Introduction
Lung cancer is the most commonly diagnosed cancer
worldwide, and is generally classified into small-cell lung cancer
and non-small cell lung cancer (NSCLC); the latter accounts for
~80% of all cases of lung cancer (1–3). NSCLC
is an aggressive carcinoma with poor prognosis; it accounted for
~27% of all cases of cancer associated-mortality in the United
States in 2017 (1). Previously,
unresectable NSCLC was primarily treated by chemotherapeutic
methods, with a median overall survival (OS) time of 8–10 months
(4). Advanced NSCLC with epidermal
growth factor receptor (EGFR) gene mutation, which accounts for
30–50% of NSCLC cases in East Asia, is often treated using several
small tyrosine kinase inhibitors (TKIs), such as gefitinib and
erlotinib, which results in a median OS of ~2 years (5–7).
Previous studies have also revealed that NSCLC was
closely associated with inflammation and chronic infection
(8,9). Obstructive pneumonia frequently occurs
in patients with advanced NSCLC, and obstruction of a proximal
airway may lead to recurrent pneumonias in the same location of the
lung lobe (10). Since pneumonia can
be a considerable cause of mortality in patients with lung cancer,
antibiotics may be used for those patients in clinical settings
(10). However, an association
between antibiotic use and inferior efficacy of antitumor drugs in
advanced NSCLC has been reported (11). Chemotherapy may alter microbiotic
distribution in the gut as a result of gastrointestinal mucositis,
which may cause bacterial translocation to the bloodstream; and
thus, may cause severe infection requiring antibiotic treatment
(12,13).
Targeted therapies, such as EGFR-TKIs, often have
fewer and relatively mild side effects compared with chemotherapy
and immunotherapy (14,15). Unlike chemotherapy, targeted
treatment rarely causes myelosuppression-related infection.
However, antibiotic use is very prevalent in the clinic for several
reasons, and it is unknown whether antibiotics may influence the
efficacy of targeted therapy in patients with advanced NSCLC. Since
multidrug-resistance of antibiotics is currently emerging as a
major challenge, it is important to investigate the relationship
between antibiotics and EGFR-TKI treatment.
Therefore, the present study was performed to
investigate whether antibiotics could affect the efficacy and
toxicity of EGFR-TKI treatment, with the aim of restricting the use
of antibiotics in combination with targeted therapy in patients
with advanced NSCLC in the near future, thus reducing the
probability of treatment failure and the associated healthcare
costs.
Materials and methods
Patients and data collection
The present study was approved by the Ethics
Committee of Dongguan People's Hospital (Dongguan, China) and was
conducted according to the Declaration of Helsinki. Patients
provided informed written consent at the time of data collection. A
total of 102 patients with EGFR mutations, treated with EGFR-TKIs
at Dongguan People's Hospital, Southern Medical University
(Dongguan, China) between May 2014 and December 2017 were included
in this study. The inclusion criteria were: i) Patients who were
≥18 years old; ii) patients with cytological or histological
confirmation of stage IIIB and IV EGFR gene-mutated NSCLC based on
The International Association for the Study of Lung Cancer 7th
edition of Tumor Node Metastasis Staging classification; iii)
patients who had not previously received any antitumor regimens.
The exclusion criteria were: i) Patients who were pregnant; ii)
patients who were allergic to the drugs; iii) patients who had
primary organ failure; iv) patients whose clinical information
could not be obtained in full.
Patients with different Eastern Cooperative Oncology
Group Performance Status were investigated in the present study
(16). A score of 0 meant that
patients had completely normal activity. A score of 1 meant that
patients had the ability to move about freely and engage in light
physical activities, including general household or office work,
but not heavier physical activities. A score of 2 meant that
patients had the ability to walk freely and take care of
themselves, at least half of the time during the daytime, but lost
the ability to work. A score of 3 meant that patients could take
care of themselves partially, and spent more than half of the day
in bed or in a wheelchair. A score of 4 meant that patients were
usually bedridden, and unable to take care of themselves at all.
Clinical data, such as patient history, physical examination and
hematological examination were recorded within 1 week prior to
EGFR-TKI treatment. Antibiotic types and treatment time were also
recorded. Tumor response was evaluated by computed tomography
scans, according to the Response Evaluation Criteria in Solid
Tumors criteria. Disease control was defined as complete response,
partial response or stable disease. Further disease progression was
defined as progressive disease. Adverse events were recorded and
classified according to the National Cancer Institute Common
Terminology Criteria for Adverse Events version 3.0 (NCI-CTCAE 3.0)
(17).
Progression-free survival (PFS) was defined as time
between the start of the treatment and disease progression or
death, with censoring for patients alive without progression at
last contact. The cutoff date for PFS data was 28 June, 2018, when
the last patient had undergone treatment for 6 months. By that
time, enough data were collected to analyze the efficacy and
adverse events for each arm of the study.
EGFR status and grouping
EGFR mutations were identified in tumor tissues
using the peptide nucleic acid-locked nucleic acid polymerase chain
reaction clamp method (Sanger), the scorpion amplification
refractory mutation system method or next-generation sequencing
technology, as previously described (18). Patients were retrospectively divided
into two groups: Group A, which were treated with EGFR-TKIs and
antibiotics, and Group B, which were treated with EGFR-TKIs alone.
Antibiotic use 6 months prior to EGFR-TKI therapy was included in
Group A.
Statistical analysis
The aim of this retrospective study was to compare
the efficacy of EGFR-TKI co-treatment with antibiotics with that of
EGFR-TKI treatment alone as a first-line therapy for patients with
advanced NSCLC. The primary endpoint was PFS. The secondary end
points were objective response rate (ORR) and disease control rate
(DCR). All patients with EGFR-TKI treatment were evaluable for
response. The safety population consisted of all patients who
received at least one week of treatment. Average response rate and
95% confidence intervals were calculated separately for each arm of
the study.
Statistical analyses were performed using SPSS 22.0
software (IBM Corp.). The relationships between treatment groups
and patient characteristics were performed using Pearson's
χ2 test or Fisher's exact test. Estimates of PFS and OS
were calculated using the Kaplan-Meier method and two-sided 95%
confidence intervals were obtained. A two-sided Breslow test was
used to compare PFS between the study groups. The Cox proportional
hazards model was used to estimate the hazard ratios for each study
group.
Results
Baseline characteristics and
treatment
A total of 102 eligible patients with NSCLC were
treated with EGFR-TKIs at Dongguan People's Hospital, Southern
Medical University (Dongguan, China). The clinicopathological
characteristics of the patients are presented in Table I; no statistically significant
differences were identified between the two study groups. The
median age of Group A was 63 years (range, 36–83 years) and 52.3%
patients were women, and that of Group B was 62 years (range, 27–82
years) and 63.8% were women. The majority of patients had an
Eastern Cooperative Oncology Group Performance Status of 0–2 and
had sensitive EGFR mutations, including Exon 19 deletion, Exon 21
L858R and Exon 18 G719X. All patients with advanced NSCLC received
treatment with first-line EGFR-TKIs: Gefitinib (250 mg/day),
icotinib (375 mg/day), erlotinib (150 mg/day) or afatinib (40
mg/day) until disease progression, unacceptable toxicity or other
factors, including death, pregnancy or unwillingness to further
receive targeted therapy, were observed.
 | Table I.Patient clinicopathological
characteristics. |
Table I.
Patient clinicopathological
characteristics.
| Characteristic | Group A (n=44) | Group B (n=58) | P-value |
|---|
| Age (years) |
|
|
|
|
Median | 63 | 62 |
|
|
Range | 36–83 | 27–82 |
|
| Age groups
(years) |
|
| 0.22 |
|
18–39 | 5 (11.4%) | 2 (3.4%) |
|
|
40–64 | 20 (45.5%) | 33 (56.9%) |
|
|
65–85 | 19 (43.1%) | 23 (39.7%) |
|
| Sex |
|
| 0.24 |
| Male | 21 (47.7%) | 21 (36.2%) |
|
|
Female | 23 (52.3%) | 37 (63.8%) |
|
| ECOG PS |
|
| 0.57 |
| 0 | 1 (2.3%) | 3 (5.2%) |
|
| 1–2 | 38 (86.3%) | 51 (87.9%) |
|
| ≥3 | 5 (11.4%) | 4 (6.9%) |
|
| Lung cancer
stage |
|
| 1.00 |
| IIIB or
lower | 1 (2.3%) | 2 (3.4%) |
|
| IV | 43 (97.7%) | 56 (96.6%) |
|
| Smoking |
|
| 0.46 |
| Yes | 11 (25) | 11 (18.9%) |
|
| No | 33 (75%) | 47 (81.1%) |
|
| Number of
metastases |
|
| 0.81 |
|
0–1 | 13 (29.5%) | 20 (34.5%) |
|
| 2 | 12 (27.3%) | 13 (22.4%) |
|
| ≥3 | 19 (43.2%) | 25 (43.1%) |
|
| Brain
metastasis |
|
| 0.27 |
|
Yes | 15 (34.1%) | 26 (44.8%) |
|
| No | 29 (65.9%) | 32 (55.2%) |
|
| EGFR mutation
status |
|
| 0.34 |
| Exon 19
deletion | 22 (48.9%) | 24 (41.5%) |
|
| Exon 21
L858R | 17 (45.7%) | 31 (53.4%) |
|
| Exon 18
G719X | 1 (2.9%) | 1 (1.7%) |
|
|
Other | 4 (2.9%) | 2 (3.4%) |
|
| Drugs |
|
| 0.46 |
|
Gefitinib | 20 (45.5%) | 31 (53.4%) |
|
|
Erlotinib | 5 (11.4%) | 7 (12.1%) |
|
|
Icotinib | 17 (38.6%) | 20 (34.5%) |
|
|
Afatinib | 2 (4.5%) | 0 (0%) |
|
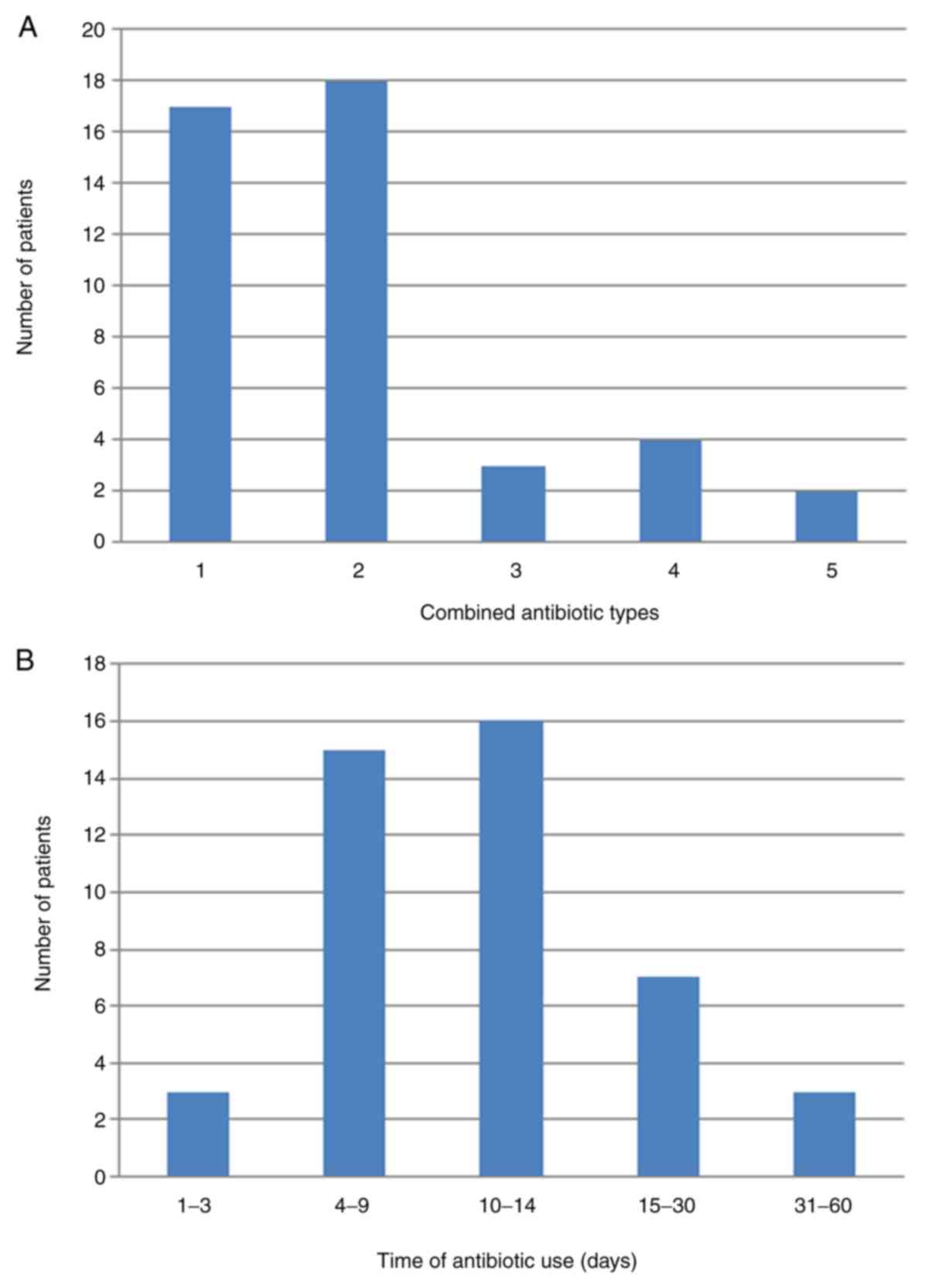
Of the 102 patients with EGFR mutations, 44 patients
received antibiotic treatment prior to or during the targeted
therapy period. Antibiotic therapy rarely exceeded three types of
drug in these patients (Fig. 1A).
