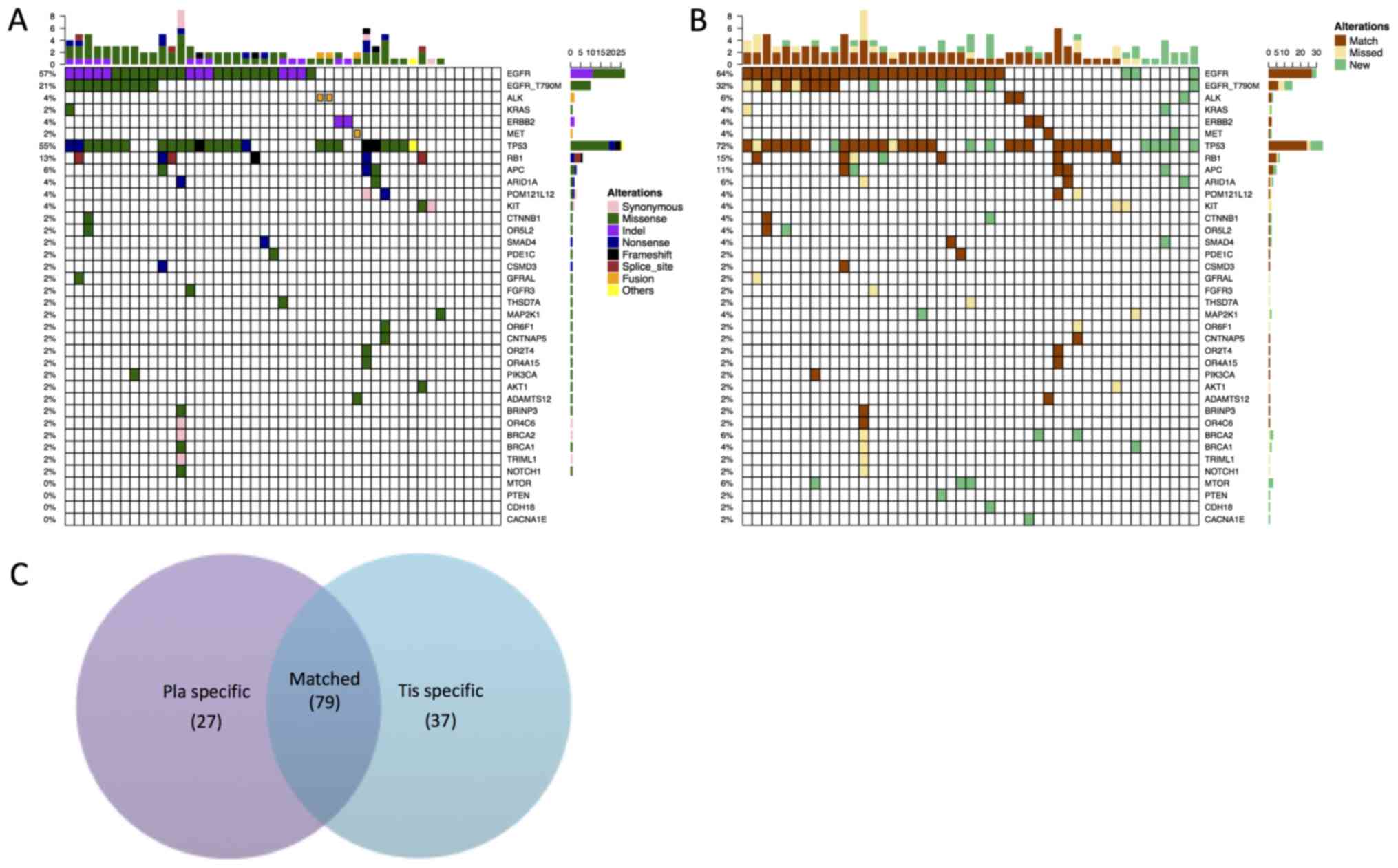
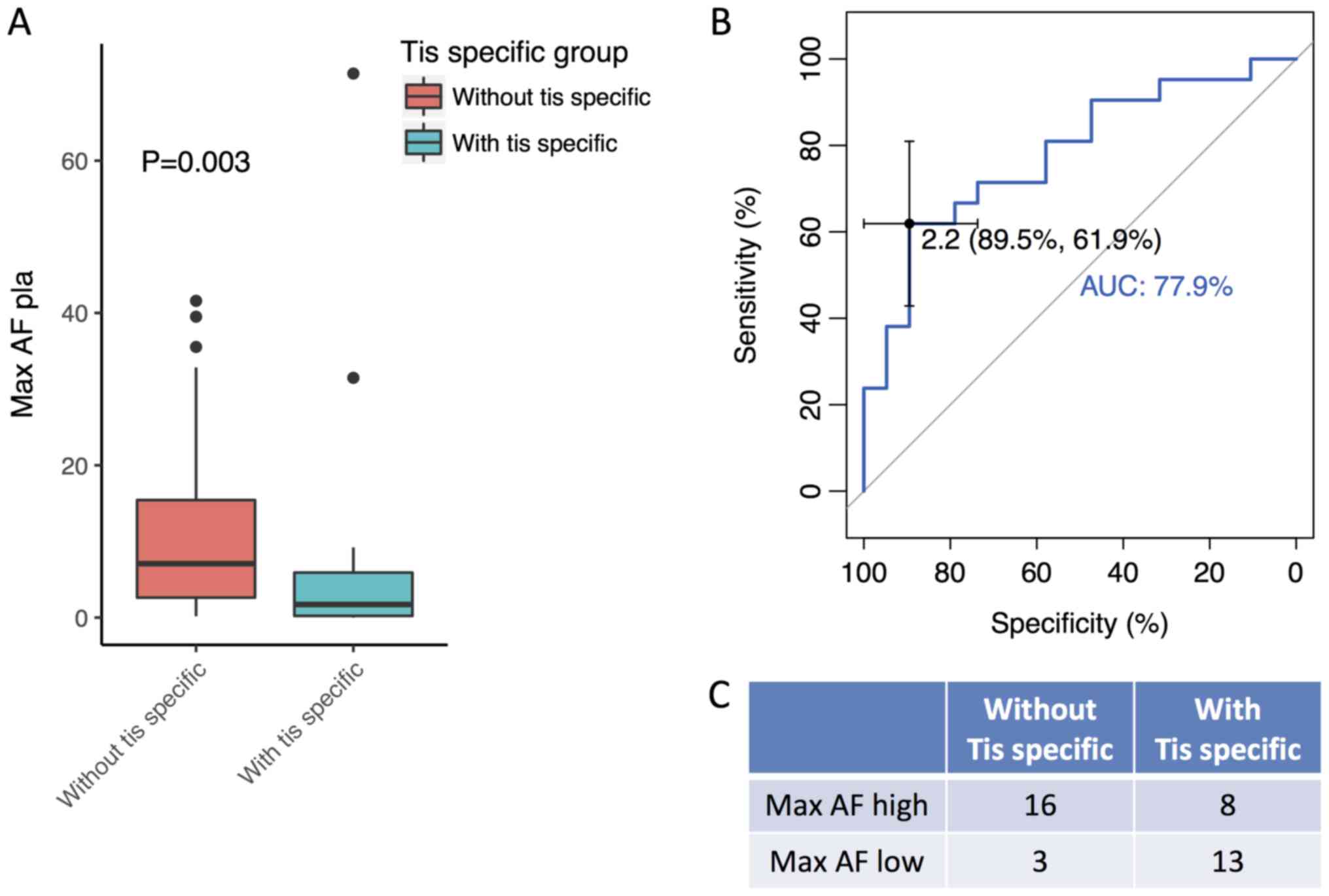
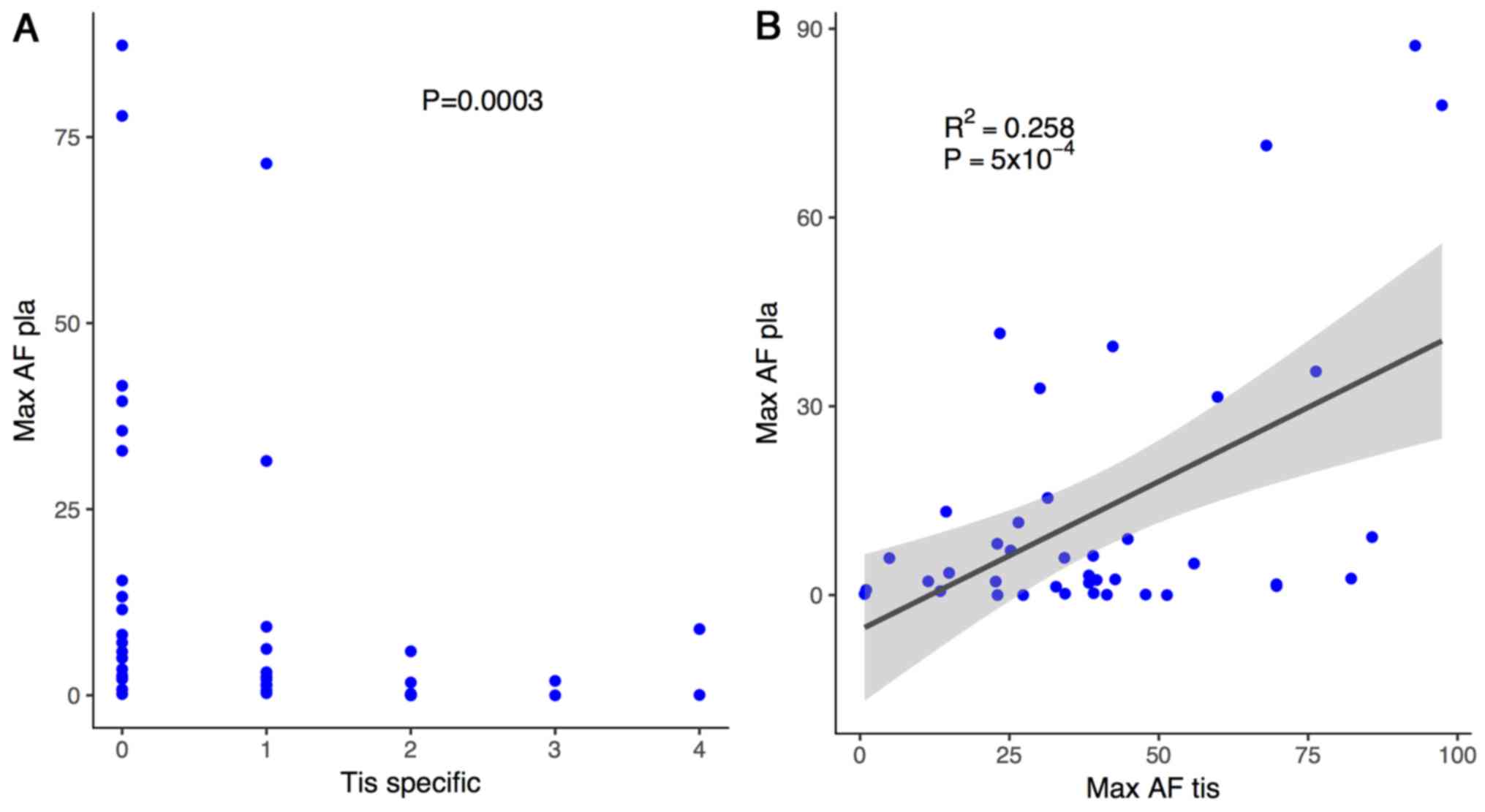
|
1
|
Morash M, Mitchell H, Beltran H, Elemento
O and Pathak J: The role of next-generation sequencing in precision
medicine: A review of outcomes in oncology. J Pers Med. 8:E302018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziogas DE, Kyrochristos ID and Roukos DH:
Next-generation sequencing: From conventional applications to
breakthrough genomic analyses and precision oncology. Expert Rev
Med Devices. 15:1–3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horak P, Fröhling S and Glimm H:
Integrating next-generation sequencing into clinical oncology:
Strategies, promises and pitfalls. ESMO Open. 1:e0000942016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazières J, Zalcman G, Crinò L, Biondani
P, Barlesi F, Filleron T, Dingemans AM, Léna H, Monnet I,
Rothschild SI, et al: Crizotinib therapy for advanced lung
adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1
cohort. J Clin Oncol. 33:992–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohtsuka K, Ohnishi H, Furuyashiki G,
Nogami H, Koshiishi Y, Ooide A, Matsushima S, Watanabe T and Goya
T: Clinico-pathological and biological significance of tyrosine
kinase domain gene mutations and overexpression of epidermal growth
factor receptor for lung adenocarcinoma. J Thorac Oncol. 1:787–795.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan CS, Gilligan D and Pacey S: Treatment
approaches for EGFR-inhibitor-resistant patients with
non-small-cell lung cancer. Lancet Oncol. 16:e447–e459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garraway LA: Genomics-driven oncology:
Framework for an emerging paradigm. J Clin Oncol. 31:1806–1814.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HK, Ku BM, Lee H, Heo MH, Hong JH, Sun
J-M, Lee SH, Ahn JS, Park K and Ahn M-J: The feasibility of using
small biopsy samples from lung cancer for targeted next-generation
sequencing. J Clin Oncol. 35:e20584. 2017. View Article : Google Scholar
|
|
11
|
Sundaresan TK, Sequist LV, Heymach JV,
Riely GJ, Jänne PA, Koch WH, Sullivan JP, Fox DB, Maher R,
Muzikansky A, et al: Detection of T790M, the acquired resistance
EGFR mutation, by tumor biopsy versus noninvasive blood-based
analyses. Clin Cancer Res. 22:1103–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alix-Panabières C and Pantel K: Clinical
applications of circulating tumor cells and circulating tumor DNA
as liquid biopsy. Cancer Discov. 6:479–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brugger W, Triller N, Blasinska-Morawiec
M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R,
Ward C, Mayne K, et al: Prospective molecular marker analyses of
EGFR and KRAS from a randomized, placebo-controlled study of
erlotinib maintenance therapy in advanced non-small-cell lung
cancer. J Clin Oncol. 29:4113–4120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee J, Cho SM, Kim MS, Lee SH, Chung YJ
and Jung SH: Circulating tumor DNA in a breast cancer patient's
plasma represents driver alterations in the tumor tissue. Genomics
Inform. 15:48–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwaederle M, Husain H, Fanta PT,
Piccioni DE, Kesari S, Schwab RB, Patel SP, Harismendy O, Ikeda M,
Parker BA and Kurzrock R: Use of liquid biopsies in clinical
oncology: Pilot experience in 168 patients. Clin Cancer Res.
22:5497–5505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fenizia F, De Luca A, Pasquale R, Sacco A,
Forgione L, Lambiase M, Iannaccone A, Chicchinelli N, Franco R,
Rossi A, et al: EGFR mutations in lung cancer: From tissue testing
to liquid biopsy. Future Oncol. 11:1611–1623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karlovich C, Goldman JW, Sun JM, Mann E,
Sequist LV, Konopa K, Wen W, Angenendt P, Horn L, Spigel D, et al:
Assessment of EGFR mutation status in matched plasma and tumor
tissue of NSCLC patients from a phase I study of rociletinib
(CO-1686). Clin Cancer Res. 22:2386–2395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thress KS, Brant R, Carr TH, Dearden S,
Jenkins S, Brown H, Hammett T, Cantarini M and Barrett JC: EGFR
mutation detection in ctDNA from NSCLC patient plasma: A
cross-platform comparison of leading technologies to support the
clinical development of AZD9291. Lung Cancer. 90:509–515. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii H, Azuma K, Sakai K, Kawahara A,
Yamada K, Tokito T, Okamoto I, Nishio K and Hoshino T: Digital PCR
analysis of plasma cell-free DNA for non-invasive detection of drug
resistance mechanisms in EGFR mutant NSCLC: Correlation with paired
tumor samples. Oncotarget. 6:30850–30858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chai YJ, Song J, Kang J, Woo JW, Song RY,
Kwon H, Kim SJ, Choi JY and Lee KE: A comparative study of
postoperative pain for open thyroidectomy versus bilateral
axillo-breast approach robotic thyroidectomy using a self-reporting
application for iPad. Ann Surg Treat Res. 90:239–245. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han JY, Lee KH, Kim SW, Min YJ, Cho E, Lee
Y, Lee SH, Kim HY, Lee GK, Nam BH, et al: A Phase II study of
poziotinib in patients with epidermal growth factor receptor
(EGFR)-mutant lung adenocarcinoma who have acquired resistance to
EGFR-tyrosine kinase inhibitors. Cancer Res Treat. 49:10–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahama T, Sakai K, Takeda M, Azuma K,
Hida T, Hirabayashi M, Oguri T, Tanaka H, Ebi N, Sawa T, et al:
Detection of the T790M mutation of EGFR in plasma of advanced
non-small cell lung cancer patients with acquired resistance to
tyrosine kinase inhibitors (West Japan oncology group 8014LTR
study). Oncotarget. 7:58492–58499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Douillard JY, Ostoros G, Cobo M, Ciuleanu
T, McCormack R, Webster A and Milenkova T: First-line gefitinib in
caucasian EGFR mutation-positive NSCLC patients: A phase-IV,
open-label, single-arm study. Br J Cancer. 110:55–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perdigones N and Murtaza M: Capturing
tumor heterogeneity and clonal evolution in solid cancers using
circulating tumor DNA analysis. Pharmacol Ther. 174:22–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in advanced non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheung AH, Chow C and To KF: Latest
development of liquid biopsy. J Thorac Dis. 10 (Suppl
14):S1645–S1651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao X, Zhang Z, Zheng X, Xie F, Duan F,
Jiang L, Chuai S, Han-Zhang H, Han B and Sun J: Capture-based
targeted ultradeep sequencing in paired tissue and plasma samples
demonstrates differential subclonal ctDNA-releasing capability in
advanced lung cancer. J Thorac Oncol. 12:663–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crowley E, