Introduction
Duodenal adenocarcinoma that originates in the
mucosal epithelium is the most common type of duodenal tumor and in
2014 accounted for 15–25% of cases of cancer of the small intestine
in the United States (1). However,
<1% of all cases of gastrointestinal cancer are diagnosed as
duodenal adenocarcinoma, and this may be due to the duodenum being
the shortest part of the small intestine (2,3). The
primary treatment for duodenal adenocarcinoma is surgery, with
pancreatoduodenectomy and segmental resection being the most
commonly used (4). The rarity of
this condition means that there is no consensus about the best
adjuvant treatment strategy (5), and
the scope of resection for duodenal adenocarcinoma remains
controversial (6). Previous studies
have suggested that regional lymph node metastasis is associated
with lower survival rates in patients with duodenal adenocarcinoma
(7–9). However, prognostic factors such as age,
sex, tumor size, pathology grade and American Joint Committee on
Cancer (AJCC) Tumor-Node-Metastasis (TNM) stage were not consistent
with that result (10). Furthermore,
relatively little is known about the effect of marital status on
the outcome of duodenal adenocarcinoma, and the data that is
available may be affected by the small sample size (11).
Extra emphasis is now being placed on the role of
social determinants in disease development (12). Social support forms an important part
of patient screening, treatment and follow-up care. It has been
suggested that spouses tend to encourage early screening and
adherence to treatment, thereby improving outcomes (13). Therefore, the potential importance of
social conditions should not be ignored in patients with duodenal
adenocarcinoma, especially given that married patients reportedly
have improved survival outcomes in breast, ovarian, colon, and head
and neck cancer (11,14–16).
According to a previous study based on information in the
Surveillance, Epidemiology and End Results (SEER) database, married
patients are more likely to be diagnosed with earlier stages of
cancer and therefore, the treatment regimens may be more effective
compared with those in unmarried patients (11).
The aim of the present study was to elucidate the
effects of marital status on the prognosis of patients with
duodenal adenocarcinoma. The study investigated the impact of
marital status on both overall survival (OS) and cause-specific
survival (CSS) by analyzing demographic data obtained from the SEER
database.
Materials and methods
Patient population and study
design
Sponsored by the National Cancer Institute, the SEER
program collects demographic, clinicopathological and survival data
on a per-patient basis from 18 cancer registries across the United
States, Hawaii and Alaska (17). The
SEER database (https://seer.cancer.gov/seerstat/software/)was used to
identify 2,018 patients with adenocarcinoma of the duodenum
diagnosed between January 2004 and December 2015. Primary cancer
site and histology were coded according to the criteria in the
third edition of the International Classification of Diseases for
Oncology (18). Major loci and
morphological code C17.0 were used to define tumors localized to
duodenum, and the histological recode broad groupings were used to
identify the nature of the tumor. Codes 8140-8389 were used to
define adenomas and adenocarcinomas. Patients were excluded if the
duodenal adenocarcinoma was not the first primary tumor, the
marital status was unknown, the age at diagnosis was <18 years
or there was missing information on ethnicity, pathological grade,
surgery, AJCC TNM stage or follow-up time. The primary outcomes of
the present study were OS and CSS, which were defined as time until
death from any cause and time until death caused by duodenal
adenocarcinoma after diagnosis of duodenal adenocarcinoma,
respectively. Death attributed to duodenal adenocarcinoma was
regarded as an event. Patients who died from other causes or were
still alive at the end of the last follow-up in December 2015 were
treated as censored observations.
Study variables
The demographic and clinicopathological data were
extracted from the SEER database, including year of diagnosis, age,
sex, ethnicity, marital status, pathological grade, AJCC TNM stage
and surgery versus no surgery. Patients were divided into three
groups according to the age at diagnosis (≤58, 59–75 and >75
years), into four groups according to the year of diagnosis
(2004–2006, 2007–2009, 2010–2012 and 2013–2015) and into three
groups according to ethnicity (Caucasian, African descent and
others). The AJCC TNM stage was established according to the
criteria described in the 6th edition of the AJCC Cancer Staging
Manual (19), and as this staging
system was publicly released in 2004, the study was limited to
patients diagnosed between 2004 and 2015. Marital status is coded
in the SEER database as married, divorced, widowed, separated,
never married, or domestic partner. The codes were combined by
classifying patients as either married (including married and
domestic partner) or unmarried (including never married, unmarried,
divorced, separated and widowed).
Statistical analysis
Patient characteristics between the two groups were
compared, and it was determined whether the continuous variables
conformed to a normal distribution; those that did are expressed as
the mean ± SD, whereas those that did not are expressed as median
values with the 25 and 75th percentiles provided. Continuous
variables with a normal distribution were statistically compared
using Student's t-test. Continuous variables that were not normally
distributed were statistically compared using a Mann-Whitney U
test. The categorical variables were compared using Pearson's
χ2 test. OS and CSS were calculated using Kaplan-Meier
plots method and a log-rank test was used to compare differences
between the groups in the Kaplan-Meier plots. A Cox
proportional-hazards model was constructed to identify factors that
were independently associated with the prognosis. Cox multivariate
analysis included age as a categorical variable (≤58, 59–75 and
>75 years). The two-sided probability values were calculated.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
(version 24.0; IBM Corp.).
Results
Baseline characteristics
A total of 2,018 eligible patients with duodenal
adenocarcinoma diagnosed between 2004 and 2015 were identified in
the SEER database. Table I shows the
baseline characteristics of the patients stratified by marital
status. Of the 2,018 patients, 1,227 (60.80%) were married and 791
(39.20%) were unmarried, with median ages of 67 and 69 years,
respectively. The married group was comprised of considerably more
males than females (63.33 vs. 36.67%), whereas the opposite was
true in the unmarried group (37.04 vs. 62.96%). There were large
proportions of white patients in the married and unmarried groups
(79.38 and 66.62%, respectively), and also large proportions of
patients who had received surgical interventions (64.38 and 56.89,
respectively). There were statistically significant intergroup
differences with regard to age (P<0.001), sex (P<0.001),
ethnicity (P<0.001), AJCC TNM stage (P<0.001) and surgical
details (P=0.001).
 | Table I.Baseline demographic and tumor
characteristics of patients in the Surveillance, Epidemiology and
End Results database. |
Table I.