Only six patients (13.6%) received ≥4 types of antibiotics. The
majority of patients (77.3%) received antibiotic treatment for ≤1
month when co-treated with EGFR-TKIs (Fig. 1B). In addition, 26 different
antibiotic types were used in the present study, the most commonly
prescribed of which was cefmetazole, followed by
imipenem-cilastatin and moxifloxacin (data not shown).
Treatment efficacy
The response rate of patients with NSCLC co-treated
with EGFR-TKIs and antibiotics (Group A) was 52.3%, whereas that of
patients treated with EGFR-TKIs alone (Group B) was 56.9% (Table II). No significant differences in
ORR or DCR were observed between the two groups. However, the
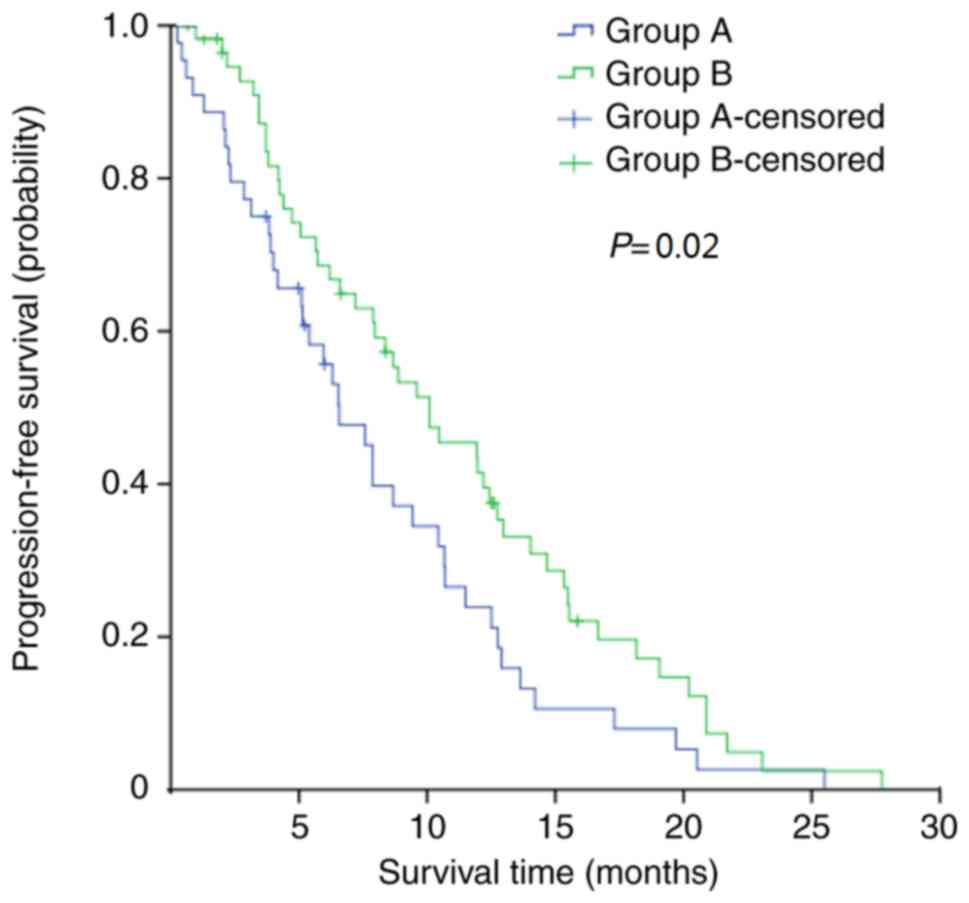
median PFS of Group A was 6.6 months compared with 10.1 months in
Group B (P=0.02; Fig. 2). The 1-year
PFS rates in the two groups were 20.5% and 37.9%, respectively
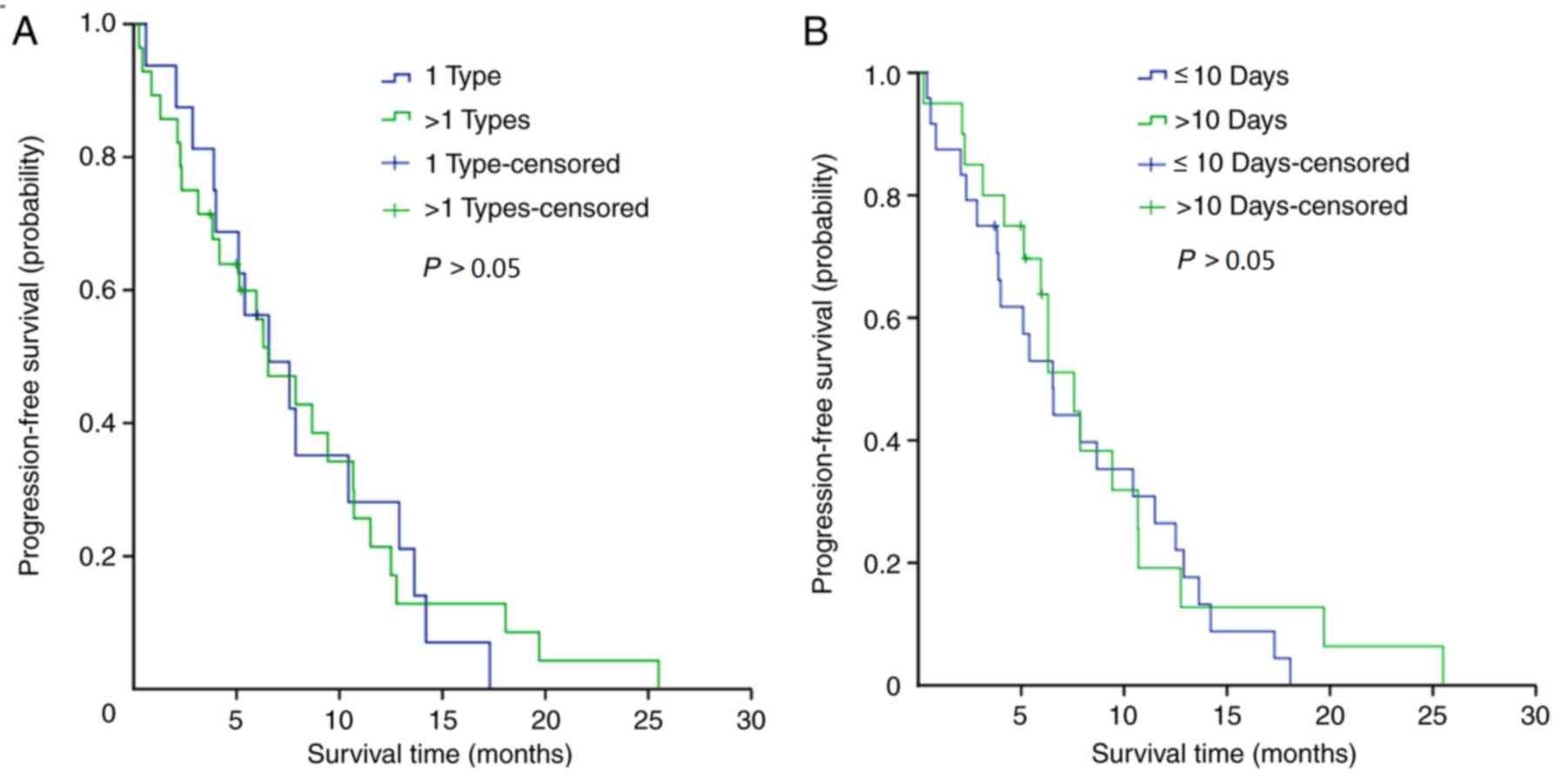
(P=0.03). The effects of different numbers of antibiotics and the
duration of the treatment period among patients in group A were
also investigated; analyses of PFS revealed that there were no
statistically significant differences between the patient subgroups
