status or age was observed. Notably, some contrasting results were
revealed when compared with other similar studies. It is widely
accepted that tumor stage, grade of differentiation and myometrial
invasion are independent prognostic factors for endometrial cancer.
Chang et al (18) reported
that the high prevalence of CD4+CD25+ Tregs
in the cancer stroma and in the peripheral blood of 57 patients
with endometrial cancer was closely associated with clinical
features, including tumor grade, stage, lymph node metastasis and
myometrium invasion. Sawan et al (26) revealed an increased proportion of
Tregs in the peripheral blood of 24 patients with endometrial
cancer, which may have been attributed to their postmenopausal
status or age, but not with cancer stage or grade. The lack of
agreement among researchers on the associations between the Treg
frequency in the peripheral blood and clinical prognostic
parameters may be attributed to different cancer types and/or
different sample sizes. In the present study, all of the 82 samples
collected were EA, whilst patients with non-endometrioid
adenocarcinoma (NEA) were excluded. Different mechanisms are
involved in the pathogenesis of EA and NEA (3,27). It
was therefore hypothesized that the inhibition of the immune
response in patients with endometrial cancers may be influenced by
several inhibitory mechanisms, including the number of Tregs in the
peripheral blood, the tumor microenvironment and the cancer
type.
The association between age and circulating Tregs in
humans has been investigated in numerous studies (26,28–30), and
the function of Tregs is thought to be age-dependent (25). However, there is conflicting evidence
as to whether Treg frequency in the peripheral blood increases with
age or not (29,30). A number of studies concerning Tregs
in the peripheral blood of patients with cancer did not investigate
the association between age and Treg frequency, and more studies
did not report the age of the control group (11,12,15,31).
Although others provided this data, the healthy controls were
significantly younger compared with patients (26,28–30). A
lack of age-matched controls in such studies may mean that their
results may be misleading. The present study investigated the
effect of aging on Treg frequency by analyzing the correlation
between age and Treg frequency; the mean age of patients with EA
was similar to the healthy controls, and the difference was not
significant. As endometrial cancer usually occurs in postmenopausal
women (23), and women in China
predominantly reach the menopause at ~50 years of age (1),
CD4+CD25+CD127− Treg frequency
between EA patients <50 years old and those ≥50 years old was
compared; however, no statistically significant difference was
observed. Therefore, differences in Treg frequency between patients
with EA and healthy women were not associated with age.
To the best of our knowledge, there has been only
one study (21) regarding the role
of menopause in Treg frequency or function in patients with EA.
Menopause is characterized by cessation of ovarian functions,
including hormonal release, representing the end of a woman's
reproductive life. The majority of cases of endometrial cancer are
diagnosed in post-menopausal patients when blood estrogen levels
are low, and >80% of cases are EA (6,27).
Increased exposure to estrogen is considered to be an important
risk factor of EA, and previous studies (32,33) have
reported that Treg frequency changed during the menstrual cycle;
the number of Tregs increased in the proliferative phase when
compared with that in the secretory phase (32,33). In
addition, estrogen has been reported to induce Treg proliferation
in vitro (1). In the present
study, a higher proportion of patients with EA were postmenopausal.
However, there was no association between Treg frequency in the
peripheral blood and menopausal status in either patients with EA
or in healthy women. Therefore, the observed difference in Treg
frequency between patients with EA and healthy women did not appear
to associate with menopausal status. These findings were in
contradiction to a previous study (21). In order to investigate the true
association between EA and Treg frequency, a larger cohort of
patients is required with appropriately matched controls.
IFN-γ is an inflammatory cytokine that can exhibit
direct cytotoxic and cytostatic activity toward tumor cells, and
inhibits the peripheral induction of naive CD4+ T cells
to FOXP3+ Tregs (34,35).
IL-10, a cytokine produced by Tregs, can directly and indirectly
inhibit effector T cell responses in cancer (36–38). It
is suggested that IL-10 production by Tregs may mediate immune
suppression in the human tumor microenvironment. Tumor-derived
Tregs are reported to suppress DC function by producing IL-10
(39). The results of the present
study suggest that Tregs produced large amounts of IL-10,
confirming their suppressive activity. Tumorigenesis is correlated
with increased prevalence of Tregs in the peripheral blood
(8). Previous studies have reported
that Tregs suppress the antigen presentation function of DCs, the
proliferation and activation of CD8+ T cells,
CD4+ T cells, NK cells and NKT cells (11,40,41). In
patients with melanoma, Tregs can regulate tumor-specific
CD4+ T cell responses. Therefore, it was hypothesized
that increased Treg frequency in patients with EA may be
responsible for impaired cellular immunity. It was revealed that
CD4+CD25+CD127− Tregs suppress the
proliferation of CD4+CD25− T cells, which
subsequently confirmed that
CD4+CD25+CD127− Tregs isolated
from patients with EA suppressed the T cell response. However, the
level of IL-10 secreted by Tregs from patients and healthy controls
was not significantly different. There was also no significant
difference in the suppressive activity of Tregs between patients
and healthy controls. It was therefore concluded that the
suppressive function of Tregs might be relatively stable in
different situations, though the number of Tregs alters in patients
with EA, which may influence the regulation of the antitumor immune
response by direct and indirect contact with other immune
cells.