Baseline demographic and tumor
characteristics of patients in the Surveillance, Epidemiology and
End Results database.
| Characteristic | Total | Married, n (%) | Unmarried, n
(%) | P-value |
|---|
| Patients, n
(%) | 2,018 (100.00) | 1,227 (60.80) | 791 (39.20) |
|
| Median age
(25th-75th percentile), years | 68 (58–76) | 67 (57–75) | 69 (59–79) | <0.001 |
| Year of diagnosis,
n (%) |
|
|
| 0.053 |
|
2004–2006 | 409 (20.27) | 262 (21.35) | 147 (18.58) |
|
|
2007–2009 | 466 (23.09) | 261 (21.27) | 205 (25.92) |
|
|
2010–2012 | 556 (27.55) | 351 (28.61) | 205 (25.92) |
|
|
2013–2015 | 587 (29.09) | 353 (28.77) | 234 (29.58) |
|
| Sex, n (%) |
|
|
| <0.001 |
|
Female | 948 (46.98) | 450 (36.67) | 498 (62.96) |
|
|
Male | 1,070 (53.02) | 777 (63.33) | 293 (37.04) |
|
| Ethnicity, n
(%) |
|
|
| <0.001 |
|
Caucasian | 1,501 (74.38) | 974 (79.38) | 527 (66.62) |
|
| African
descent | 358 (17.74) | 156 (12.71) | 202 (25.54) |
|
|
Other | 159 (7.88) | 97 (7.91) | 62 (7.83) |
|
| Grade, n (%) |
|
|
| 0.357 |
| I | 376 (18.63) | 216 (17.60) | 160 (20.23) |
|
| II | 908 (45.00) | 569 (46.37) | 339 (42.86) |
|
|
III | 705 (34.94) | 425 (34.64) | 280 (35.40) |
|
| IV | 29 (1.44) | 17 (1.39) | 12 (1.52) |
|
| AJCC TNM stage, n
(%) |
|
|
| <0.001 |
| I | 406 (20.12) | 217 (17.69) | 189 (23.89) |
|
| II | 387 (19.18) | 248 (20.21) | 139 (17.57) |
|
|
III | 609 (30.18) | 406 (33.09) | 203 (25.66) |
|
| IV | 616 (30.53) | 356 (29.01) | 260 (32.87) |
|
| Surgery, n (%) |
|
|
| <0.001 |
|
Yes | 1,240 (61.45) | 790 (64.38) | 450 (56.89) |
|
| No | 778 (38.55) | 437 (35.62) | 341 (43.11) |
|
Marital status and OS
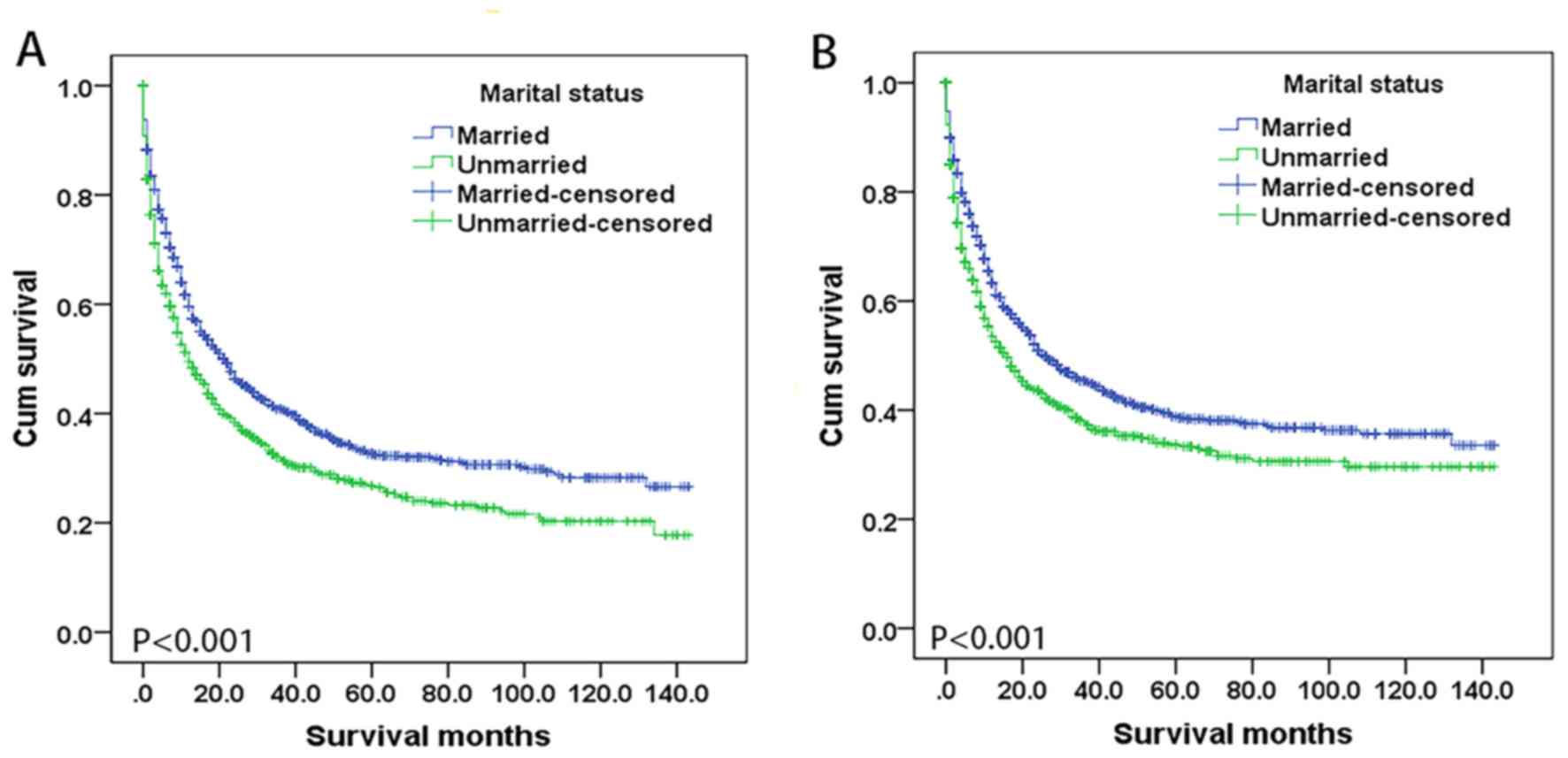
Survival differed according to marital status
(P<0.001), as shown by the Kaplan-Meier curve for OS in Fig. 1A. OS time was higher in married
patients compared with that in unmarried patients, with median
values of 22 and 12 months, respectively. Similarly, the 5-year OS
rate was higher in married patients compared with that in unmarried
patients (32.6 vs. 26.8%). In univariate analysis, all variables
were identified as significantly predictive factors for OS, aside
from ethnicity. After adjustment in multivariate analysis, all
aforementioned variables retained independent significance in OS,
except for year of diagnosis between 2007 and 2009 (P=0.562), while
African descent (P=0.387) or other ethnicity (P=0.296) variables
remained non-significant. Unmarried status had a validated negative
effect on survival outcomes compared with married status [hazard
ratio (HR), 1.259; 95% CI, 1.118–1.419; P<0.001] (Table II).
 | Table II.Univariate and multivariate survival
analysis of OS in patients with duodenal adenocarcinoma. |
Table II.
