Introduction
The treatment of cancer in the peritoneal cavity is
a major problem in gastrointestinal and gynecological oncology
worldwide (1,2). Globally, patients with gastric cancer
with peritoneal carcinomatosis have a median survival time of 3–6
months, when the disease is left untreated (3). In the majority of newly diagnosed
ovarian cancer cases, peritoneal metastases are already present
(4).
Hyperthermic intraperitoneal chemotherapy (HIPEC) is
a promising treatment option for intraperitoneally disseminated
cancer (5–7). It has been reported to be more
efficient compared with intravenous chemotherapy, however, as a
treatment of peritoneal metastases it is limited by the
plasma-peritoneal barrier (8,9). It has
been previously suggested that hyperthermia can enhance the
intraperitoneal application of cytotoxic agents (10,11).
Residual microtumors, following cytoreductive surgery, are treated
by intraperitoneal chemotherapy in combination with hyperthermia up
to 42–43°C (12). Overall,
hyperthermia as part of HIPEC is thought to boost pharmacokinetics
and increase DNA damage induced by the cytostatic agent (13). Cisplatin is an alkylating drug used
to treat residual gastric and ovarian cancer in the peritoneal
cavity (14). Previous in
vitro studies produced controversial data about the additivity
of hyperthermia to cisplatin (15,16).
The response and resistance of cancer cells to
chemotherapy and hyperthermia depend on the induction and
expression of a number of cytoprotective proteins, including Hsp70
and Hsp27 (17–19). Therefore, the modulation of
cytoprotective proteins may serve a crucial role in cancer
treatment. One potential target is heme oxygenase (HO)-1,
particularly in HIPEC, since high temperatures are a component of
HIPEC, and it has been reported that, under hyperthermia, cells
enhance HO-1 expression for self-protective purposes
(19). HO-1 is normally
expressed at low levels in the majority of tissues, including the
gastrointestinal tract, female reproductive organs, brain and bone
marrow (20); however, it is highly
inducible by a variety of stimuli, including cytokines,
lipopolysaccharides (21) and
serine/threonine kinases (22).
Cellular levels of HO-1 are known to be
temperature-dependent (23,24). HO-1 is overexpressed under
hyperthermic conditions, exerting a protective function (25,26).
An in vitro study was conducted to clarify
the underlying mechanism of how cisplatin and hyperthermia induce
HO-1 expression in ovarian and gastric cancer cells. In
addition, the present study investigated the response of these
cancer cells to cisplatin and hyperthermia following the modulation
of HO-1 protein expression.
Materials and methods
Cell lines and conditions
Human gastric adenocarcinoma, AGS, and ovarian
adenocarcinoma, OVCAR-3, cell lines were purchased from the
American Type Tissue Culture Collection (Manassas, VA, USA).
OVCAR-3 cells were cultivated in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 20% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin/streptomycin and 0.01 mg/ml bovine insulin (Gibco;
Thermo Fisher Scientific, Inc.). AGS cells were harvested in Ham's
F-12K medium with 10% FBS and 1% penicillin/streptomycin at 37°C
and 5% CO2.
Experimental design
Cells were harvested for 24 h in the conditions
described previously. The cells were subjected to conditions of
normothermia (37°C) or 43°C and/or an IC50 dose of
cisplatin. The IC50 dose was determined for each cell
line individually in an experimental manner. The IC50
doses of cisplatin for AGS and OVCAR-3 cells were determined (at
37°C) to be 111 and 152 µM, respectively. Hyperthermia and/or
cisplatin exposure lasted for 1 h; this step began once the media
reached the desired temperature (37 or 43°C), as measured by a
digital thermometer in a humid incubator with a set temperature of
43°C. Following treatment, the medium was changed and cells were
harvested after 48 h of incubation in a humidified atmosphere at
37°C with 5% CO2. AGS and OCAR-3 cell viability,
apoptosis, and cell index were all subsequently measured.
Additionally, these cell lines were used in real time cell
analysis, western blotting and semi-quantitative polymerase chain
reaction (qPCR) assays.
Silencing of HO-1
HO-1 small interfering RNA (siRNA; 30 nM
HMOX1; sense 5′-UGAACACUCUGGAGAUGAC-3′, and antisense
5′-GUCAUCUCCAGAGUGUCCA-3′) was obtained from Ambion; Thermo Fisher
Scientific, Inc., and negative control (30 nM AllStars Negative
Control siRNA; sense 5′-UUCUCCGAACGUGUCACGU-3′ and antisense
5′-ACGUGACACGUUCGGAGAA-3′) was obtained from Qiagen GmbH (Hilden,
Germany). Lipofectamine® RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.) and Opti-MEM™ media (Gibco; Thermo Fisher
Scientific, Inc.) were used according to the manufacturer's
protocols. The efficiency of transfection was verified using
BLOCK-iT Alexa Fluor (Invitrogen; Thermo Fisher Scientific, Inc.).
The efficiency of knockdown was verified by western blot analysis.
HO-1-silencing was performed 72 h prior to implementation of
the experimental temperature and treatment with cisplatin.
MTT assay
Cell viability was determined using an MTT assay
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were incubated
for 4 h at 37°C following the addition of 5 mg/ml MTT reagent. The
supernatant was subsequently removed and dimethyl sulfide was added
(Carl Roth GmbH Co KG, Karlsruhe, Germany). Absorbance was measured
at a wavelength of 570 nm and the reference was measured at 650 nm
using a Sunrise spectrophotometer (Tecan Austria GmbH, Grödig,
Austria).
qPCR
Cellular RNA was extracted using a
PureLink® RNA Mini kit (Ambion; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
Purified RNA was measured and verified for purity using ultraviolet
(UV) spectrophotometry (NanoDrop; Thermo Fisher Scientific, Inc.).
