Introduction
Lung cancer is the most common type of malignant
tumor, accounting for 11.6% of total global cancer incidence and
18.4% of cancer-associated mortalities (1). As lung cancer has no specific symptoms
and signs, patients usually present with advanced stage disease at
primary diagnosis. Despite the availability of comprehensive
treatments, including active surgery and radiotherapy, a large
percentage of patients inevitably succumb to the disease due to
recurrence and distant metastasis. A study of risk factors
associated with lung cancer and a comprehensive understanding of
its pathogenesis is vital in the prevention and treatment of this
disease.
The tumor microenvironment is the environment
surrounding a tumor that may promote immune escape and induce tumor
resistance (2–4). Macrophages are an important component
of the tumor microenvironment. They may be divided into two primary
groups, termed M1 and M2, which may be identified by their cell
surface markers and functional phenotypes (5). Tumor-associated macrophages (TAMs) are
recruited to tumors as monocytes from the blood, and as they escape
across the vasculature of the tumor, they differentiate into
macrophages. Immature monocytes have good plasticity and may
therefore be induced by interleukin (IL)-4 and IL-13 to form M2
macrophages, which have similar functions to TAMs (6,7). In
general, increased levels of M1-like infiltrates in the tumor are
correlated with an improved prognosis, exhibiting an antitumoral
effect, whereas increased levels of M2-like TAM infiltrate are
correlated with poor prognoses, demonstrating pro-tumoral effects
(8,9). TAMs are abundant in all the stages of
tumor progression (4). In early
phases, macrophages recognize the malignant cells and present their
antigens to the effectors of the immune system. In later stage
disease, TAMs serve a role in tumor progression by stimulating
tumor growth, angiogenesis, metastasis and immunosuppression
(10). By producing growth factors,
they stimulate carcinoma cell proliferation (11). They are also able to produce
proteolytic enzymes that digest the extracellular matrix to assist
with tumor cell dissemination. Finally, they provide a supportive
niche for metastatic tumor cells at distant sites (12).
Previous studies have indicated that TAMs may be a
diagnostic and prognostic indicator for malignant pleural effusion
(13,14), thereby suggesting a close association
with lung cancer invasion and metastasis. Stromal macrophages
expressing macrophage scavenger receptor 1 are associated with
tumor aggressiveness in lung adenocarcinoma (15). A previous study hypothesized that the
developmental origin of TAMs dictates their relative distribution,
function and response to cancer therapies in lung tumors (16). Furthermore, the distribution patterns
of M1 and M2 macrophages in tumor islets and stroma were
demonstrated to be associated with the prognosis of non-small cell
lung cancer (NSCLC); high levels of M1 macrophage infiltration in
the tumor islets and low levels of total tumor-infiltrating M2
macrophages were associated with improved survival of patients with
NSCLC (17). However, the mechanism
of its effect on lung cancer invasion and metastasis remains
unclear. The aim of the present study was to investigate the
effects of TAMs on the proliferation, invasion and migration of
lung adenocarcinoma A549 cells, and to explore its molecular
mechanism.
Materials and methods
Cell culture and treatment
A549 and THP-1 human mononuclear leukemia cell lines
were provided by the Jiangxi Provincial Key Laboratory of Molecular
Medicine. A549 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Biological Industries Ltd.) supplemented with 10%
fetal bovine serum (FBS; Biological Industries Ltd.) and maintained
in a humidified atmosphere of 5% CO2 and 95% air at
37°C. THP-1 cells were cultured in RPMI-1640 medium supplemented
with 10% FBS and 0.05 mM β-mercaptoethanol in a humidified
atmosphere of 5% CO2 and 95% air at 37°C.
Induced differentiation of THP-1
cells
The THP-1 cells were cultured and differentiation
was induced (β-mercaptoethanol was removed during induction, and
the serum was inactivated). Initially, the cells were cultured in
RPMI-1640 medium supplemented with 320 nM phorbol 12-myristate
13-acetate (PMA, ab120297). After 48 h, the cells adapted from
suspension to adherent growth (cultured M0 macrophages).
Thereafter, the original medium was discarded and changed to
RPMI-1640 medium supplemented with 20 ng/ml IL-4 and IL-13 for 72
h. The majority of the cells protruded from pseudopods, and were
identified as TAMs. Following repeated washing with aseptic PBS,
subsequent co-cultivation was possible. The cytokine recombinant
human IL-4 (200–04)/IL-13 (200–13) was purchased from
PeproTech, Inc.
ELISA assay
Following normal culture of THP-1 and TAMs for 48 h,
cell culture supernatant was centrifuged for 20 min at 1,000 × g
and at a temperature of 2–8°C. Cell debris and impurities were
removed and supernatant was collected. Samples were stored at −20°C
to avoid repeated freezing and thawing. These assays were performed
according to the manufacturer's instructions, using the Invitrogen
ELISA Kit (cat. no., KHC0101; Thermo Fisher Scientific, Inc.) for
human IL-10. The standard curve assay range was 7.8–500 pg/ml,
sensitivity <1 pg/ml.
Flow cytometry
M0 and TAMs were digested with trypsin without
phenol red. THP-1 cells grown normally without treatment were used
as normal controls. They were resuspended following low-speed (600
× g) centrifugation at room temperature for 5 min. A total of
1×106 cells were counted in each flow tube, then washed
3 times with sterile PBS. Cluster of differentiation (CD) 14 (cat.
no., 555399; dilution 1:5) and CD163 (cat. no., 556018; 1:5) flow
and isotype control antibodies (BD Biosciences) were then added and
incubated for 45 min in the dark at room temperature. Following
washing, they were resuspended in 500 µl PBS and examined using a
flow cytometer. The results were analyzed using BD FACSDiva 6.1
software (BD Biosciences).
Transwell chamber indirect coculture
and experimental grouping
Using a 0.4 µm Transwell coculture system
(Sigma-Aldrich; Merck KGaA), THP-1 cells and TAMs were indirectly
cocultured with A549 cells, respectively. A549 cells in the
logarithmic growth phase were plated in 6- or 24-well plates (lower
chamber) and untreated THP-1 cells or induced TAMs were placed in
the Transwell chamber (upper chamber). The experimental groups were
as follows: Blank control (A549 cells were cultured in 1640-RPMI
medium containing 1% FBS); THP-1 cell coculture (A549 cells
cocultured with THP-1 cells); and TAMs coculture (A549 cells
cocultured with TAMs).
EDU proliferation experiment
Each group contained 3 duplicate wells. A549 cells
were seeded at a density of 1.0×104/600 µl in DMEM
medium and placed in a 24-well plate. The normal culture was at
70–80% confluence. The old medium was removed via a Transwell
chamber membrane (0.4 µM). THP-1 cells and TAMs cells were seeded
at a density of 1×105/200 µl according to the
aforementioned experimental coculture groups. The Transwell
membrane was removed after 24 h and suspended in DMEM medium
containing 50 µM EDU reagent A (Guangzhou RiboBio Co., Ltd.) for 2
h, following removal of the lower chamber culture medium. Cells
were fixed with 4% formaldehyde for 15 min at room temperature and
treated with 0.5% Triton ×100 for 20 min at room temperature to
permeabilize cells. After being washed with PBS three times, cells
were incubated with 1X Apollo reaction cocktail (200 µl/well) for
30 min. DNA was stained with 10 µg/ml of Hoechst 33342 stain (200
µl/well) for 20 min and visualized with fluorescence microscopy.
Images were captured with a fluorescence microscope (magnification,
×100). Five groups of confluent cells were randomly selected from
each sample image. EdU-positive cells were counted in the
fluorescent image, and the relative positive ratio was calculated
as the number of red positive cells as a percentage of blue
DAPI-stained cells from the average of the five group values.
Transwell invasion and migration
assay
A549 cells cocultured with THP-1 cells or TAMs for
48 h were trypsinized, centrifuged at 600 × g for 5 min at room
temperature and resuspended in serum-free DMEM medium, and cell
density was adjusted to 1×105/ml. The cell suspension
(200 µl) was added to the upper chamber, which was precoated with
50 µg/ml Matrigel gel at 37°C for 30 min, and 600 µl DMEM
supplemented with 10% FBS was added to the lower chamber. After 24
h, the Transwell membrane (8 µm) was removed and fixed with 100%
methanol at room temperature for 30 min. The cells were wiped with
a cotton swab to avoid penetration of the membranes of the upper
chamber, and then stained with 0.1% crystal violet at room
temperature for 20 min. A light microscope (magnification, ×100)
was used to capture images and count the number of transmembrane
cells and examine the invasive ability of A549 cells. The protocols
for the Transwell migration and invasion experiments were
identical, with the exception of the use of Matrigel gel in the
invasion experiments only.
Scratch wound assay
A549 cells were seeded in 6-well plates. At a
confluence of 80–90%, a sterile 200 µl pipette tip was used to
scratch cells. Following 2 washing steps with sterile PBS, fresh
DMEM high glucose medium containing 1% FBS was added. In the upper
chambers of Transwell (membrane pore size, 0.4 µm), THP-1 or TAM
cells (1×105 cells/well) were plated in 500 µl RPMI-1640
medium supplemented with 1% FBS, and incubated at 37°C for 2 h.
Transwells were then placed into the 6-well plates containing A549
cells which had been previously prepared. Images were captured
under an light microscope (magnification, ×100) at 0 and 24 h,
respectively, and the wound area was measured.
Western blot analysis
Following treatment, A549 cells were harvested and
lysed using radioimmunoprecipitation assay lysis buffer (Thermo
Fisher Scientific, Inc.) supplemented with PMSF (1 mM). The whole
cell lysates were centrifuged at 12,000 × g for 15 min at 4°C, and
protein content in the supernatant was determined by the
bicinchoninic acid protein assay kit (Applygen Technologies, Inc.).
Proteins samples (30 µg per lane) were separated by 10% SDS-PAGE
and transferred to PVDF membranes at 4°C for 90 min. After blocking
with 5% non-fat milk at room temperature for 60 min, membranes were
incubated with the following primary antibodies overnight at 4°C:
Anti-PI3K p85α (cat. no., ab191606; 1:1,000); anti-phosphorylated
(p)-PI3K p85α (Y607; cat. no., ab182651; 1:1,000); anti-AKT1/2/3
(cat. no., ab179463; 1:10,000); anti-p-AKT1 (Ser473; cat. no.,
ab81283; 1:1,000); and anti-GAPDH (cat. no., ab181602; 1:10,000).
All primary antibodies were purchased from Abcam. The membranes
were then probed with IgG-horseradish peroxidase-conjugated goat
anti-rabbit (cat. no., ab2057; Abcam; 1:10,000) and goat anti-mouse
(cat. no., ab6789; Abcam; 1:10,000) secondary antibodies for 1 h at
room temperature. Finally, the proteins were detected using ECL
Enhanced Chemiluminescent kit (Thermo Fisher Scientific, Inc.).
Densitometric analysis was performed using ImageJ 1.44p software
(National Institutes of Health).
Statistical analysis
Experiments were performed in triplicate. Data are
presented as the mean ± standard deviation. Statistical analysis
was performed using GraphPad Prism 6 software (GraphPad Software,
Inc.). Data were evaluated using one-way ANOVA with Tukey's post
hoc test or unpaired two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Formation of TAMs
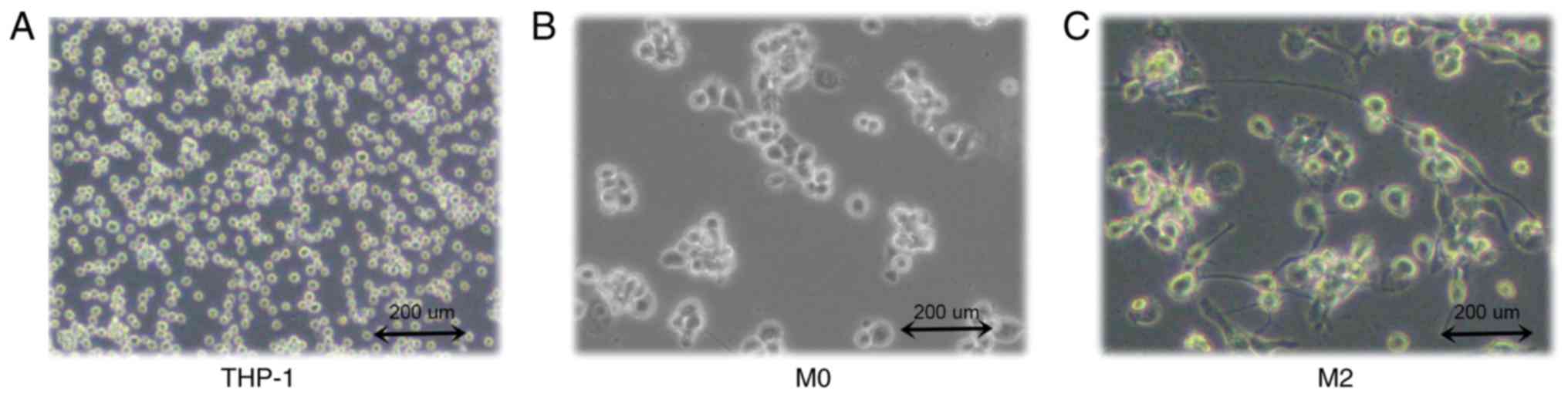
THP-1 human mononuclear leukemia cells were
successfully induced to form TAMs (Fig.
1A). After 48 h of PMA stimulation, the cells adhered to the
wall of the culture plate but no longer proliferated. Cell became
large, with round or elliptical morphologies, and there was a
pseudopod extension (Fig. 1B). After
72 h of stimulation with IL-4 and IL-13, the majority of the cells
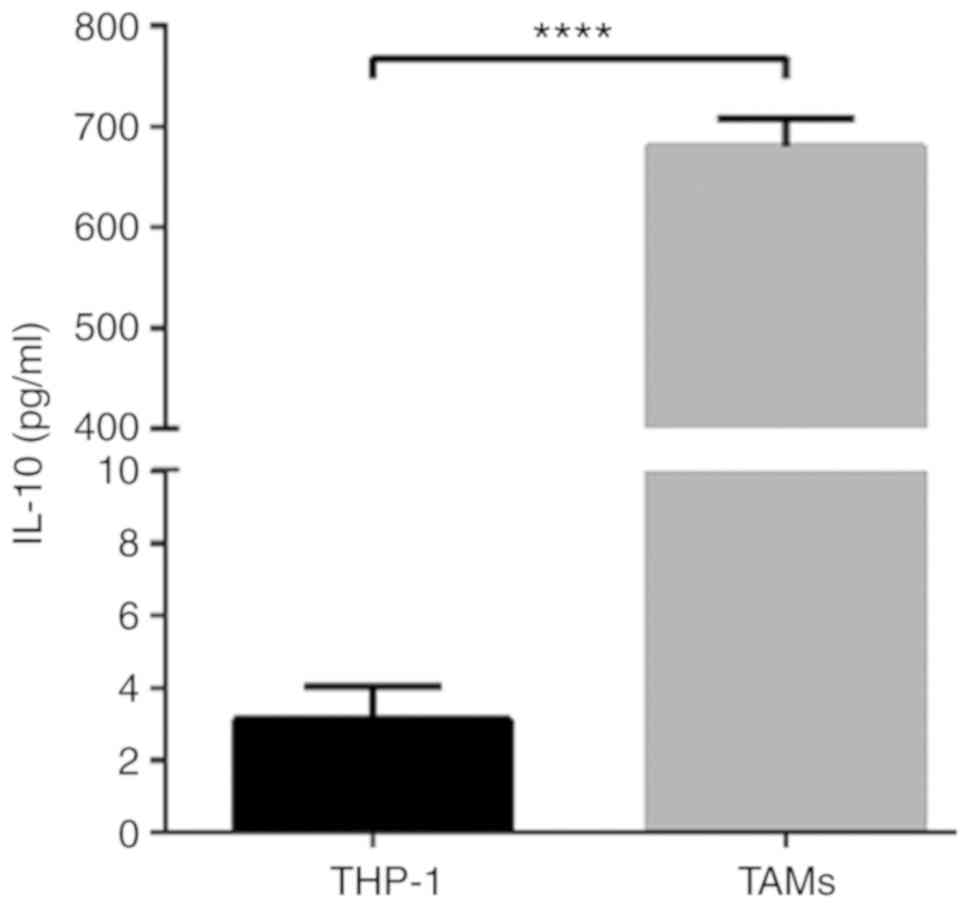
were spindle-shaped, protruding from the pseudopod (Fig. 1C). Then, ELISA was used to detect the
expression of IL-10 in THP-1 and TAM culture supernatants. The
results indicated that IL-10 expression levels in the TAM culture
supernatant were significantly increased compared with the THP-1
supernatant (P<0.0001; Fig. 2).
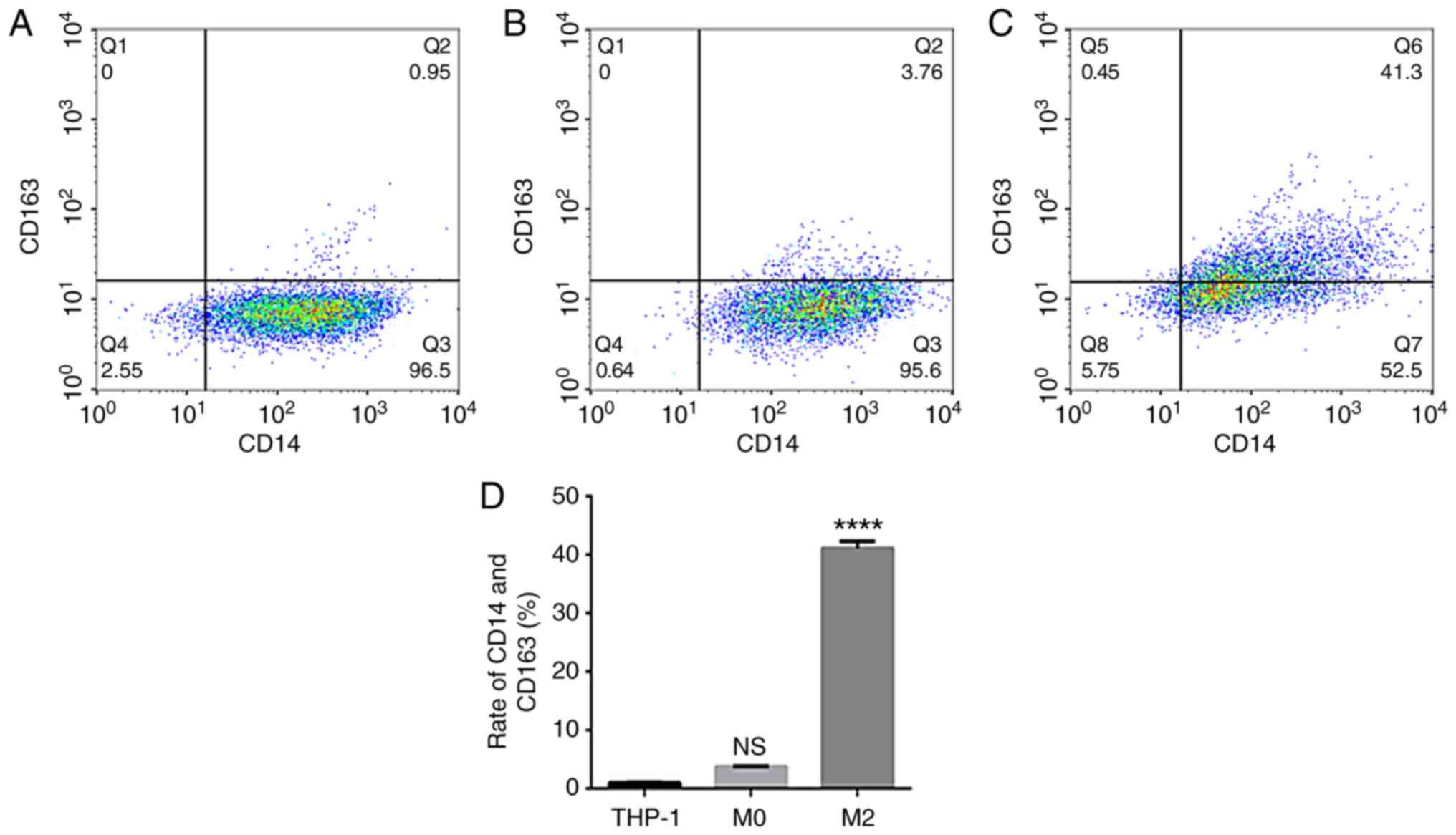
The flow cytometry data suggested that the expression rate of
uninduced THP-1 CD14+/CD163+ cells was
0.96±0.09% (Fig. 3A). The expression
rate of CD14+/CD163+ cells after 48 h of PMA
stimulation was 3.77±0.10% (Fig.
3B). The expression rate of CD14+/CD163+
cells following stimulation with IL-4 and IL-13 was 41.10±1.21%
(Fig. 3C), and the successful
induction of CD14+/CD163+ cells was
demonstrated in the TAMs culture group (Fig. 3D). The rate of expression was
significantly increased compared with that of uninduced THP-1
cells, and the difference was statistically significant
(P<0.0001).
TAMs affect the biological activity of
A549 cells
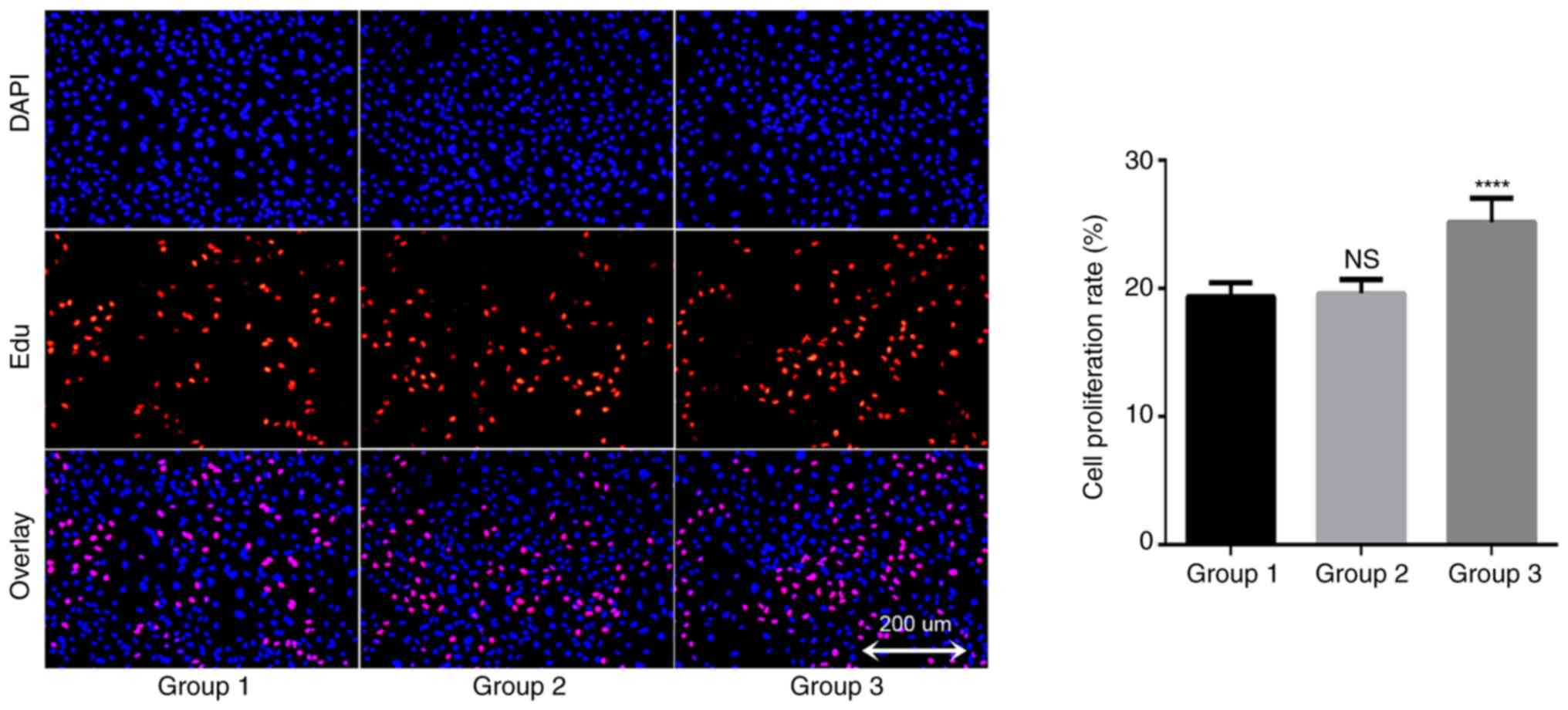
In order to understand the potential role of TAMs in
the growth of A549 cells, an EDU proliferation kit was used to
detect changes in the proliferation rate of lung cancer A549 cells.
The results demonstrated that the proliferation rate of the blank
control group was 19.4±1.1% after 24 h of A549 cell culture; the
proliferation rate of the THP-1 coculture group was 19.6±1.1%, and
the proliferation rate of TAMs coculture group was 25.2±1.9%
(Fig. 4). There was no significant
difference between the THP-1 coculture and blank control groups
(P=0.945), but the proliferation rate of the TAMs coculture group
was significantly increased compared with that of the blank control
group. These data suggested that TAMs in coculture system may
promote the proliferation of lung adenocarcinoma A549 cells.
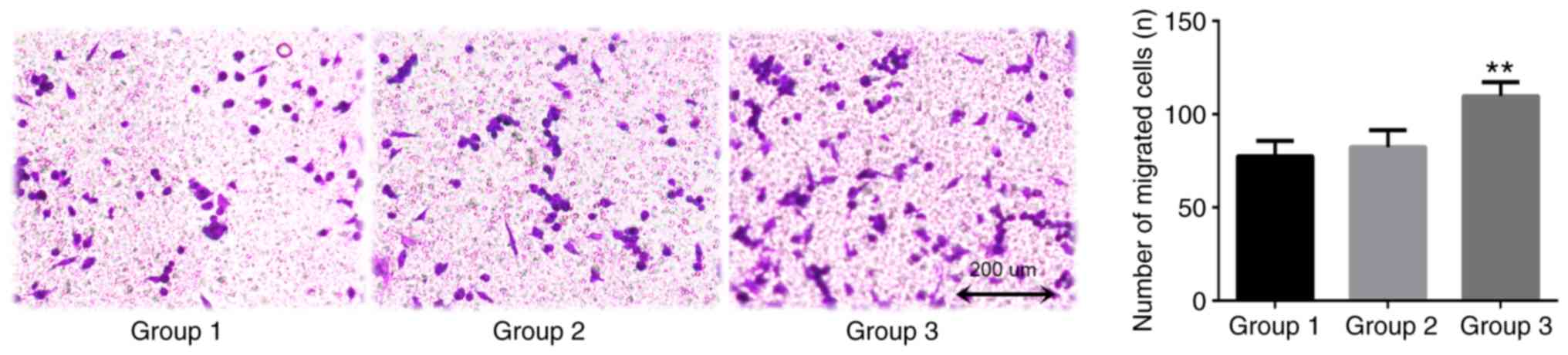
TAMs in the coculture system may promote the
invasion of lung adenocarcinoma A549 cells, as indicated by data
from the Transwell assay conducted in the presents study (Fig. 5). After 24 h coculture, the number of
transmembrane cells in the blank control group was 77.3±8.3. The
number of transmembrane cells in the THP-1 coculture group (group
2) was 82.3±9.1. The difference between these groups was not
statistically significant (P=0.753). The number of transmembrane
cells in the TAMs coculture group was 109.7±7.5, which was
significantly increased compared with that of the blank control
group (P=0.007). This confirmed that TAMs promoted the invasive
ability of A549 cells.
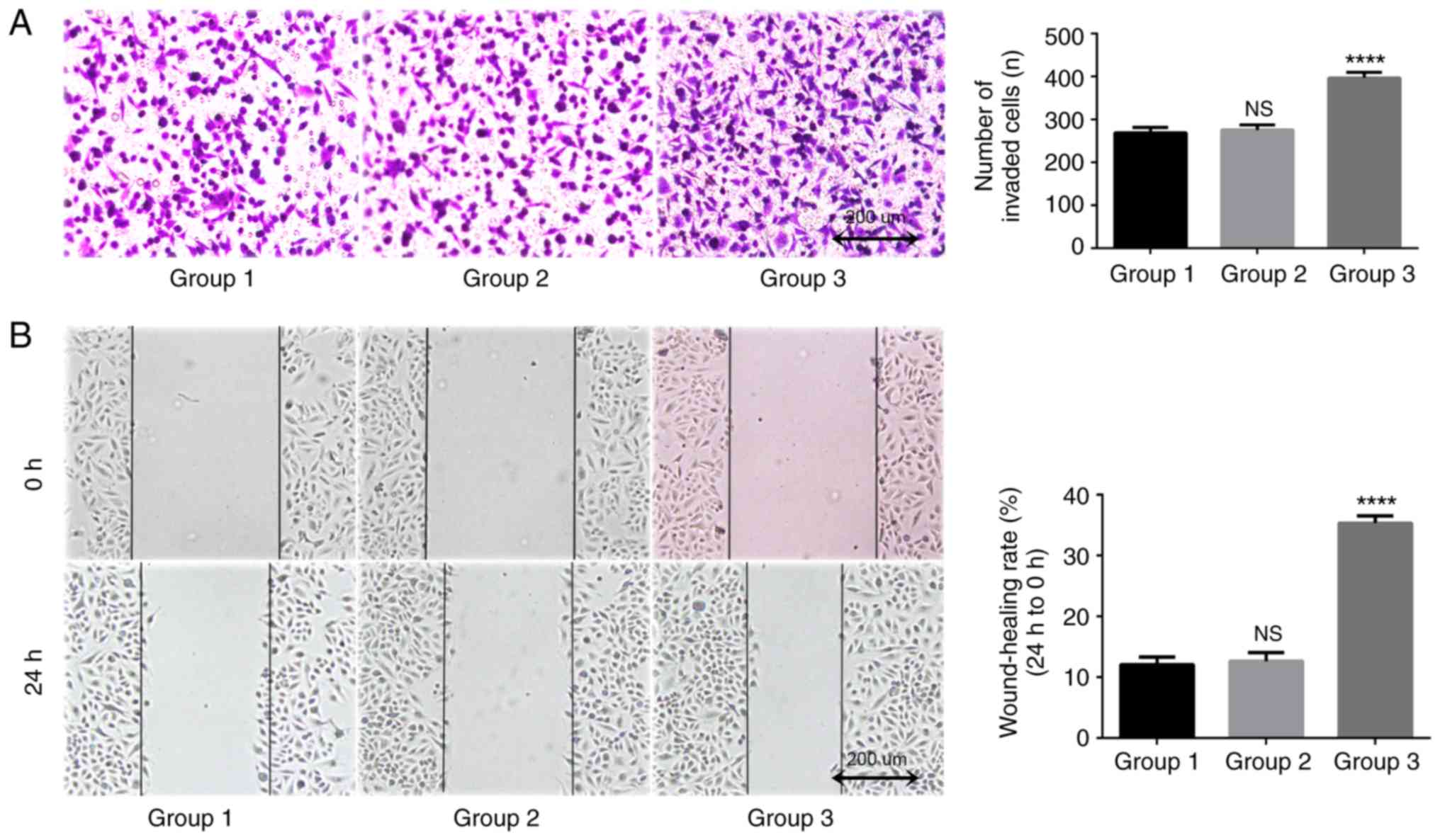
TAMs in coculture system may promote the migration
of lung adenocarcinoma A549 cells. In the present study, a
Transwell assay was used to detect longitudinal migration ability
of A549 cells. The results indicated that after 24 h of coculture,
the number of transmembrane cells in the blank control group was
269.0±12.5, and the number in the THP-1 coculture group was
275.3±12.1. The difference between these two groups was
statistically significant (P=0.816; Fig.
6A). The number of transmembrane cells in the TAMs coculture
group was 396.7±13.0, which was significantly increased compared
with that in the blank control group and the THP-1 coculture group
(P<0.0001). A scratch wound assay was used to detect lateral
migration ability of A549 cells. The results indicated that the
differences between the TAMs coculture group compared with the
blank control group was 12.0±1.3%, and between the THP-1 coculture
group and the blank control group was 12.6±1.4% (Fig. 6B). The migration rate of A549 cells
after 24 h (35.3±1.2%) was significantly increased (P<0.0001).
These data suggested that TAMs may improve the migration ability of
A549 cells in the coculture system.
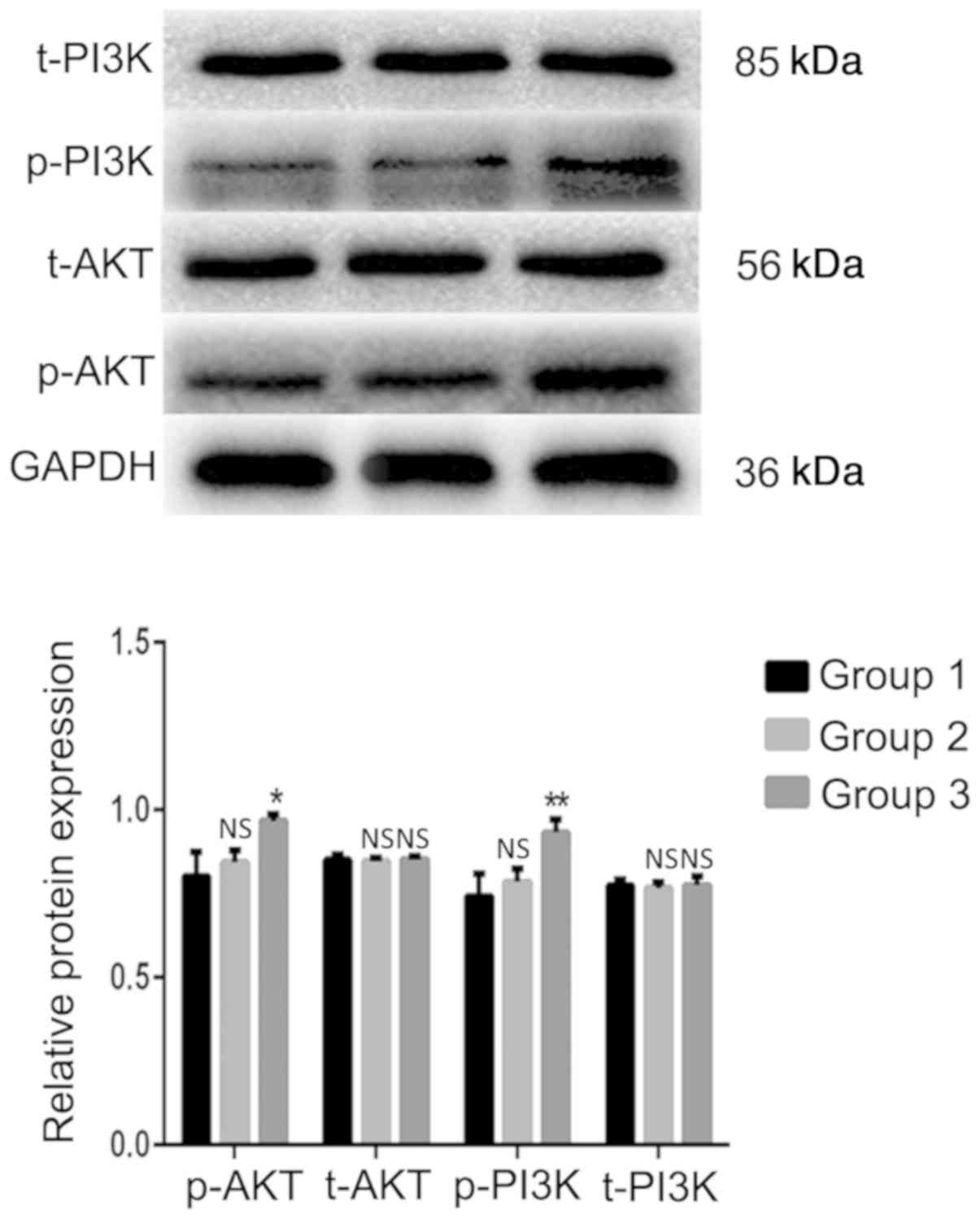
PI3K/AKT signaling pathway is
activated in A549 cells by indirect coculture with TAMs
The western blot analysis results indicated that the
expression levels of the p-AKT and p-PI3K proteins in the TAMs
coculture group were significantly increased compared with those in
the blank control group (Fig. 7).
The differences were statistically significant (P=0.014 and
P=0.008, respectively). The expression levels of the total (t)-AKT
and t-PI3K proteins were not significantly different from those in
the blank control group (P>0.05). These results demonstrated
that TAMs may affect the biological activity of lung adenocarcinoma
A549 cells by activating the PI3K/AKT signaling pathway in
coculture systems.
Discussion
The tumor microenvironment is the internal
environment on which tumor cells depend for their survival and
development. In previous years, the interaction between immune
cells and other cells or factors in the tumor microenvironment has
been demonstrated. Immature monocytes in the blood are recruited by
various cytokines in the tumor microenvironment and induced to
undergo a phenotypic and functional transformation, thereby forming
TAMs. Furthermore, chemical factors including chemokines, growth
factors, angiogenic factors and matrix proteases are produced to
act on tumor cells in order to promote tumor growth, invasion and
metastasis. It is hypothesized that immature monocytes may be
induced into different functional macrophages by different
cytokines, including M1 macrophages induced by granulocyte-
macrophage-colony-stimulating factor/lipopolysaccharide to promote
inflammation and tumor suppressor activity via NF-κB and p-signal
transducer and activator of transcription 1 pathways, and induced
M2 macrophages by IL-4 and IL-13 in order to promote tumor growth
(6,7). As TAMs have a similar function to M2
macrophages, M2 macrophages are usually induced in vitro to
replace TAMs in research models (18). The present study also used specific
concentrations of PMA, IL-4 and IL-13 to stimulate the
differentiation of THP-1 cells into M2 macrophages as opposed to
TAMs, and used ELISA to detect the expression of IL-10 in THP-1
cells and TAM culture supernatants. In addition, flow cytometry was
used to detect the expression ratio of
CD14+/CD163+ cells to verify that the model
was successfully established. The experimental results indicated
that following induction, the growth characteristics of THP-1 cells
changed from suspension to adherence, and their morphology changed
from round to fusiform with no proliferative activity. The flow
cytometry data suggested that the expression ratio of
CD14+/CD163+ pattern in the induced cells was
significantly increased, thereby confirming that the in
vitro study model of TAMs was successfully established in the
present study.
Previous studies have demonstrated that TAMs may
affect the efficacy of epidermal growth factor receptor-tyrosine
kinase inhibitors (EGFR-TKI) targeted therapy in NSCLC, and its
pathological score may be used as an effective prognostic biomarker
(19–21). However, at a cellular level, there is
no relevant basic research to confirm that TAMs directly affect the
the ability of TKIs to induce cell death in NSCLC cells. Additional
studies are vital to explore the potential involvement of TAMs in
mediating NSCLC TKI-targeted drug resistance and TKI resistance
reversal via immunotherapy. In the present study, a Transwell
coculture method was used to simulate a tumor microenvironment, and
the effects of cytokines secreted by TAMs on the biological
activity of lung adenocarcinoma A549 cells were explored. The
molecular mechanism was initially examined on a preliminary basis.
In the present study, an indirect coculture model of TAMs and lung
adenocarcinoma A549 cells was successfully established and an EDU
proliferation assay kit was used to detect the proliferation rate
of A549 cells. The results demonstrated that TAMs significantly
promoted the proliferation and clonality of A549 cells. Through
Transwell invasion, migration and scratch wound assays, it was also
identified that TAMs also significantly promoted the invasion and
migration of A549 cells.
The PI3K/AKT cell signal transduction pathway a
signaling pathways closely associated with tumorigenesis and
development. In lung cancer, it not only promotes proliferation,
invasion and migration of tumor cells through cascade
phosphorylation of a series of proteins, but it may also affect the
efficacy of EGFR-targeted therapy for non-small NSCLC (22,23).
Therefore, the present study aimed to verify whether TAMs affect
the biological activity of lung cancer cells by activating the
PI3K/AKT signaling pathway. In the present study, the expression
levels of t-PI3K, t-AKT and corresponding phosphorylated p-AKT and
p-PI3K proteins were detected by western blot analysis. The results
suggested that TAMs may significantly promote the expression of
phosphorylated PI3K and AKT proteins. In other words, cytokines
secreted by TAMs may activate the PI3K/AKT signaling pathway in
order to promote the proliferation, invasion and migration of A549
cells. Whether TAMs are involved in the TKI resistance observed in
lung cancer cells exhibiting EGFR-activating mutations would be an
interesting topic of future study.
The data from the present study is inconclusive in
certain aspects, for example: TAMs in the microenvironment may
affect tumor cells through paracrine effects and direct contact,
but the present study only established an indirect coculture
environment. The proportion of TAMs in the in vitro TAMs
study model was high, but it was not pure. Although the present
study established the THP-1 coculture group to exclude the effects
of undifferentiated or other different types of macrophages,
purification of TAMs by specific antibody-modified magnetic beads
would produce more convincing results. In conclusion, TAMs promoted
the proliferation, invasion and migration of lung adenocarcinoma
A549 cells, and this may be associated with the activation of the
PI3K/AKT signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by Key Projects of
External Scientific and Technological Cooperation of Jiangxi
Province Science and Technology Department (grant no.
20181BBH80005) and the Natural Science Foundation of Jiangxi
Province (grant no. 20161BAB205265).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
JX designed the experiment and revised the
manuscript. SY and YD performed the experiments and were major
contributors in writing the manuscript. LP, MY, ZL and LN collected
and collated the relevant literature and data and processed the
experimental data and images. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for approval
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerkar SP and Restifo NP: Cellular
constituents of immune escape within the tumor microenvironment.
Cancer Res. 72:3125–3130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrand-Rosenberg S: Tolerance and immune
suppression in the tumor microenvironment. Cell Immunol. 299:23–29.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prenen H and Mazzone M: Tumor-associated
macrophages: A short compendium. Cell Mol Life Sci. 76:1447–1458.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goswami KK, Ghosh T, Ghosh S, Sarkar M,
Bose A and Baral R: Tumor promoting role of anti-tumor macrophages
in tumor microenvironment. Cell Immunol. 316:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aras S and Zaidi MR: TAMeless traitors:
Macrophages in cancer progression and metastasis. Br J Cancer.
117:1583–1591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Yang L, Gao Q, Huang L, Wang L,
Wang J, Wang S, Zhang B and Zhang Y:
CD163+CD14+ macrophages, a potential immune
biomarker for malignant pleural effusion. Cancer Immunol
Immunother. 64:965–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Wang F, Wang L, Huang L, Wang J,
Zhang B and Zhang Y: CD163+ tumor-associated macrophage
is a prognostic biomarker and is associated with therapeutic effect
on malignant pleural effusion of lung cancer patients. Oncotarget.
6:10592–10603. 2015.PubMed/NCBI
|
|
15
|
Ohtaki Y, Ishii G, Nagai K, Ashimine S,
Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I and Ochiai
A: Stromal macrophage expressing CD204 is associated with tumor
aggressiveness in lung adenocarcinoma. J Thorac Oncol. 5:1507–1515.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loyher PL, Hamon P, Laviron M,
Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici
N, Baudesson de Chanville C, et al: Macrophages of distinct origins
contribute to tumor development in the lung. J Exp Med.
215:2536–2553. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackute J, Zemaitis M, Pranys D,
Sitkauskiene B, Miliauskas S, Vaitkiene S and Sakalauskas R:
Distribution of M1 and M2 macrophages in tumor islets and stroma in
relation to prognosis of non-small cell lung cancer. BMC Immunol.
19:32018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung FT, Lee KY, Wang CW, Heh CC, Chan
YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, et al:
Tumor-associated macrophages correlate with response to epidermal
growth factor receptor-tyrosine kinase inhibitors in advanced
non-small cell lung cancer. Int J Cancer. 131:E227–E235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Zhang Y, Zhao J, Wang Z, Wu T, Ou
W, Wang J, Yang B, Zhao Y, Rao Z and Gao J: M2-polarized
macrophages contribute to the decreased sensitivity of EGFR-TKIs
treatment in patients with advanced lung adenocarcinoma. Med Oncol.
31:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng H, Chen B, Huang W, Tang Y, Jiang Y,
Zhang W and Huang Y: Reprogramming tumor-associated macrophages to
reverse EGFRT790M resistance by dual-targeting
codelivery of gefitinib/vorinostat. Nano Lett. 17:7684–7690. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gadgeel SM and Wozniak A: Preclinical
rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for
epidermal growth factor receptor inhibitor-resistant non-small-cell
lung cancer. Clin Lung Cancer. 14:322–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pérez-Ramírez C, Cañadas-Garre M, Molina
MÁ, Faus-Dáder MJ and Calleja-Hernández MÁ: PTEN and PI3K/AKT in
non-small-cell lung cancer. Pharmacogenomics. 16:1843–1862. 2015.
View Article : Google Scholar : PubMed/NCBI
|