(Fig. 3A and B).
 | Table II.Treatment efficacy. |
Table II.
Treatment efficacy.
| Variable | Group A (n=44) | Group B (n=58) | P-value |
|---|
| Response |
|
|
|
| PR
(%) | 23 (52.3%) | 33 (56.9%) |
|
| SD
(%) | 13 (29.5%) | 18 (31.0%) |
|
| PD
(%) | 8 (18.2%) | 7 (12.1%) |
|
| Response rate
(%) | 52.3 | 56.9 | 0.64 |
| 95%
CI | 37.3–67.2 | 44.1–69.8 |
|
| Disease control
rate (%) | 81.8 | 87.9 | 0.39 |
| 95%
CI | 70.3–93.3 | 79.5–96.4 |
|
| Median PFS
(months) | 6.6 | 10.1 | 0.04 |
| 95%
CI | 4.7–8.4 | 6.4–13.8 |
|
| 1-year PFS rate
(%) | 20.5 | 37.9 | 0.03 |
Adverse events
The most common side effects that were possibly
related to the treatment are presented in Table III. The majority of the adverse
events in the two study groups were mild; no patients in the
present study exhibited severe adverse events. The most common
grade 1/2 adverse events (NCI-CTCAE 3.0) in Group A were anorexia,
rash, raised aminopherase and fatigue, whereas those in Group B
were raised aminopherase, rash, anorexia and nausea; no
statistically significant differences in treatment-related
toxicities other than fever were observed between the two groups.