In conclusion, an increased frequency of Tregs with
suppressive function in patients with EA may serve a role in
regulating the antitumor immune response. However, no correlation
between Treg frequency and tumor stage, differentiation grade,
menopausal status or age was observed. As peripheral Tregs may be
recruited to tumor sites and proliferate as a result of
self-antigen recognition by memory Tregs, it may be more
informative to monitor tumor-infiltrating Tregs in patients with
EA, rather than circulating Tregs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LQ18H160019), the Zhejiang Provincial Medical Science and
Technology Plan (grant no. 2016KYA035) and the Zhejiang Provincial
Science and Technology Planning Project for Chinese Medicine (grant
no. 2016ZA040).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and HL contributed to the conception of this
study and performed the preliminary experiments. All authors
participated in the design of the study and implemented the
research. DY and LL enrolled participants in the study and
collected clinical data. JZ performed the flow cytometry
experiments. LL, YL, ZY and JZ determined cytokine levels and cell
proliferation. All authors participated in the statistical analysis
and contributed to the interpretation of the results as well as the
writing of the study. All authors reviewed all data and approved
the final manuscript.
Ethics approval and consent to
participate
This study followed international and national
regulations in accordance with the Declaration of Helsinki. The
present study was approved by the Ethical Committee of Zhejiang
Cancer Hospital (Hangzhou, China). Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Tregs
|
regulatory T cells
|
|
EA
|
endometrioid adenocarcinoma
|
References
|
1
|
Li L, Wu J, Pu D, Zhao Y, Wan C, Sun L,
Shen C, Sun W, Yuan Z, Shen Q, et al: Factors associated with the
age of natural menopause and menopausal symptoms in Chinese women.
Maturitas. 73:354–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–e278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fung-Kee-Fung M, Dodge J, Elit L, Lukka H,
Chambers A and Oliver T; Cancer Care Ontario Program in
Evidence-based Care Gynecology Cancer Disease Site, : Follow-up
after primary therapy for endometrial cancer: A systematic review.
Gynecol Oncol. 101:520–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lajer H, Elnegaard S, Christensen RD,
Ortoft G, Schledermann DE and Mogensen O: Survival after stage IA
endometrial cancer; can follow-up be altered? A prospective
nationwide Danish survey. Acta Obstet Gynecol Scand. 91:976–982.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vanderstraeten A, Tuyaerts S and Amant F:
The immune system in the normal endometrium and implications for
endometrial cancer development. J Reprod Immunol. 109:7–16. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wing K and Sakaguchi S: Regulatory T cells
exert checks and balances on selftolerance and autoimmunity. Nat
Immunol. 11:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shevach EM: Mechanisms of foxp3+ T
regulatory cell-mediated suppression. Immunity. 30:636–645. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beyer M and Schultze JL: Regulatory T
cells in cancer. Blood. 108:804–811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller AM, Lundberg K, Ozenci V, Banham
AH, Hellstrom M, Egevad L and Pisa P: CD4+CD25high T cells are
enriched in the tumor and peripheral blood of prostate cancer
patients. J Immunol. 177:7398–7405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perez SA, Karamouzis MV, Skarlos DV,
Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos
G, Baxevanis CN, Rigatos G and Papamichail M: CD4+CD25+ Regulatory
T-cell frequency in HER-2/neu (HER)-positive and HER-negative
advanced-stage breast cancer patients. Clin Cancer Res.
13:2714–2721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanagiri T, Shigematsu Y, Shinohara S,
Takenaka M, Oka S, Chikaishi Y, Nagata Y, Iwata T, Uramoto H, So T
and Tanaka F: Clinical significance of the frequency of regulatory
T cells in regional lymph node lymphocytes as a prognostic factor
for non-small-cell lung cancer. Lung Cancer. 81:475–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sellitto A, Galizia G, De Fanis U, Lieto
E, Zamboli A, Orditura M, De Vita F, Giunta R, Lucivero G and
Romano C: Behavior of circulating CD4+CD25+Foxp3+ regulatory t
cells in colon cancer patients undergoing surgery. J Clin Immunol.