Using the Super Script Vilo Master Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) with 2 µg RNA, cDNA was generated, according to
the manufacturer's protocols. RNA amplification was performed in a
20 µl reaction volume, which contained 1X PCR Master Mix, primers,
and 2 µl cDNA template. Thermocycling conditions were as follows:
initial step at 95°C for 10 min (1 cycle), denaturation at 95°C for
15 sec and annealing/extending at 60°C for 1 min (40 cycles),
followed by a final extension step at 72°C for 2 min. HO-1
primers were obtained from Invitrogen (Thermo Fisher Scientific,
Inc.): forward, 5′-TGCTCAACATCCAGCTCTTTGAGGA-3′; and reverse,
5′-CAGGCAGAGAATGCTGAGTTC-3′. The products were loaded on 1.5%
agarose gels. Ethidium bromide staining and UV light (Gel Doc™ XR+
Gel Documentation System; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) were used for visualization. Analysis was performed using
ImageLab software (version 6.0.0; Bio-Rad Laboratories, Inc.).
Flow cytometry
Apoptosis was evaluated by flow cytometry using
Annexin V-PE and 7-aminoactinomycin D. A Guava Nexin Annexin V
Assay kit (Merck KGaA, Darmstadt, Germany) was used according to
the manufacturer's protocols. Analysis was performed with the Guava
Personal Cell Analysis Flow Cytometer (Guava; EMD Millipore,
Billerica, MA, USA) and CytoSoft software (version 2.1.4; Guava;
EMD Millipore).
Western blot analysis
Lysates were prepared using radioimmunoprecipitation
lysis buffer (Abcam, Cambridge, UK) containing protease inhibitors
(Roche Diagnostics, Basel, Switzerland). A bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.) was used to
determine the protein concentration, according to the
manufacturer's protocols. Following heating at 97°C for 5 min,
protein samples (50 µg) were subjected to 4–12% SDS-PAGE and
transferred to polyvinylidene fluoride membranes at 30 V for 50
min. Membranes were blocked with a blocking buffer (20% diluent A,
30% diluent B; WesternBreeze Blocker/Diluent; Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 1 h and incubated
with the primary antibodies rabbit anti-HO-1 (dilution,
1:2,000; cat. no., EP1391Y; Abcam) and mouse anti-GAPDH (dilution,
1:1,000; cat. no., AM4300; Ambion; Thermo Fisher Scientific, Inc.)
at 4°C overnight. The following day, the blots were incubated with
ready to use secondary antibodies against rabbit (cat. no. WP20007;
Invitrogen, Thermo Fisher Scientific, Inc.) or mouse immunoglobulin
G (cat. no. WP20006; Invitrogen; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. Chemiluminescence substrate (CDP-Star;
Invitrogen; Thermo Fisher Scientific, Inc.) was added and the
ChemiDoc imaging system (Bio-Rad Laboratories, Inc.) was used for
visualization. ImageJ software (version 1.48; National Institutes
of Health, Bethesda, MD, USA) was used for quantification of
western blots (27).
Real time cell analysis
The xCELLigence® RTCA DP Real-Time
Analyzer (ACEA Biosciences Inc., San Diego, CA, USA) was used to
investigate the PCR cellular response to treatment. To present the
real time cell analysis data obtained by xCELLigence system, the
cell index was used. The determination of the cell index parameter
is an automatic system feature and is based on the rapid
measurements of impedance between gold electrodes in the analyzer
wells (28). Cell-free medium
(RPMI-1640; Gibco; Thermo Fisher Scientific, Inc.) and medium with
cells have different cell impedance and proliferation indices,
which is reflected in changes in impedance value and CI; this is
calculated as follows: (impedancetime point
n-impedancebackground)/nominal impedance value. Delta cell index
was measured immediately when cells reached the electronic
microplate. Cells were evaluated for 48 h following the experiment
and/or 72 h following HO-1-silencing.
Statistical analysis
SPSS v.21.0 software (IBM Corp., Armonk, NY, USA)
and GraphPad (version 6.01; GraphPad Software Inc., La Jolla, CA,
USA) were used for statistical evaluation. The Mann-Whitney test
and one-way analysis of variance with a Bonferroni post hoc test
were performed to assess clinical significance. Data are presented
as the mean ± standard deviation of three independent experiments,
performed in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hyperthermia and cisplatin
differentially induce HO-1 mRNA and protein expression in ovarian
and gastric cancer cells
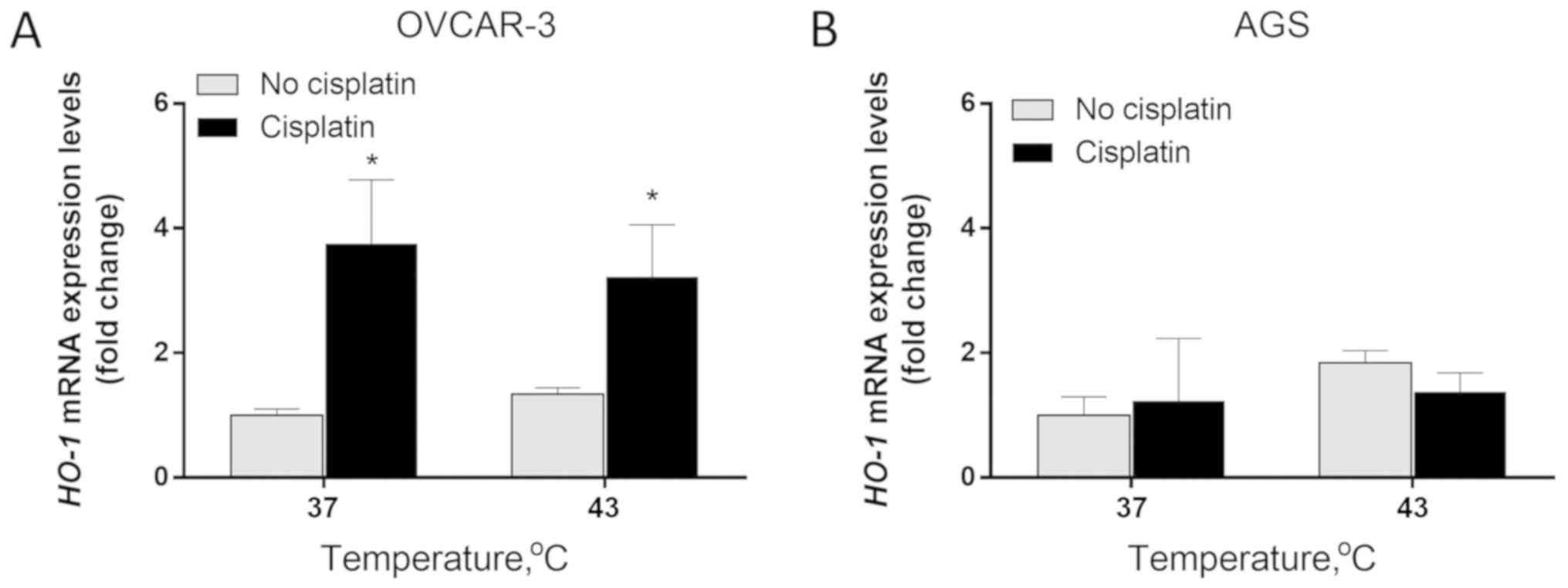
Enhanced expression levels of HO-1 mRNA were
only observed in OVCAR-3 cells. The exposure of OVCAR-3 cells to
cisplatin resulted in a significant increase of HO-1 mRNA
expression. Cisplatin induced a 3.75- and 2.4-fold increase of
HO-1 expression in conditions of normothermia (37°C) and
hyperthermia (43°C), respectively (P<0.05). While hyperthermia
at 43°C boosted HO-1 expression by 1.34-fold, the addition
of cisplatin increased the effect on HO-1 expression by
3.22-fold (P<0.05; Fig. 1A). In
AGS cells, HO-1 expression was not significantly affected by
temperature or cisplatin (Fig.
1B).
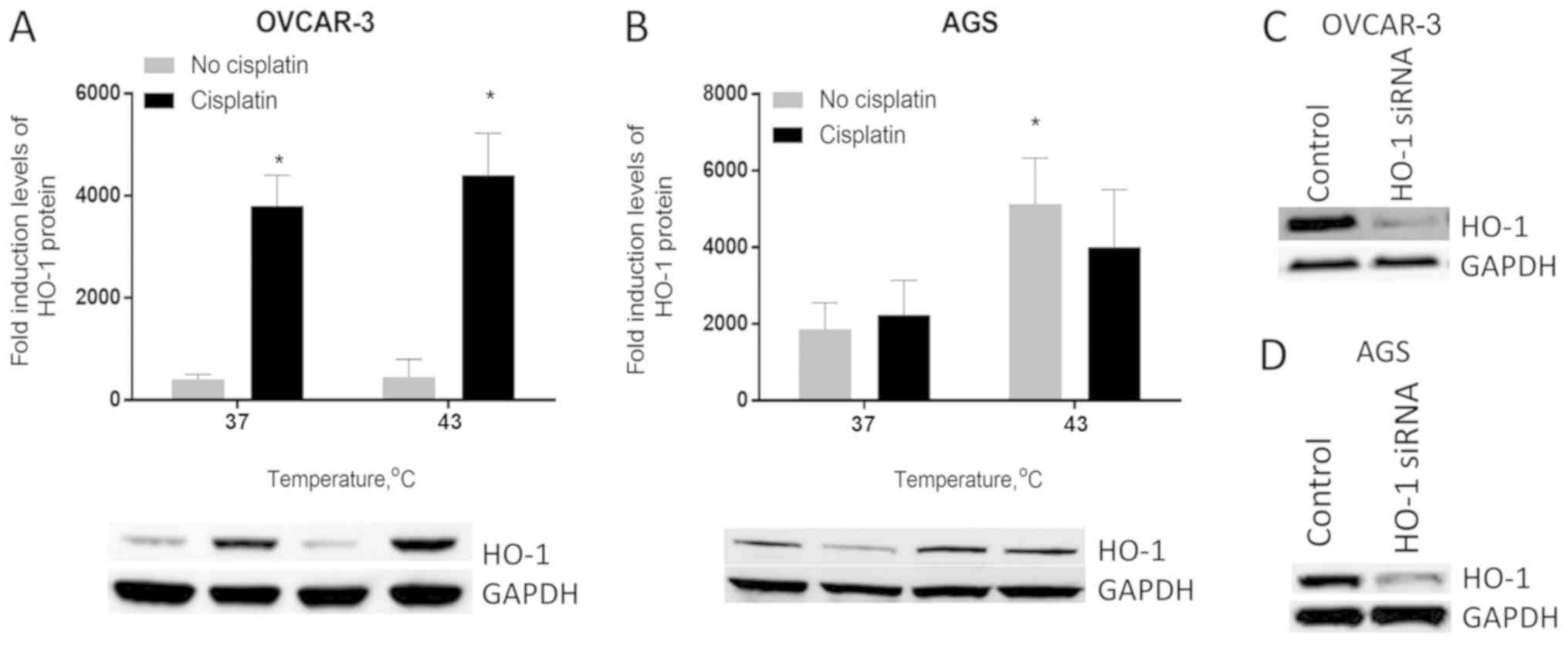
Furthermore, cisplatin significantly increased
HO-1 protein expression in OVCAR-3 cells (P<0.05).
HO-1 expression was increased 9.5-fold, following cisplatin
treatment under normothermia. At 43°C, this effect was slightly
higher, with a 9.77-fold increase (P<0.05). The exposure of
OVCAR-3 cells to hyperthermia alone had no effect on HO-1
protein expression levels. However, the combination of cisplatin
and hyperthermia increased HO-1 protein expression by
11-fold compared with the control (untreated cells in normothermia;
P<0.01; Fig. 2A).
In AGS cells, cisplatin had no notable effect on
HO-1 expression. At 37°C, cisplatin increased HO-1
protein expression in AGS cells by 1.2-fold; however, this increase
was not statistically significant, whereas the exposure of cells to
43°C in the absence of cisplatin increased HO-1 protein
expression levels by 2.75-fold (P<0.05). HO-1 expression
dropped slightly when cisplatin was added at 43°C. Therefore,
concomitant treatment of AGS cells with cisplatin and hyperthermia
at 43°C resulted in a 2.14-fold increase in HO-1 protein
compared with the control (Fig. 2B).
Furthermore, HO-1 knockdown was assessed by western blotting
(Fig. 2C and D).
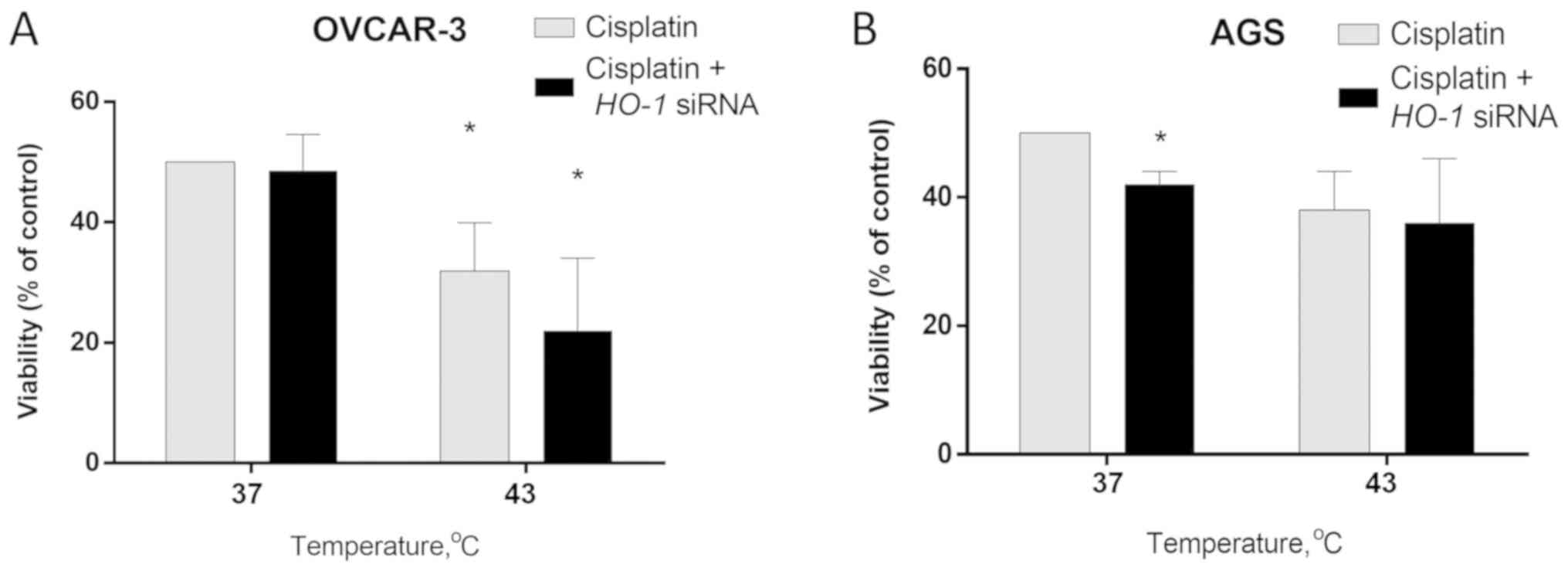
HO-1-silencing does not influence AGS
cell viability
The results of the MTT assay revealed that
HO-1-silencing in OVCAR-3 cells does not affect viability in
response to cisplatin at 37°C. The exposure of OVCAR-3 cells to
cisplatin and hyperthermia (43°C) resulted in a 36% drop in cell
viability (P<0.05). HO-1-silencing enhanced this effect
by an additional 20% (P<0.05; Fig.
3A).
HO-1-silencing in AGS cells enhanced the
cisplatin effect and reduced cell viability by 16% at 37°C
(P<0.05). Hyperthermia potentiated cisplatin cytotoxicity in AGS
cells: viability dropped by 24% compared with 37°C. However,
HO-1-silencing had no significant additional effect, whereas
viability rates were similar in HO-1-silenced or unsilenced
AGS cells following cisplatin treatment at 43°C (Fig. 3B).
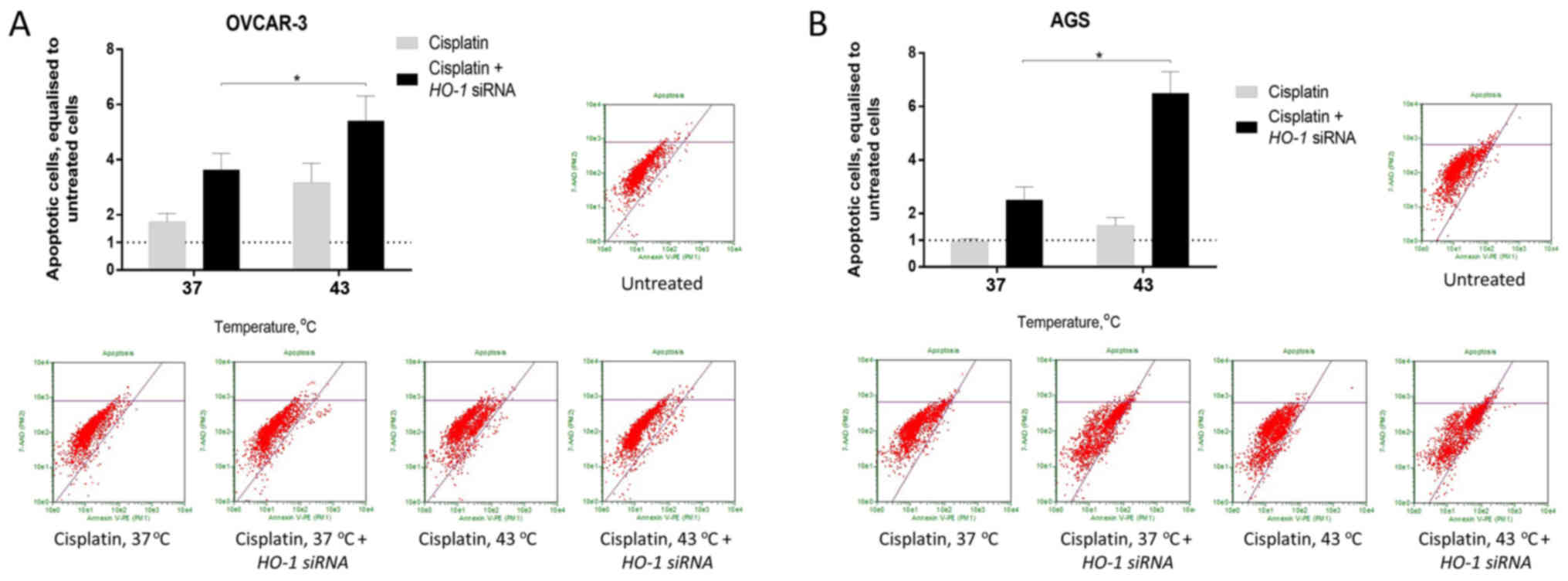
HO-1-silencing prior to concomitant
hyperthermia and cisplatin treatment increases ovarian and gastric
cancer cell apoptosis
The exposure of OVCAR-3 and AGS cells to
hyperthermia resulted in a better cell response to cisplatin with
respect to apoptosis. Prior HO-1-silencing under
normothermia increased cisplatin-induced apoptosis in OVCAR-3 and
AGS cells by 2.07- and 2.63-fold, respectively. In addition,
silencing of HO-1 under hyperthermia enhanced the apoptosis
of OVCAR-3 and AGS cells by 3.09- and 6.84-fold, respectively
(P<0.05; Fig. 4).
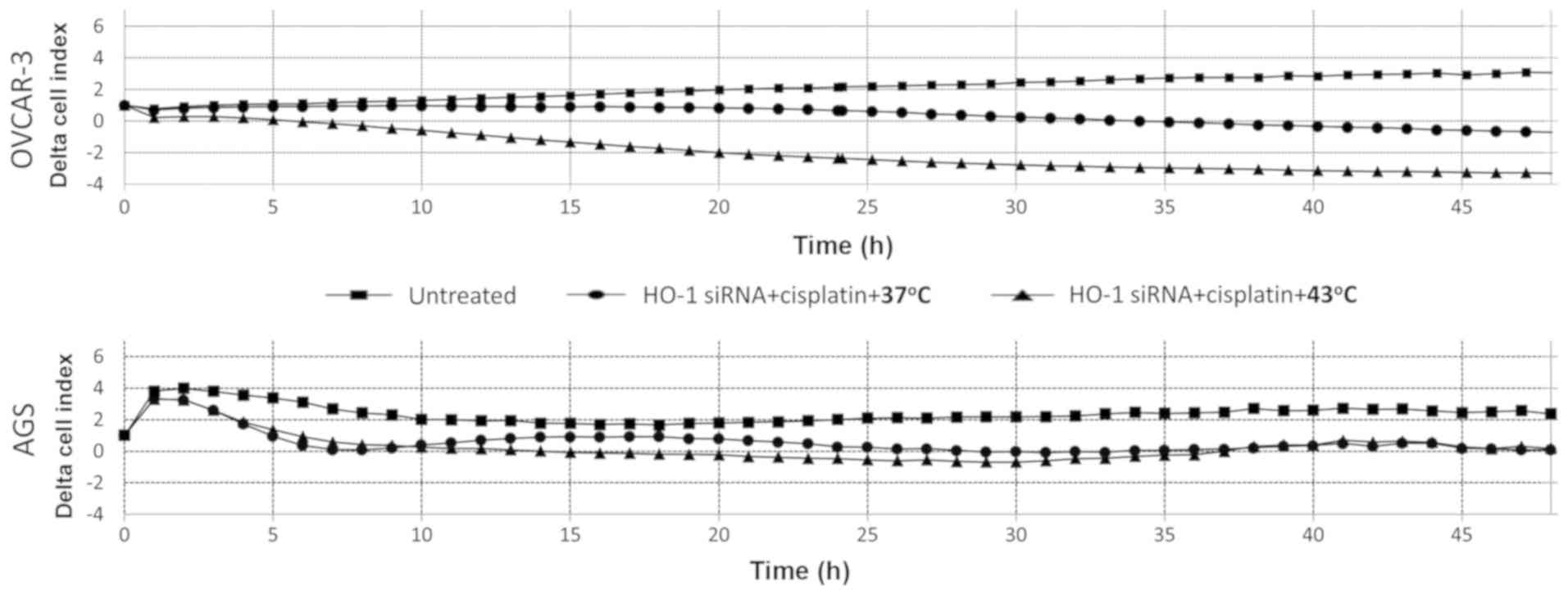
Hyperthermia enhances the effect of
cisplatin on OVCAR-3, but not on AGS cells, following modulation of
HO-1 expression
PCR analysis for 48 h following treatment indicated
that exposure to cisplatin resulted in a gradual decrease in the
cell index of AGS and OVCAR-3 (HO-1-silenced) cells at 37°C.
Hyperthermia at 43°C boosted this effect by inducing a gradual
decrease of the OVCAR-3 (HO-1-silenced) cell index. However,
the cell index of AGS (HO-1-silenced) cells following
cisplatin treatment at 37 or 43°C remained similar (Fig. 5).
Discussion
In the present study, the HO-1 protein was
variably expressed at the basal level and variably induced
following exposure to cisplatin and hyperthermia in OVCAR-3 and AGS
cells. Cisplatin increased the expression levels of HO-1 in
OVCAR-3 cells, while hyperthermia at 43°C had no effect. In AGS
cells, HO-1 expression was slightly increased under
hyperthermia, with no significant induction following exposure to
cisplatin, indicating that the modulation of HO-1 may serve
a role in the response of cancer cells to cisplatin and
hyperthermia and affect cancer treatment outcomes.