Univariate and multivariate survival
analysis of OS in patients with duodenal adenocarcinoma.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | 5-year OS, % | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Year of
diagnosis |
|
|
|
|
|
|
|
|
2004–2006 | 26.1 | Reference |
|
| Reference |
|
|
|
2007–2009 | 25.7 | 0.948 | 0.814–1.103 | 0.488 | 0.955 | 0.819–1.115 | 0.562 |
|
2010–2012 | 34.0 | 0.770 | 0.660–0.898 | 0.001 | 0.852 | 0.729–0.995 | 0.043 |
|
2013–2015 | N/A | 0.772 | 0.652–0.914 | 0.003 | 0.805 | 0.679–0.955 | 0.013 |
| Age, years |
|
|
|
|
|
|
|
|
≤58 | 46.0 | Reference |
|
| Reference |
|
|
|
59–75 | 31.3 | 1.519 | 1.312–1.760 | <0.001 | 1.544 | 1.330–1.791 | <0.001 |
|
>75 | 12.8 | 2.753 | 2.358–3.215 | <0.001 | 2.585 | 2.199–3.039 | <0.001 |
| Sex |
|
|
|
|
|
|
|
|
Female | 33.4 | Reference |
|
| Reference |
|
|
|
Male | 27.6 | 1.129 | 1.010–1.261 | 0.032 | 1.176 | 1.047–1.322 | 0.006 |
| Race |
|
|
|
|
|
|
|
|
Caucasian | 30.8 | Reference |
|
| Reference |
|
|
| African
descent | 29.7 | 1.038 | 0.898–1.199 | 0.614 | 1.068 | 0.920–1.239 | 0.387 |
|
Other | 26.0 | 0.976 | 0.789–1.206 | 0.820 | 0.892 | 0.721–1.105 | 0.296 |
| Marital status |
|
|
|
|
|
|
|
|
Married | 32.6 | Reference |
|
| Reference |
|
|
|
Unmarried | 26.8 | 1.303 | 11.651.457 | <0.001 | 1.259 | 1.118–1.419 | <0.001 |
| Grade |
|
|
|
|
|
|
|
| I | 56.0 | Reference |
|
| Reference |
|
|
| II | 29.0 | 2.073 | 1.723–2.494 | <0.001 | 1.552 | 1.278–1.884 | <0.001 |
|
III | 19.1 | 2.963 | 2.457–3.547 | <0.001 | 2.105 | 1.722–2.572 | <0.001 |
| IV | 22.3 | 2.740 | 1.731–4.336 | <0.001 | 2.349 | 1.473–3.745 | <0.001 |
| AJCC TNM stage |
|
|
|
|
|
|
|
| I | 53.0 | Reference |
|
| Reference |
|
|
| II | 36.3 | 1.433 | 1.171–1.752 | <0.001 | 1.451 | 1.171–1.798 | 0.001 |
|
III | 36.4 | 1.386 | 1.153–1.667 | 0.001 | 1.722 | 1.405–2.110 | <0.001 |
| IV | 6.2 | 4.375 | 3.665–5.223 | <0.001 | 2.427 | 1.994–2.953 | <0.001 |
| Surgery |
|
|
|
|
|
|
|
|
Yes | 46.4 | Reference |
|
| Reference |
|
|
| No | 3.9 | 4.610 | 4.097–5.188 | <0.001 | 3.399 | 2.914–3.964 | <0.001 |
Marital status and CSS
Representative Kaplan-Meier curves for CSS are
presented in Fig. 1B. The 5-year CSS
rate was higher for married patients compared with unmarried
patients (38.8 vs. 33.7%) and a log-rank test indicated that the
difference was significant (P<0.001). In univariate analysis,
all variables were identified as significantly predictive factors
for CSS, aside from ethnicity and sex. After adjustment in
multivariate analysis, all aforementioned variables retained
independent significance in CSS, except for year of diagnosis
between 2007 and 2009 (P=0.612), year of diagnosis between 2010 and
2012 (P=0.221), African ethnicity (P=0.825) or other ethnicity
(P=0.092), and male patients (P=0.071). Unmarried status had a
validated negative effect on survival outcomes compared with
married status (HR, 1.236; 95% CI, 1.086–1.407; P<0.001;
Table III).
 | Table III.Univariate and multivariate survival
analysis of CSS in patients with duodenal adenocarcinoma. |
Table III.
Univariate and multivariate survival
analysis of CSS in patients with duodenal adenocarcinoma.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | 5-year CSS, % | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Year of
diagnosis |
|
|
|
|
|
|
|
|
2004–2006 | 32.0 | Reference |
|
| Reference |
|
|
|
2007–2009 | 32.9 | 0.934 | 0.792–1.103 | 0.421 | 0.985 | 0.810–1.133 | 0.612 |
|
2010–2012 | 40.1 | 0.790 | 0.669–0.932 | 0.005 | 0.901 | 0.762–1.065 | 0.221 |
|
2013–2015 | N/A | 0.766 | 0.639–0.919 | 0.004 | 0.823 | 0.685–0.989 | 0.038 |
| Age, years |
|
|
|
|
|
|
|
|
≤58 | 49.7 | Reference |
|
| Reference |
|
|
|
59–75 | 38.1 | 1.377 | 1.179–1.609 | <0.001 | 1.390 | 1.187–1.627 | <0.001 |
|
>75 | 20.2 | 2.478 | 2.102–2.921 | <0.001 | 2.325 | 1.958–2.760 | <0.001 |
| Sex |
|
|
|
|
|
|
|
|
Female | 39.4 | Reference |
|
| Reference |
|
|
|
Male | 34.5 | 0.102 | 0.978–1.242 | 0.111 | 1.123 | 0.990–1.274 | 0.071 |
| Race |
|
|
|
|
|
|
|
|
Caucasian | 36.5 | Reference |
|
| Reference |
|
|
| African
descent | 37.5 | 0.969 | 0.827–1.136 | 0.697 | 0.982 | 0.833–1.156 | 0.825 |
|
Other | 38.9 | 0.885 | 0.698–1.121 | 0.310 | 0.815 | 0.642–1.034 | 0.092 |
| Marital status |
|
|
|
|
|
|
|
|
Married | 38.8 | Reference |
|
| Reference |
|
|
|
Unmarried | 33.7 | 1.273 | 1.128–1.436 | <0.001 | 1.236 | 1.086–1.407 | <0.001 |
| Grade |
|
|
|
|
|
|
|
| I | 69.5 | Reference |
|
| Reference |
|
|
| II | 35.0 | 2.690 | 2.153–3.362 | <0.001 | 1.919 | 1.523–2.419 | <0.001 |
|
III | 22.9 | 4.071 | 3.254–5.093 | <0.001 | 2.717 | 2.145–3.441 | <0.001 |
| IV | 23.3 | 3.923 | 2.418–6.365 | <0.001 | 3.237 | 1.979–5.294 | <0.001 |
| AJCC TNM stage |
|
|
|
|
|
|
|
| I | 67.3 | Reference |
|
| Reference |
|
|
| II | 44.8 | 1.762 | 1.390–2.234 | <0.001 | 1.666 | 1.298–2.140 | <0.001 |
|
III | 42.2 | 1.786 | 1.435–2.222 | <0.001 | 2.116 | 1.668–2.685 | <0.001 |
| IV | 7.3 | 6.143 | 4.984–7.571 | <0.001 | 3.057 | 2.435–3.838 | <0.001 |
| Surgery |
|
|
|
|
|
|
|
|
Yes | 54.6 | Reference |
|
| Reference |
|
|
| No | 5.9 | 5.163 | 4.544–5.866 | <0.001 | 3.746 | 3.165–4.434 | <0.001 |
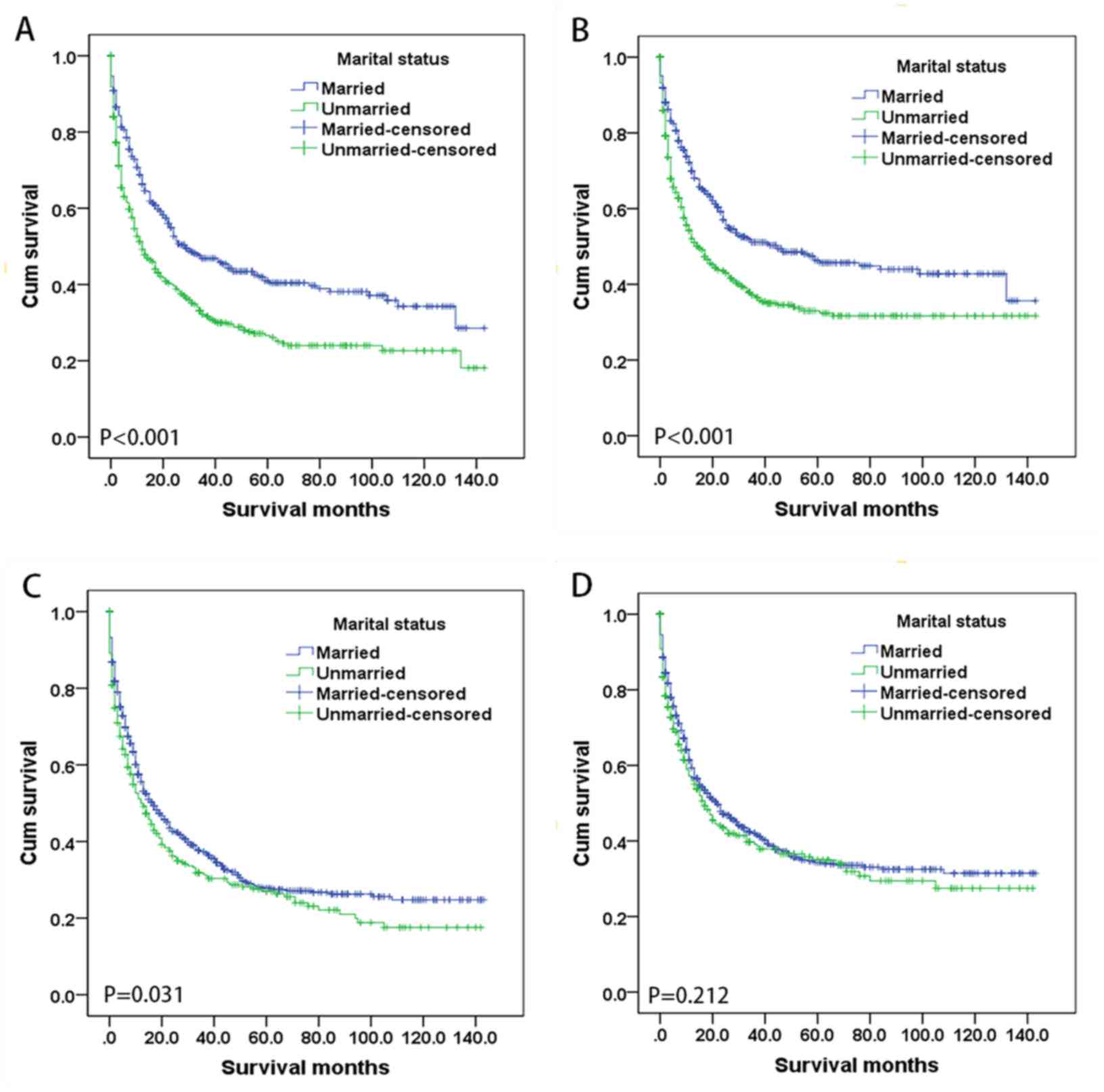
Subgroup analysis of the effect of
marital status according to sex
The association between marital status and survival
was analyzed separately for the two sexes. Fig. 2 shows Kaplan-Meier survival curves
according to marital status and sex. The 5-year OS rate among
female patients was higher in the married group (41.0%) compared
with the unmarried group (26.6%) (P<0.001), as was the 5-year
CSS rate (46.3 vs. 33.0%; P<0.001). The 5-year OS rate was also
higher for the married group (27.8%) compared with the unmarried
group (27.0%) (P=0.031) in male patients, whereas their CSS rate
did not differ significantly with marital status (P=0.212).
Multivariate analysis indicated that marital status affects OS in
both females (HR, 1.220; 95% CI, 1.024–1.454; P=0.026) and males
(HR, 1.273; 95% CI, 1.078–1.503; P=0.004), and also CSS in both
females (HR, 1.218; 95% CI, 1.009–1.470; P=0.040) and males (HR,
1.218; 95% CI, 1.014–1.463; P=0.035; Table IV).
 | Table IV.Univariate and multivariate survival
analysis of patients with duodenal adenocarcinoma based on sex. |
Table IV.
Univariate and multivariate survival
analysis of patients with duodenal adenocarcinoma based on sex.
| A, OS |
|---|
|
|---|
|
|
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | 5-year survival,
% | Median survival
time, months | Log rank
χ2 | P-value | HR | 95% CI | P-value |
|---|
| Female |
|
| 28.356 | <0.001 |
|
|
|
|
Married | 41.0 | 29 |
|
| Reference |
|
|
|
Unmarried | 26.6 | 12 |
|
| 1.220 | 1.024–1.454 | 0.026 |
| Male |
|
| 4.648 | 0.031 |
|
|
|
|
Married | 27.8 | 17 |
|
| Reference |
|
|
|
Unmarried | 27.0 | 12 |
|
| 1.273 | 1.078–1.503 | 0.004 |
|
| B, CSS |
|
|
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
|
|
|
|
Characteristic | 5-year survival,
% | Median survival
time, months | Log rank
χ2 | P-value | HR | 95% CI | P-value |
|
| Female |
|
| 24.144 | <0.001 |
|
|
|
|
Married | 46.3 | 45 |
|
| Reference |
|
|
|
Unmarried | 33.0 | 15 |
|
| 1.218 | 1.009–1.470 | 0.040 |
| Male |
|
| 1.558 | 0.212 |
|
|
|
|
Married | 34.3 | 22 |
|
| Reference |
|
|
| Unmarried | 35.0 | 17 |
|
| 1.218 | 1.014–1.463 | 0.035 |
Subgroup analysis of the effect of
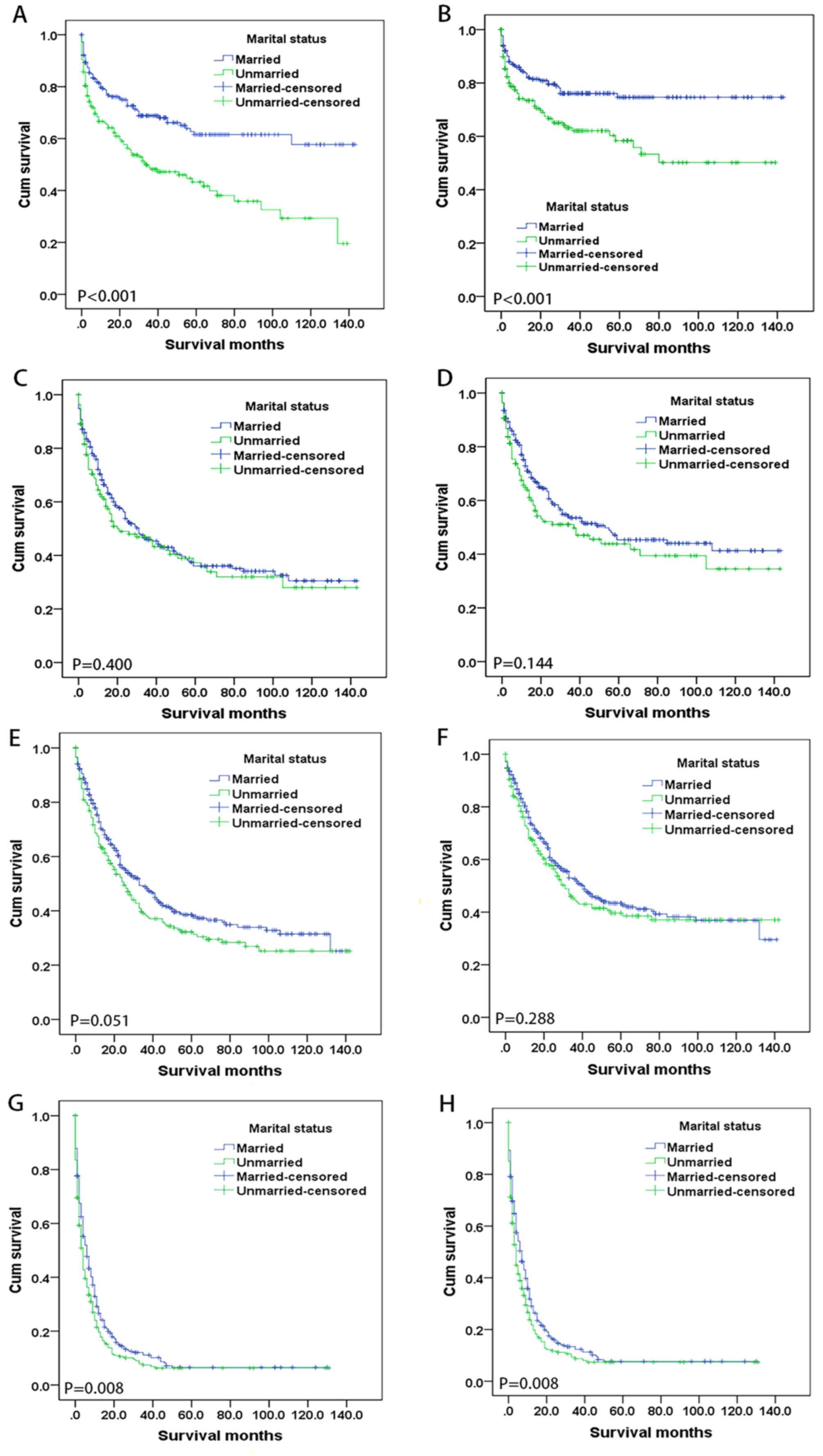
marital status according to AJCC TNM stage
Fig. 3 shows
Kaplan-Meier survival curves according to marital status of the
patients at the four AJCC TNM stages: I, n=406; II, n=387; III,
n=609; and IV, n=616. For stage-I patients, both the 5-year OS
(P<0.001) and CSS (P=0.001) rates were significantly higher in
the married group (61.5 and 74.6%, respectively) compared with the
unmarried group (43.2 and 58.4%, respectively). In addition, for
stage-IV patients, the 5-year OS rates (6.5 vs. 5.8%; P=0.008) and
5-year CSS rates (7.7 vs. 6.9%; P=0.008) were similarly higher in
the married group compared with the unmarried group, while the
survival rates did not differ significantly with marital status for
either AJCC TNM stage II or III. Multivariate analysis showed that
married status was an independent prognostic factor for OS (HR,
1.778; 95% CI, 1.286–2.459; P<0.001) and CSS (HR, 1.732; 95% CI,
1.162–2.583; P=0.007) in patients at stage I, but not in those at
stage II, III, or IV (Table V).
 | Table V.Univariate and multivariate survival
analysis of patients with duodenal adenocarcinoma based on the
American Joint Committee on Cancer Tumor-Node-Metastasis stage. |
Table V.
Univariate and multivariate survival
analysis of patients with duodenal adenocarcinoma based on the
American Joint Committee on Cancer Tumor-Node-Metastasis stage.
| A, OS |
|---|
|
|---|
|
|
|
| Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | 5-year survival,
% | Median survival
time, months | Log rank
χ2 | P-value | HR | 95% CI | P-value |
|---|
| Stage I |
|
| 19.027 | <0.001 |
|
|
|
|
Married | 61.5 | N/A |
|
| Reference |
|
|
|
Unmarried | 43.2 | 34 |
|
| 1.778 | 1.286–2.459 | <0.001 |
| Stage II |
|
| 0.707 | 0.400 |
|
|
|
|
Married | 36.0 | 31 |
|
| Reference |
|
|
|
Unmarried | 37.2 | 20 |
|
| 1.101 | 0.825–1.468 | 0.513 |
| Stage III |
|
| 3.800 | 0.051 |
|
|
|
|
Married | 38.6 | 33 |
|
| Reference |
|
|
|
Unmarried | 32.2 | 25 |
|
| 1.226 | 0.971–1.547 | 0.087 |
| Stage IV |
|
| 7.020 | 0.008 |
|
|
|
|
Married | 6.5 | 6 |
|
| Reference |
|
|
|
Unmarried | 5.8 | 4 |
|
| 1.176 | 0.972–1.423 | 0.096 |
|
| B, CSS |
|
|
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
|
|
|
|
Characteristic | 5-year survival,
% | Median survival
time, months | Log rank
χ2 | P-value | HR | 95% CI | P-value |
|
| Stage I |
|
| 10.993 | 0.001 |
|
|
|
|
Married | 74.6 | N/A |
|
| Reference |
|
|
|
Unmarried | 58.4 | N/A |
|
| 1.732 | 1.162–2.583 | 0.007 |
| Stage II |
|
| 2.130 | 0.144 |
|
|
|
|
Married | 45.3 | 53 |
|
| Reference |
|
|
|
Unmarried | 43.8 | 37 |
|
| 1.255 | 0.916–1.721 | 0.157 |
| Stage III |
|
| 1.129 | 0.288 |
|
|
|
|
Married | 43.4 | 41 |
|
| Reference |
|
|
|
Unmarried | 39.6 | 31 |
|
| 1.119 | 0.867–1.445 | 0.387 |
| Stage IV |
|
| 7.000 | 0.008 |
|
|
|
|
Married | 7.7 | 7 |
|
| Reference |
|
|
|
Unmarried | 6.9 | 4 |
|
| 1.189 | 0.976–1.447 | 0.085 |
Discussion
The present study used the SEER database to
investigate the association between marital status and survival
outcomes in patients with duodenal adenocarcinoma. OS and CSS rates
were higher in married patients compared with those in unmarried
patients in both the univariate and multivariate analyses. Marital
status may therefore be an independent prognostic factor for
survival in patients with duodenal adenocarcinoma. The results of
the present study are consistent with previous studies showing that
both OS and CSS rates are increased in patients who are married
compared with unmarried patients (including separated/divorced,
widowed and unmarried patients) (14,20–25).
In the present study, there was a larger proportion
of male patients who were married compared with female patients,
and this may be associated with differences in duodenal
adenocarcinoma development in the sexes. Generally, there were more
male patients than female patients with duodenal adenocarcinoma,
consistent with the result that there were more married male
participants compared with female participants (26). Additionally, ethnic differences and
the financial and social responsibilities associated with marriage
may provide an explanation for the differences observed. Socially,
Caucasian men are more likely to have a higher education and income
when compared with men of other ethnicities, therefore, the
marriage rate may be higher (27). A
previous study has indicated that the impact of marital status on
the survival outcomes for certain types of cancer differs between
men and women (28). Therefore, the
present cohort was stratified according to sex in order to analyze
whether the effect of marital status on the survival of patients
with duodenal adenocarcinoma differed between the sexes, and it was
found that both OS [females (HR, 1.220; 95% CI, 1.024–1.454;
P=0.026) and males (HR, 1.273; 95% CI, 1.078–1.503; P=0.004)] and
CSS [females (HR, 1.218; 95% CI, 1.009–1.470; P=0.040) and males
(HR, 1.218; 95% CI, 1.014–1.463; P=0.035)] were increased in
married patients compared with those in unmarried patients in each
sex. The results of the subgroup analyses were similar to those
found by Zhou et al (19).
Previous studies have suggested that the prognosis
may be worse in unmarried patients due to a delayed diagnosis at
more advanced tumor stages in such patients (16,29).
However, the present subgroup analysis of AJCC TNM stages showed
that marital status is only an independent prognostic factor for OS
and CSS in patients with stage-I disease, but not in those with
disease at stage II, III or IV. Therefore, marital status was a
protective factor for patients with early-stage disease, and its
impact should not be ignored.
The generally accepted explanations as to why the
survival rate of cancer is higher in married patients compared with
that in unmarried patients involve improved mental health and
socioeconomic status (30). It has
been proposed that psychology, living habits and economic
conditions, as well as certain biological factors, are all strongly
associated with marriage and lead to different degrees of
physiological changes (31,32). From the physiological viewpoint,
there have been numerous reasonable explanations for the mechanism
underlying the impact of psychosocial factors on cancer survival
rates. The positive effect of marital status on the cancer-related
death rate is usually attributable to social support behaviors,
including the spouse encouraging healthy behaviors, seeking
curative treatment for the patient, and helping to reduce anxiety
and stress both during and after treatment (13,33). The
socioeconomic status is generally higher for married patients
compared with that for unmarried individuals. High medical expenses
associated with receiving care usually put the patient under
greater financial pressure, and a family member with another source
of income may significantly ameliorate this situation (34,35);
this is particularly applicable in non-European countries, where
the financial resources of patients have greater effects on their
access to health care. Without sufficient funds to pay for
treatment, some unmarried patients may be unwilling to seek the
care they need in a timely manner (36).
In comparing the two groups of patients in this
study, it can be seen that unmarried and married patients are
comparable with regard to disease progression at the time of
diagnosis, that is, there were no significant differences in
pathological grades between the two groups. However, when analyzing
the ratio of surgical treatment, unmarried patients did not undergo
such treatment modalities as frequently as their married
counterparts, and this translates into poor survival for widowed
patients whose death risk increased by 101.4% and 5-year CSS was
59.8% compared to married patients (76.1%) (13). Therefore, although unmarried and
married patients may present with similar disease processes, they
differ in subsequent treatment options, with married patients being
more likely to receive active treatment. In addition, there are
reports that for unmarried individuals, compliance with clinical
appointments is poor (37).
Previous studies have shown that a lack of
psychosocial support and increased psychological stress can affect
the normal functioning of the immune system, which may promote
tumor progression and mortality (38,39).
Moreover, inadequate social support can reportedly reduce the
activity of natural-killer cells and lead to disorders of various
endocrine hormones such as cortisol and catecholamines (35–40).
Other studies have shown that cortisol and catecholamines can
accelerate the growth and metastasis of malignant tumors via
immunosuppression (41–43). In addition, psychological stress can
elicit prolonged release of cortisol and lead to cytokine-mediated
inflammation, which is considered to be a poor prognostic factor
for patients with cancer (44,45).
Although the majority of the previous studies involved patients
with adenocarcinoma outside the duodenum (14,22,24), the
psychosocial and physiological mechanisms underlying the effect of
marital status on survival may be similar. Additionally, these
previous studies support the presence of links between marriage,
social support, and the immune response, and their impact on cancer
mortality (30,31).
Some limitations of the present study should be
considered: i) The SEER database only provides the marital status
at the time of diagnosis, and it was not possible to determine if
this had changed after the diagnosis, and any such changes might
have influenced the results; ii) patients who were not legally
married may still have been in same-sex or heterosexual
partnerships; iii) The SEER database lacks detailed information on
the quality of marriage (for example, the degree of trust between
husband and wife), and whether divorced or widowed patients are in
different family relationships, and both of these aspects may
affect the prognosis of patients with duodenal adenocarcinoma; and
iv) it was not possible to obtain detailed information about
disease recurrence, comorbidities, radiochemotherapy regimens and
surgical procedures from the SEER database. The absence of these
covariates may partially bias the observations made in the present
study.
In summary, marital status may be an independent
prognostic factor for OS and CSS in patients with duodenal
adenocarcinoma. The survival rate of married patients was higher
compared with that of unmarried patients, irrespective of sex, and
thus, marital status plays an important role as a protective factor
in patients with early-stage disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Social Science Foundation of China (grant no. 16BGL183) and The
Clinical Research Award of the First Affiliated Hospital of Xi'an
Jiaotong University of China (grant no. XJTU1AF-CRF-2016-024).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
NW and QB contributed equally to the work. NW, QB
and JLy conceived the study and completed the manuscript. NW and
JLi analyzed and interpreted the data. XR, JY, QL and HH reviewed,
collected and analyzed data. XR and JLy supervised the whole study,
revised the manuscript and gave final approval of the version to be
published. All authors reviewed, edited and approved the present
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suh CH, Tirumani SH, Shinagare AB, Kim KW,
Rosenthal MH, Ramaiya NH and Baheti AD: Diagnosis and management of
duodenal adenocarcinomas: A comprehensive review for the
radiologist. Abdom Imaging. 40:1110–1120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Overman MJ, Hu CY, Kopetz S, Abbruzzese
JL, Wolff RA and Chang GJ: A population-based comparison of
adenocarcinoma of the large and small intestine: Insights into a
rare disease. Ann Surg Oncol. 19:1439–1445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cloyd JM, George E and Visser BC: Duodenal
adenocarcinoma: Advances in diagnosis and surgical management.
World J Gastrointest Surg. 8:212–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solaini L, Jamieson NB, Metcalfe M, Abu
Hilal M, Soonawalla Z, Davidson BR, McKay C and Kocher HM; UK
Duodenal Cancer Study Group, : Outcome after surgical resection for
duodenal adenocarcinoma in the UK. Br J Surg. 102:676–681. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onkendi EO, Boostrom SY, Sarr MG, Farnell
MB, Nagorney DM, Donohue JH, Kendrick ML, Reid-Lombardo KM, Harmsen
WS and Que FG: 15-year experience with surgical treatment of
duodenal carcinoma: A comparison of periampullary and
extra-ampullary duodenal carcinomas. J Gastrointest Surg.
16:682–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cloyd JM, Norton JA, Visser BC and
Poultsides GA: Does the extent of resection impact survival for
duodenal adenocarcinoma? Analysis of 1,611 cases. Ann Surg Oncol.
22:573–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Delcore R, Thomas JH, Forster J and
Hermreck AS: Improving resectability and survival in patients with
primary duodenal carcinoma. Am J Surg. 166:626–631. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Struck A, Howard T, Chiorean EG, Clarke
JM, Riffenburgh R and Cardenes HR: Non-ampullary duodenal
adenocarcinoma: Factors important for relapse and survival. J Surg
Oncol. 100:144–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han SL, Cheng J, Zhou HZ, Zeng QQ and Lan
SH: The surgical treatment and outcome for primary duodenal
adenocarcinoma. J Gastrointest Cancer. 41:243–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryder NM, Ko CY, Hines OJ, Gloor B and
Reber HA: Primary duodenal adenocarcinoma: A 40-year experience.
Arch Surg. 135:1070–1075. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aizer AA, Chen MH, McCarthy EP, Mendu ML,
Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE,
et al: Marital status and survival in patients with cancer. J Clin
Oncol. 31:3869–3876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adler RH: Engel's biopsychosocial model is
still relevant today. J Psychosom Res. 67:607–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu SG, Zhang QH, Zhang WW, Sun JY, Lin Q
and He ZY: The effect of marital status on nasopharyngeal carcinoma
survival: A Surveillance, Epidemiology and End Results study. J
Cancer. 9:1870–1876. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Wilson SE, Stewart DB and
Hollenbeak CS: Marital status and colon cancer outcomes in US
Surveillance, Epidemiology and End Results registries: Does
marriage affect cancer survival by gender and stage? Cancer
Epidemiol. 35:417–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahdi H, Kumar S, Munkarah AR, Abdalamir
M, Doherty M and Swensen R: Prognostic impact of marital status on
survival of women with epithelial ovarian cancer. Psychooncology.
22:83–88. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osborne C, Ostir GV, Du X, Peek MK and
Goodwin JS: The influence of marital status on the stage at
diagnosis, treatment, and survival of older women with breast
cancer. Breast Cancer Res Treat. 93:41–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu C, Liu X, Chen YP, Mao YP, Guo R, Zhou
GQ, Tang LL, Lin AH, Sun Y and Ma J: Impact of marital status at
diagnosis on survival and its change over time between 1973 and
2012 in patients with nasopharyngeal carcinoma: A propensity
score-matched analysis. Cancer Med. 6:3040–3051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilhelm A, Galata C, Beutner U, Schmied
BM, Warschkow R, Steffen T, Brunner W, Post S and Marti L: Duodenal
localization is a negative predictor of survival after small bowel
adenocarcinoma resection: A population-based, propensity
score-matched analysis. J Surg Oncol. 117:397–408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Zhang Y, Song Y, Tan W, Qiu Z, Li
S, Chen Q and Gao S: Marital status is an independent prognostic
factor for pancreatic neuroendocrine tumors patients: An analysis
of the Surveillance, Epidemiology, and End Results (SEER) database.
Clin Res Hepatol Gastroenterol. 41:476–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jatoi A, Novotny P, Cassivi S, Clark MM,
Midthun D, Patten CA, Sloan J and Yang P: Does marital status
impact survival and quality of life in patients with non-small cell
lung cancer? Observations from the mayo clinic lung cancer cohort.
Oncologist. 12:1456–1463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Denberg TD, Beaty BL, Kim FJ and Steiner
JF: Marriage and ethnicity predict treatment in localized prostate
carcinoma. Cancer. 103:1819–1825. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Wang X, Huang R, Jin K, Zhangyuan
G, Yu W, Yin Y, Wang H, Xu Z and Sun B: Prognostic value of marital
status on stage at diagnosis in hepatocellular carcinoma. Sci Rep.
7:416952017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nelles JL, Joseph SA and Konety BR: The
impact of marriage on bladder cancer mortality. Urol Oncol.
27:263–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Liu Y, Wang Y, Ruan C, Wang H, Liang
X, Sun Y and Hu Z: The influence of marital status on survival of
gallbladder cancer patients: A population-based study. Sci Rep.
7:53222017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Gan L, Wu Z, Yan S, Liu X and Guo
W: The influence of marital status on the stage at diagnosis,
treatment, and survival of adult patients with gastric cancer: A
population-based study. Oncotarget. 8:22385–22405. 2017.PubMed/NCBI
|
|
26
|
Kakushima N, Ono H, Yoshida M, Takizawa K,
Tanaka M, Kawata N, Ito S, Imai K, Hotta K, Ishiwatari H and
Matsubayashi H: Characteristics and risk factors for sporadic
non-ampullary duodenal adenocarcinoma. Scand J Gastroenterol.
52:1253–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Griffith DM, Gunter K and Allen JO: Male
gender role strain as a barrier to African American men's physical
activity. Health Educ Behav. 38:482–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaplan RM and Kronick RG: Marital status
and longevity in the United States population. J Epidemiol
Community Health. 60:760–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nayeri K, Pitaro G and Feldman JG: Marital
status and stage at diagnosis in cancer. N Y State J Med. 92:8–11.
1992.PubMed/NCBI
|
|
30
|
Shi RL, Qu N, Lu ZW, Liao T, Gao Y and Ji
QH: The impact of marital status at diagnosis on cancer survival in
patients with differentiated thyroid cancer. Cancer Med.
5:2145–2154. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aarstad AK, Aarstad HJ and Olofsson J:
Quality of life, drinking to cope, alcohol consumption and smoking
in successfully treated HNSCC patients. Acta Otolaryngol.
127:1091–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheungpasitporn W, Thongprayoon C, Srivali
N, Vijayvargiya P, Andersen CA, Kittanamongkolchai W, Sathick IJ,
Caples SM and Erickson SB: The effects of napping on the risk of
hypertension: A systematic review and meta-analysis. J Evid Based
Med. 9:205–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pinquart M and Duberstein PR: Associations
of social networks with cancer mortality: A meta-analysis. Crit Rev
Oncol Hematol. 75:122–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Neill CB, Atoria CL, O'Reilly EM,
LaFemina J, Henman MC and Elkin EB: Costs and trends in pancreatic
cancer treatment. Cancer. 118:5132–5139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baker MS, Kessler LG, Urban N and Smucker
RC: Estimating the treatment costs of breast and lung cancer. Med
Care. 29:40–49. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alamanda VK, Song Y and Holt GE: Effect of
marital status on treatment and survival of extremity soft tissue
sarcoma. Ann Oncol. 25:725–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wheeler E, Prettyman A, Lenhard MJ and
Tran K: Adherence to outpatient program postoperative appointments
after bariatric surgery. Surg Obes Relat Dis. 4:515–520. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moreno-Smith M, Lutgendorf SK and Sood AK:
Impact of stress on cancer metastasis. Future Oncol. 6:1863–1881.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garssen B and Goodkin K: On the role of
immunological factors as mediators between psychosocial factors and
cancer progression. Psychiatry Res. 85:51–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levy SM, Herberman RB, Whiteside T, Sanzo
K, Lee J and Kirkwood J: Perceived social support and tumor
estrogen/progesterone receptor status as predictors of natural
killer cell activity in breast cancer patients. Psychosom Med.
52:73–85. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sephton SE, Lush E, Dedert EA, Floyd AR,
Rebholz WN, Dhabhar FS, Spiegel D and Salmon P: Diurnal cortisol
rhythm as a predictor of lung cancer survival. Brain Behav Immun.
30 (Suppl):S163–S170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lointier P, Wildrick DM and Boman BM: The
effects of steroid hormones on a human colon cancer cell line in
vitro. Anticancer Res. 12:1327–1330. 1992.PubMed/NCBI
|
|
43
|
McEwen BS, Biron CA, Brunson KW, Bulloch
K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH,
Spencer RL and Weiss JM: The role of adrenocorticoids as modulators
of immune function in health and disease: Neural, endocrine and
immune interactions. Brain Res Brain Res Rev. 23:79–133. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miller GE, Cohen S and Ritchey AK: Chronic
psychological stress and the regulation of pro-inflammatory
cytokines: A glucocorticoid-resistance model. Health Psychol.
21:531–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Formica V, Luccchetti J, Cunningham D,
Smyth EC, Ferroni P, Nardecchia A, Tesauro M, Cereda V, Guadagni F
and Roselli M: Systemic inflammation, as measured by the
neutrophil/lymphocyte ratio, may have differential prognostic
impact before and during treatment with fluorouracil, irinotecan
and bevacizumab in metastatic colorectal cancer patients. Med
Oncol. 31:1662014. View Article : Google Scholar : PubMed/NCBI
|