31:1095–1104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wertel I, Surówka J, Polak G, Barczyński
B, Bednarek W, Jakubowicz-Gil J, Bojarska-Junak A and Kotarski J:
Macrophage-derived chemokine CCL22 and regulatory T cells in
ovarian cancer patients. Tumour Biol. 36:4811–4817. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Turk MJ, Guevara-Patiño JA, Rizzuto GA,
Engelhorn ME Sakaguchi S and Houghton AN: Concomitant tumor
immunity to a poorly immunogenic melanoma is prevented by
regulatory T cells. J Exp Med. 200:771–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang WC, Li CH, Huang SC, Chang DY, Chou
LY and Sheu BC: Clinical significance of regulatory T cells and
CD8+ effector populations in patients with human endometrial
carcinoma. Cancer. 116:5777–5788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fattorossi A, Battaglia A, Ferrandina G,
Buzzonetti A, Legge F, Salutari V and Scambia G: Lymphocyte
composition of tumor draining lymph nodes from cervical and
endometrial cancer patients. Gynecol Oncol. 92:106–115. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamagami W, Susumu N, Tanaka H, Hirasawa
A, Banno K, Suzuki N, Tsuda H, Tsukazaki K and Aoki D:
Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory t
cells is relevant to the prognosis of patients with endometrial
cancer. Int J Gynecol Cancer. 21:1628–1634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawan S, Burt DJ, Stern PL, Holland C and
Elkord E: Circulating regulatory T cells in endometrial cancer: A
role for age and menopausal status. Immunol Invest. 40:62–75. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1092009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong A, Johnson N, Kitchener HC and Lawrie
TA: Adjuvant radiotherapy for stage I endometrial cancer: An
updated cochrane systematic review and meta-analysis. J Natl Cancer
Inst. 104:1625–1634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee
MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St
Groth B, et al: CD127 expression inversely correlates with FoxP3
and suppressive function of human CD4+ T reg cells. J Exp Med.
203:1701–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sawan S, Burt DJ, Stern PL, Holland C and
Elkord E: Circulating regulatory T cells in endometrial cancer: A
role for age and menopausal status. Immunol Invest. 40:62–75. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsaknaridis L, Spencer L, Culbertson N,
Hicks K, LaTocha D, Chou YK, Whitham RH, Bakke A, Jones RE, Offner
H, et al: Functional assay for human CD4+CD25+ Treg cells reveals
an age-dependent loss of suppressive activity. J Neurosci Res.
74:296–308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santner-Nanan B, Seddiki N, Zhu E, Quent
V, Kelleher A, Fazekas de St Groth B and Nanan R: Accelerated
age-dependent transition of human regulatory T cells to effector
memory phenotype. Int Immunol. 20:375–383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dejaco C, Duftner C and Schirmer M: Are
regulatory T-cells linked with aging? Exper Gerontol. 41:339–345.
2006. View Article : Google Scholar
|
|
31
|
Yao X, Ahmadzadeh M, Lu YC, Liewehr DJ,
Dudley ME, Liu F, Schrump DS, Steinberg SM, Rosenberg SA and
Robbins PF: Levels of peripheral CD4(+)Foxp3(+) regulatory T cells
are negatively associated with clinical response to adoptive
immunotherapy of human cancer. Blood. 119:5688–5696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arruvito L, Sanz M, Banham AH and Fainboim
L: Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the
follicular phase of the menstrual cycle: Implications for human
reproduction. J Immunol. 178:2572–2578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Hamarneh T, Hey-Cunningham AJ, Berbic
M, Al-Jefout M, Fraser IS and Black K: Cellular immune environment
in endometrial polyps. Fertil Steril. 100:1364–1372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murphy KM, Ouyang W, Szabo SJ, Jacobson
NG, Guler ML, Gorham JD, Gubler U and Murphy TL: T helper
differentiation proceeds through Stat1-dependent, Stat4-dependent
and Stat4-independent phases. Curr Top Microbiol Immunol.
238:13–26. 1999.PubMed/NCBI
|
|
35
|
Caretto D, Katzman SD, Villarino AV, Gallo
E and Abbas AK: Cutting edge: The Th1 response inhibits the
generation of peripheral regulatory T cells. J Immunol. 184:30–34.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asseman C, Mauze S, Leach MW, Coffman RL
and Powrie F: An essential role for interleukin 10 in the function
of regulatory T cells that inhibit intestinal inflammation. J Exp
Med. 190:995–1004. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Belkaid Y, Piccirillo CA, Mendez S,
Shevach EM and Sacks DL: CD4+CD25+ regulatory T cells control
Leishmania major persistence and immunity. Nature. 420:502–507.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Loser K, Apelt J, Voskort M, Mohaupt M,
Balkow S, Schwarz T, Grabbe S and Beissert S: IL-10 controls
ultraviolet-induced carcinogenesis in mice. J Immunol. 179:365–371.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Larmonier N, Marron M, Zeng Y, Cantrell J,
Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S and
Katsanis E: Tumor-derived CD4(+)CD25(+) regulatory T cell
suppression of dendritic cell function involves TGF-beta and IL-10.
Cancer Immunol Immunother. 56:48–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vieweg J, Su Z, Dahm P and Kusmartsev S:
Reversal of tumor-mediated immunosuppression. Clin Cancer Res.
13:727s–732s. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Curr Opin Immunol. 27:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|