HIPEC is widely used in clinical settings, and
promising results have been reported in the treatment of peritoneal
dissemination of gastric and ovarian cancer (29,30). To
the best of our knowledge, to date, there has been a lack of
evidence regarding the synergy of chemotherapy and hyperthermia. In
our previous studies, it was observed that gastrointestinal and
ovarian cancer cells responded unpredictably following exposure to
cisplatin and hyperthermia (16,31). One
of the limits of this response may be the induction of
cytoprotective enzymes associated with chemotherapy and/or
hyperthermia, in particular HO-1. HO-1 is known to be
highly expressed in human gastric and ovarian cancer tissue
(32). Anticancer treatment options,
including chemotherapy and radiotherapy may increase HO-1
expression (33). HO-1 serves
an important role in a number of pathophysiological conditions,
including temperature rise and inflammation, and has been reported
to be associated with cancer (34,35).
HO-1 expression is associated with cancer growth and
progression by promoting angiogenesis in the tumor itself and
metastases and pro-proliferation in different types of tumors,
including renal cell carcinoma, prostate and pancreatic cancer,
melanoma, and hepatoma (36–40). Numerous studies have highlighted that
cancer cells with high expression levels of HO-1 are less
sensitive to cisplatin treatment compared with cancer cells with
low HO-1 expression levels (41,42).
The mechanism underlying this cytoprotective effect
relies on the ability of HO-1 to catabolize free heme and
prevent it from sensitizing cells to undergo programmed cell death
(43). HO-1 under normal
conditions has various cellular functions, including catalyzing the
heme molecule to form bile pigments (44). When stimulus (heat) is present,
cellular HO-1 synthesis is enhanced (45). Therefore, the present study suggests
that HO-1 is crucially important when dealing with
intraperitoneally-spread cancer, particularly treating it with
HIPEC. Following the administration of heated chemotherapy drugs
into the abdominal cavity, tumor cells should start to defend
themselves, by activating heat shock proteins, particularly
HO-1. The aim of the present study is to achieve better
treatment results by downregulating HO-1 expression.
To the best of our knowledge, there are no published
data on the efficacy of HIPEC treatment while modulating the
expression of HO-1. The results of our study demonstrate the
impact of HO-1 expression modulation in the combination
treatment of hyperthermia at 43°C and cisplatin in OVCAR-3
cells.
Zhao et al (46) reported that the basal level of
HO-1 expression is higher in ovarian cancer cells compared
with normal ovarian tissues. A high level of HO-1 expression
has also been associated with aggressive tumors and poor clinical
outcomes (46). The ability of
cisplatin to increase the expression of HO-1 was also
observed in different cancer types, including pancreatic and
hepatic cancer (43,47,48). Was
et al (37) reported the
different abilities of tumor tissues to produce heat shock
proteins. Nonetheless, a high level of HO-1 is known to be
associated with the reduced tumor growth observed in some types of
cancers, including breast and prostate cancer and non-small-cell
lung carcinoma (37).
The results of the present study indicate that the
viability of HO-1-silenced OVCAR-3 cells decreased
significantly following cisplatin treatment at 43°C. However, in
AGS cells, the inhibition of HO-1 did not improve the
response to cisplatin treatment. These results are associated with
the expression of HO-1. It is possible that the inhibition
of HO-1 only increases the effect of cisplatin in cancer
cells, where HO-1 is highly expressed. This is in accordance
with the data reported by Lv et al (41), where cisplatin significantly induced
the expression of HO-1. The study modulated HO-1
expression using hemin (an inducer of HO-1) and ZnPPIX (an
inhibitor of HO-1), and reported that hemin strongly
inhibited cisplatin-induced cell death, while ZnPPIX significantly
increased cell death (41,49). These effects following HO-1
modulation can be explained by the cytoprotective ability of this
protein. HO-1 activates a cellular defense mechanism against
oxidative stress through its catalytic products, including ferrous
iron, carbon monoxide, and biliverdin (37). In addition, growing evidence has
suggested that HO-1 protects cells from chemotherapeutic
agent-induced apoptosis, and the targeted knockdown of HO-1
gene expression or suppression of HO-1 activity in
vitro significantly enhances the chemosensitivity of cancer
cells (50). Furthermore, it has
been reported that the inhibition of HO-1 can increase
cellular response to anticancer treatment (26).
Cisplatin can effectively induce and promote
apoptosis in a wide range of solid tumors, including head and neck
cancer, esophageal carcinoma, non-small cell lung carcinoma, and
testicular, cervical, and ovarian cancer (51). Inhibition of HO-1 may
strengthen the pro-apoptotic effects of cisplatin (41). In the present study, the inhibition
of HO-1 increased the number of apoptotic cells in OVCAR-3
and AGS cell lines, however these results did not indicate any
significant differences associated with HO-1 expression and
cell viability. This could be explained by the fact that the
present study measured the number of cells in both early and late
apoptosis, and early apoptosis can be reversible (52). Geske et al (52) reported that the early stages of
apoptosis are reversible if the apoptotic stimulus is removed,
which is the reason that PCR analysis was performed in the present
study.
In this experimental model, hyperthermia alone did
not induce the upregulation of HO-1 expression in the tested
cancer cell lines. Nevertheless, HO-1-silencing resulted in
the optimal response to cisplatin treatment in terms of cell
viability in OVCAR-3 cells, and apoptosis in both OVCAR-3 and AGS
cells, under conditions of hyperthermia. Therefore, a novel finding
regarding the role of HO-1 in HIPEC is presented in this
study. In conclusion, the cytoprotective protein HO-1 is
induced in cancer cells by different stressors in a variable
manner. In tumors with highly inducible HO-1, the present
study indicated that prior silencing of this gene may significantly
improve the cellular response to hyperthermia and cisplatin.
Acknowledgements
Not applicable.
Funding
This study was supported by the Research Council of
Lithuania (grant no. SEN-01/2015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VC wrote the manuscript and analyzed the data. AS
performed the western blot analysis and analyzed the data. GS and
AJ performed semi-quantitative PCR and siRNA transfection. SP
performed the PCR cell analysis and analyzed the data. ZD and AG
revised the data and manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shariff U, Seretis C and Youssef H:
Management of colorectal cancer patients at high risk of peritoneal
metastases. J BUON. 20 (Suppl 1):S71–S79. 2015.PubMed/NCBI
|
|
2
|
Zivanovic O, Chi DS, Filippova O, Randall
LM, Bristow RE and O'Cearbhaill RE: It's time to warm up to
hyperthermic intraperitoneal chemotherapy for patients with ovarian
cancer. Gynecol Oncol. 151:555–561. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yonemura Y, Endou Y, Sasaki T, Hirano M,
Mizumoto A, Matsuda T, Takao N, Ichinose M, Miura M and Li Y:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart JH IV, Shen P and Levine EA:
Intraperitoneal hyperthermic chemotherapy for peritoneal surface
malignancy: Current status and future directions. Ann Surg Oncol.
12:765–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stirrups R: HIPEC improves survival in
stage III epithelial ovarian cancer. Lancet Oncol. 19:e1382018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tentes AA, Pallas N, Karamveri C,
Kyziridis D and Hristakis C: Cytoreduction and HIPEC for peritoneal
carcinomatosis of pancreatic cancer. J BUON. 23:482–487.
2018.PubMed/NCBI
|
|
7
|
Tonello M, Ortega-Perez G, Alonso-Casado
O, Torres-Mesa P, Guiñez G and Gonzalez-Moreno S: Peritoneal
carcinomatosis arising from rectal or colonic adenocarcinoma
treated with cytoreductive surgery (CRS) hyperthermic
intraperitoneal chemotherapy (HIPEC): Two different diseases. Clin
Transl Oncol. 20:1268–1273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacquet P and Sugarbaker PH:
Peritoneal-plasma barrier. Cancer Treat Res. 82:53–63. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markman M: Intraperitoneal chemotherapy in
the management of malignant disease. Expert Rev Anticancer Ther.
1:142–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Helm CW: The role of hyperthermic
intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Oncologist.
14:683–694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dovern E, de Hingh IH, Verwaal VJ, van
Driel WJ and Nienhuijs SW: Hyperthermic intraperitoneal
chemotherapy added to the treatment of ovarian cancer. a review of
achieved results and complications. Eur J Gynaecol Oncol.
31:256–261. 2010.PubMed/NCBI
|
|
12
|
Zhu Y, Hanna N, Boutros C and Alexander HR
Jr: Assessment of clinical benefit and quality of life in patients
undergoing cytoreduction and Hyperthermic Intraperitoneal
Chemotherapy (HIPEC) for management of peritoneal metastases. J
Gastrointest Oncol. 4:62–71. 2013.PubMed/NCBI
|
|
13
|
van de Vaart PJ, van der Vange N,
Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink
WW, Beijnen JH, Bartelink H and Begg AC: Intraperitoneal cisplatin
with regional hyperthermia in advanced ovarian cancer:
pharmacokinetics and cisplatin-DNA adduct formation in patients and
ovarian cancer cell lines. Eur J Cancer. 34:148–154. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan TD, Cao CQ and Munkholm-Larsen S: A
pharmacological review on intraperitoneal chemotherapy for
peritoneal malignancy. World J Gastrointest Oncol. 2:109–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura E and Howell SB: Analysis of the
cytotoxic interaction between cisplatin and hyperthermia in a human
ovarian carcinoma cell line. Cancer Chemother Pharmacol.
32:419–424. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sukovas A, Cesna V, Jasukaitiene A,
Barauskas G, Nadisauskiene RJ, Dambrauskas Z, Paskauskas S and
Gulbinas A: Response of OVCAR-3 cells to cisplatin and
hyperthermia: does hyperthermia really matter? Anticancer Res.
37:5011–5018. 2017.PubMed/NCBI
|
|
17
|
Leung AW, Hung SS, Backstrom I, Ricaurte
D, Kwok B, Poon S, McKinney S, Segovia R, Rawji J, Qadir MA, et al:
Combined use of gene expression modeling and siRNA screening
identifies genes and pathways which enhance the activity of
cisplatin when added at no effect levels to non-small cell lung
cancer cells in vitro. PLoS One. 11:e01506752016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skowron MA, Niegisch G, Albrecht P, van
Koeveringe G, Romano A, Albers P, Schulz WA and Hoffmann MJ:
Various mechanisms involve the nuclear factor (erythroid-derived
2)-like (NRF2) to achieve cytoprotection in long-term
cisplatin-treated urothelial carcinoma cell lines. Int J Mol Sci.
18(pii): E16802017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar S, Stokes J III, Singh UP, Scissum
Gunn K, Acharya A, Manne U and Mishra M: Targeting Hsp70: a
possible therapy for cancer. Cancer Lett. 374:156–166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thul PJ, Åkesson L, Wiking M, Mahdessian
D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM,
et al: A subcellular map of the human proteome. Science. 356(pii):
eaal33212017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rushworth SA and O'Connell MA: Haem
oxygenase-1 in inflammation. Biochem Soc Trans. 32:1093–1094. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nemmiche S, Chabane-Sari D, Kadri M and
Guiraud P: Cadmium-induced apoptosis in the BJAB human B cell line:
involvement of PKC/ERK1/2/JNK signaling pathways in HO-1
expression. Toxicology. 300:103–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibahara S, Müller RM and Taguchi H:
Transcriptional control of rat heme oxygenase by heat shock. J Biol
Chem. 262:12889–12892. 1987.PubMed/NCBI
|
|
24
|
Vile GF, Basu-Modak S, Waltner C and
Tyrrell RM: Heme oxygenase 1 mediates an adaptive response to
oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA.
91:2607–2610. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang RC, Chang CY, Lu TS and Chen SC:
Effects of hyperthermia pretreatment on expression of heme
oxygenase-1 and nitric oxide synthase in rats subjected to
experimental anaphylactic shock. Chin J Physiol. 48:193–199.
2005.PubMed/NCBI
|
|
26
|
Berberat PO, Dambrauskas Z, Gulbinas A,
Giese T, Giese N, Künzli B, Autschbach F, Meuer S, Büchler MW and
Friess H: Inhibition of heme oxygenase-1 increases responsiveness
of pancreatic cancer cells to anticancer treatment. Clin Cancer
Res. 11:3790–3798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ke N, Wang X, Xu X and Abassi YA: The
xCELLigence system for real-time and label-free monitoring of cell
viability. Methods Mol Biol. 740:33–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang LY, Mok KT, Liu SI, Tsai CC, Wang BW,
Chen IS, Chen YC, Chang BM and Chou NH: Intraoperative hyperthermic
intraperitoneal chemotherapy as adjuvant chemotherapy for advanced
gastric cancer patients with serosal invasion. J Chin Med Assoc.
76:425–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halkia E and Spiliotis J: The role of
cytoreductive surgery and HIPEC in epithelial ovarian cancer. J
BUON. 20 (Suppl 1):S12–S28. 2015.PubMed/NCBI
|
|
31
|
Cesna V, Sukovas A, Jasukaitiene A,
Naginiene R, Barauskas G, Dambrauskas Z, Paskauskas S and Gulbinas
A: Narrow line between benefit and harm: additivity of hyperthermia
to cisplatin cytotoxicity in different gastrointestinal cancer
cells. World J Gastroenterol. 24:1072–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papatheodorou I, Fonseca NA, Keays M, Tang
YA, Barrera E, Bazant W, Burke M, Füllgrabe A, Fuentes AM, George
N, et al: Expression Atlas: gene and protein expression across
multiple studies and organisms. Nucleic Acids Res. 46(D1):
D246–D251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nitti M, Piras S, Marinari UM, Moretta L,
Pronzato MA and Furfaro AL: HO-1 induction in cancer progression: a
matter of cell adaptation. Antioxidants (Basel). 6(pii): E292017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chau LY: Heme oxygenase-1: Emerging target
of cancer therapy. J Biomed Sci. 22:222015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang TY, Liu CL, Chen MJ, Lee JJ, Pun PC
and Cheng SP: Expression of haem oxygenase-1 correlates with tumour
aggressiveness and BRAF V600E expression in thyroid cancer.
Histopathology. 66:447–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loboda A, Jozkowicz A and Dulak J: HO-1/CO
system in tumor growth, angiogenesis and metabolism-Targeting HO-1
as an anti-tumor therapy. Vascul Pharmacol. 74:11–22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Was H, Dulak J and Jozkowicz A: Heme
oxygenase-1 in tumor biology and therapy. Curr Drug Targets.
11:1551–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Becker JC, Fukui H, Imai Y, Sekikawa A,
Kimura T, Yamagishi H, Yoshitake N, Pohle T, Domschke W and
Fujimori T: Colonic expression of heme oxygenase-1 is associated
with a better long-term survival in patients with colorectal
cancer. Scand J Gastroenterol. 42:852–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goodman AI, Choudhury M, da Silva JL,
Schwartzman ML and Abraham NG: Overexpression of the heme oxygenase
gene in renal cell carcinoma. Proc Soc Exp Biol Med. 214:54–61.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maines MD and Abrahamsson PA: Expression
of heme oxygenase-1 (HSP32) in human prostate: normal,
hyperplastic, and tumor tissue distribution. Urology. 47:727–733.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv X, Song DM, Niu YH and Wang BS:
Inhibition of heme oxygenase-1 enhances the chemosensitivity of
laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis.
21:489–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeon WK, Hong HY, Seo WC, Lim KH, Lee HY,
Kim WJ, Song SY and Kim BC: Smad7 sensitizes A549 lung cancer cells
to cisplatin-induced apoptosis through heme oxygenase-1 inhibition.
Biochem Biophys Res Commun. 420:288–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gozzelino R, Jeney V and Soares MP:
Mechanisms of cell protection by heme oxygenase-1. Annu Rev
Pharmacol Toxicol. 50:323–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ewing JF, Haber SN and Maines MD: Normal
and heat-induced patterns of expression of heme oxygenase-1 (HSP32)
in rat brain: Hyperthermia causes rapid induction of mRNA and
protein. J Neurochem. 58:1140–1149. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi AM and Alam J: Heme oxygenase-1:
Function, regulation, and implication of a novel stress-inducible
protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol.
15:9–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao Z, Xu Y, Lu J, Xue J and Liu P: High
expression of HO-1 predicts poor prognosis of ovarian cancer
patients and promotes proliferation and aggressiveness of ovarian
cancer cells. Clin Transl Oncol. 20:491–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Doi K, Akaike T, Fujii S, Tanaka S, Ikebe
N, Beppu T, Shibahara S, Ogawa M and Maeda H: Induction of haem
oxygenase-1 nitric oxide and ischaemia in experimental solid
tumours and implications for tumour growth. Br J Cancer.
80:1945–1954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nuhn P, Künzli BM, Hennig R, Mitkus T,
Ramanauskas T, Nobiling R, Meuer SC, Friess H and Berberat PO: Heme
oxygenase-1 and its metabolites affect pancreatic tumor growth in
vivo. Mol Cancer. 8:372009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kongpetch S, Kukongviriyapan V, Prawan A,
Senggunprai L, Kukongviriyapan U and Buranrat B: Crucial role of
heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to
chemotherapeutic agents. PLoS One. 7:e349942012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gueron G, Giudice J, Valacco P, Paez A,
Elguero B, Toscani M, Jaworski F, Leskow FC, Cotignola J, Marti M,
et al: Heme-oxygenase-1 implications in cell morphology and the
adhesive behavior of prostate cancer cells. Oncotarget.
5:4087–4102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barry MA, Behnke CA and Eastman A:
Activation of programmed cell death (apoptosis) by cisplatin, other
anticancer drugs, toxins and hyperthermia. Biochem Pharmacol.
40:2353–2362. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Geske FJ, Lieberman R, Strange R and
Gerschenson LE: Early stages of p53-induced apoptosis are
reversible. Cell Death Differ. 8:182–191. 2001. View Article : Google Scholar : PubMed/NCBI
|