In the EGFR-TKIs treatment without antibiotic treatment group, the
incidence of fever was relatively low.
 | Table III.Treatment-related toxicity. |
Table III.
Treatment-related toxicity.
|
| Grade 1/2 |
| Grade 3/4 |
|
|---|
|
|
|
|
|
|
|---|
| Toxicity | Group A (n=44)
(%) | Group B (n=58)
(%) | P-value | Group A (n=44)
(%) | Group B (n=58)
(%) | P-value |
|---|
| Rash | 15 (34.1) | 15 (25.9) | 0.37 | 5 (11.4) | 7 (12.1) | 0.91 |
| Pruritus | 8 (18.2) | 6 (10.3) | 0.26 | 0 | 0 |
|
| Dizziness | 6 (13.6) | 5 (8.6) | 0.42 | 0 | 0 |
|
| Fever | 8 (18.2) | 2 (3.4) | 0.01 | 0 | 0 |
|
| Diarrhea | 7 (15.9) | 7 (12.1) | 0.58 | 7 (15.9) | 1 (1.7) | 0.008 |
| Fatigue | 9 (20.5) | 8 (13.8) | 0.38 | 2 (4.5) | 0 | 0.10 |
| Nausea | 8 (18.2) | 9 (15.5) | 0.72 | 0 | 0 |
|
| Vomiting | 8 (17.1) | 8 (13.7) | 0.55 | 0 | 0 |
|
| Anorexia | 16 (36.4) | 13 (22.4) | 0.12 | 0 | 0 |
|
| Raised
aminopherase | 14 (31.8) | 16 (27.6) | 0.65 | 0 | 1 (1.7) | 0.380 |
| Dyspnea | 6 (13.6) | 7 (12.1) | 0.82 | 5 (11.4) | 0 | 0.008 |
| Hemorrhage | 2 (4.5) | 4 (6.9) | 0.62 | 1 (2.3) | 0 | 0.25 |
A total of 20 episodes of grade 3/4 adverse events
occurred in Group A, including diarrhea (15.9%), rash (11.4%),
dyspnea (11.4%), fatigue (4.5%) and hemorrhage (2.3%), whereas only
nine episodes occurred in Group B, including rash (12.1%), raised
aminopherase (1.7%) and diarrhea (1.7%). The adverse events of
diarrhea and dyspnea were significantly more common in Group A
compared with Group B (P<0.05).
Discussion
Advanced NSCLC is closely associated with chronic
and acute infection (8,9). A previous study revealed that the tumor
itself causes immunosuppression, which may lead to severe infection
(8). In addition, chemotherapy has a
myelosuppressive effect; therefore, the incidence of infection is
high in patients with NSCLC. Thus, antibiotics are extensively used
in the clinic to decrease the mortality rate in patients with
advanced NSCLC (19). However,
antibiotics may negatively impact the therapeutic efficacy of
antitumor agents in NSCLC (11). A
previous study has shown that antibiotics inhibit the clinical
benefit of these agents by changing the gut microbiome composition
of patients with advanced cancer, which further reduces the
recruitment of CCR9+CXCR3+CD4+ T
lymphocytes into the tumor beds (20).
EGFR-TKIs are a standard treatment method for
patients with EGFR mutated advanced NSCLC (7). Compared with patients receiving
chemotherapy, serious infection caused by myelosuppression rarely
occurs in patients during the targeted therapeutic period (6). However, EGFR-TKI treatment may damage
the gastrointestinal mucosa, inducing side effects such as nausea,
vomiting and diarrhea; this may promote bacterial translocation and
lead to bloodstream infection (21).
Therefore, antibiotics are still frequently prescribed for patients
undergoing EGFR-TKI treatment in the presence of infection.
In the present study, a similar phenomenon was
observed with antibiotic use in patients with targeted therapy.
During different periods of the antitumor treatment, ~43.1% of
patients who chose EGFR-TKIs as their initial therapy received one
or more types of antibiotics. Additionally, 25.5% of these patients
received prophylactic or empirical treatment with several
antibiotics for >10 days. Further analysis displayed that
cefmetazole, imipenem-cilastatin and moxifloxacin were the most
frequent antibiotic types used in patients who participated in this
study. Similar to previous studies, antibiotics also exerted a
negative impact on targeted therapy for the first-line treatment of
advanced NSCLC (20,22).
In the present study, antibiotics did not change the
ORR or DCR. However, antibiotic administration was associated with
shorter median PFS of EGFR-TKI treatment for NSCLC of only 6.6
months, which is lower compared with the results from multiple
randomized clinical trials (PFS, 9–10 months) (5,6). For the
patients who did not receive antibiotics, the median PFS was 10.1
months, which is similar to the results of previous clinical trials
(5,6). The 1-year PFS rates of the two study
groups were also significantly different. Since the basic patient
characteristics in the two groups were well balanced, it is
possible that antibiotics weakened the long-term efficacy of the
EGFR-TKI treatment, rather than altering the partial response to
the antitumor agents, which was commonly obtained after a few
months of targeted therapy. Antibiotics affect the number of
lymphocytes around the tumor (20);
this process requires a relatively long time, thus the effect of
antibiotics on EGFR-TKI therapy may present in a chronic way, which
corresponds with the results of PFS and 1-year PFS.
The impact of the number of antibiotics and
treatment duration on targeted therapy for advanced NSCLC was also
investigated in the present study. No significant differences were
observed among these factors. These results suggested that the
administration of antibiotics may decrease the therapeutic efficacy
of EGFR-TKI independently of antibiotic number and treatment time.
These results are consistent with a previous study, which
demonstrated that antibiotics lead to long-term microbial shifts in
feces, which in turn may influence antitumor outcomes (22). As the number of patients in the
present study was relatively small, additional studies are
necessary to further clarify the relationship between antibiotic
use and EGFR-TKI efficacy.
Antibiotics may also influence the adverse events
associated with targeted therapy for advanced NSCLC. However, the
statistical difference of the grade 1/2 incidence rate of fever may
be unrelated to the different treatment arms. It was observed in
the medical records of these patients that the majority of them
already exhibited symptoms of fever associated with infection prior
to antibiotic treatment. In the present study, the incidence rates
of grade 3/4 diarrhea and dyspnea in group A were increased
compared with group B. Previous studies demonstrated that the use
of antibiotics is associated with an altered composition of the gut
microbiome and increased occurrence of ectopic diseases, such as
asthma and eczema (23–26). Diarrhea accounts for the majority of
adverse events associated with targeted treatment, and dyspnea is a
severe symptom of advanced NSCLC. Therefore, it is strongly
suggested that the use of antibiotics without evidence should be
prohibited and that prophylactic use should be applied with caution
for patients with NSCLC receiving EGFR targeted treatment.
In conclusion, antibiotics may lead to a long-term
decrease the efficacy of first-line targeted therapy in advanced
NSCLC, with increased adverse events of diarrhea and dyspnea.
Further large randomized studies are necessary to evaluate the
impact of antibiotic use on EGFR-TKI treatment for NSCLC. Basic
research is also suggested to clarify the mechanism of this
clinical phenomenon.
Acknowledgements
The authors would like to thank the following
colleagues at Dongguan People's Hospital, Southern Medical
University: Mr. Shulin Huang, Mr. Jingtang Chen, Mrs. Shunhuan Lin
and Mrs. Yifen Wu for their kind technical help, psychological
support, theoretical guidance and writing assistance during the
present study. The authors would also like to thank Mr. Zhuanghua
Li, Mr. Qinglin Tan, Mr. Ruinian Zheng and Mrs. Liping Li (all
Dongguan People's Hospital, Southern Medical University) for their
active participation in patient follow-up and assistance with
manuscript revision.
Funding
This study was funded by The Dongguan Social Science
and Technology Development Project (grant no. 201750715001285).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL, WZ and QT interpreted the patient data regarding
antibiotic use and EGFR targeted therapy in advanced NSCLC, and
participated in drafting and revising the manuscript. GJ and JJ
designed and supervised the analysis of this retrospective study.
All authors read and approved the final manuscript and agreed to be
accountable for all aspects of the work presented in the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Dongguan People's Hospital (Dongguan, China) and was
conducted according to the Declaration of Helsinki. Patients
provided informed written consent at the time of data
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DCR
|
disease control rate
|
|
EGFR
|
epidermal growth factor receptor
|
|
NCI-CTCAE 3.0
|
National Cancer Institute Common
Terminology Criteria for Adverse Events version 3.0
|
|
NSCLC
|
non-small-cell lung cancer
|
|
ORR
|
objective response rate
|
|
PFS
|
progression-free survival
|
|
TKIs
|
tyrosine kinase inhibitors
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Visbal AL, Leighl NB, Feld R and Shepherd
FA: Adjuvant chemotherapy for early-stage non-small cell lung
cancer. Chest. 128:2933–2943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen F, Cole P and Bina WF: Time trend and
geographic patterns of lung adenocarcinoma in the United States,
1973–2002. Cancer Epidemiol Biomarkers Prev. 16:2724–2729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zatloukal P, Petruzelka L, Zemanova M,
Kolek V, Skricková J, Pesek M, Fojtů H, Grygárková I, Sixtová D,
Roubec J, et al: Gemcitabine plus cisplatin vs. gemcitabine plus
carboplatin in stage IIIb and IV non-small cell lung cancer: A
phase III randomized trial. Lung Cancer. 41:321–331. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perez-Soler R, Chachoua A, Hammond LA,
Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabárbara P
and Bonomi P: Determinants of tumor response and survival with
erlotinib in patients with non-small-cell lung cancer. J Clin
Oncol. 22:3238–3247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomes M, Teixeira AL, Coelho A, Araújo A
and Medeiros R: The role of inflammation in lung cancer. Adv Exp
Med Biol. 816:1–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shiels MS, Pfeiffer RM, Hildesheim A,
Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G,
Caporaso NE, et al: Circulating inflammation markers and
prospective risk for lung cancer. J Natl Cancer Inst.
105:1871–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu-Kim C, Hoag JB, Cheng GS and Lund ME:
The microbiology of postobstructive pneumonia in lung cancer
patients. J Bronchology Interv Pulmonol. 20:266–270. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huemer F, Rinnerthaler G, Westphal T,
Hackl H, Hutarew G, Gampenrieder SP, Weiss L and Greil R: Impact of
antibiotic treatment on immune-checkpoint blockade efficacy in
advanced non-squamous non-small cell lung cancer. Oncotarget.
9:16512–16520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Vliet MJ, Harmsen HJ, de Bont ES and
Tissing WJ: The role of intestinal microbiota in the development
and severity of chemotherapy-induced mucositis. PLoS Pathog.
6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marin M, Gudiol C, Ardanuy C, Garcia-Vidal
C, Calvo M, Arnan M and Carratalà J: Bloodstream infections in
neutropenic patients with cancer: Differences between patients with
haematological malignancies and solid tumours. J Infect.
69:417–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi YK, Wang L, Han BH, Li W, Yu P, Liu
YP, Ding CM, Song X, Ma ZY, Ren XL, et al: First-line icotinib
versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for
patients with advanced EGFR mutation-positive lung adenocarcinoma
(CONVINCE): A phase 3, open-label, randomized study. Ann Oncol.
28:2443–2450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Broderick JM, Hussey J, Kennedy MJ and
O'Donnell DM: Patients over 65 years are assigned lower ECOG PS
scores than younger patients, although objectively measured
physical activity is no different. J Geriatr Oncol. 5:49–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Liang F and Tannock I: Use and
misuse of common terminology criteria for adverse events in cancer
clinical trials. BMC Cancer. 16:3922016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beck TF, Mullikin JC; NISC Comparative
Sequencing Program, ; Biesecker LG: Systematic evaluation of sanger
validation of next-generation sequencing variants. Clin Chem.
62:647–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Timmer-Bonte JN, de Boo TM, Smit HJ,
Biesma B, Wilschut FA, Cheragwandi SA, Termeer A, Hensing CA,
Akkermans J, Adang EM, et al: Prevention of chemotherapy-induced
febrile neutropenia by prophylactic antibiotics plus or minus
granulocyte colony-stimulating factor in small-cell lung cancer: A
Dutch Randomized Phase III Study. J Clin Oncol. 23:7974–7984. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Bastard Q, Ward T, Sidiropoulos D,
Hillmann BM, Chun CL, Sadowsky MJ, Knights D and Montassier E:
Fecal microbiota transplantation reverses antibiotic and
chemotherapy-induced gut dysbiosis in mice. Sci Rep. 8:62192018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaura E, Brandt BW, Teixeira de Mattos MJ,
Buijs MJ, Caspers MP, Rashid MU, Weintraub A, Nord CE, Savell A, Hu
Y, et al: Same exposure but two radically different responses to
antibiotics: Resilience of the salivarymicrobiome versus long-term
microbial shifts in feces. MBio. 6:e01693–e01615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clemente JC, Ursell LK, Parfrey LW and
Knight R: The impact of the gut microbiota on human health: An
integrative view. Cell. 148:1258–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Modi SR, Collins JJ and Relman DA:
Antibiotics and the gut microbiota. J Clin Invest. 124:4212–4218.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts SE, Wotton CJ, Williams JG,
Griffith M and Goldacre MJ: Perinatal and early life risk factors
for inflammatory bowel disease. World J Gastroenterol. 17:743–749.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Willing BP, Russell SL and Finlay BB:
Shifting the balance: Antibiotic effects on host-microbiota
mutualism. Nat Rev Microbiol. 9:233–243. 2011. View Article : Google Scholar : PubMed/NCBI
|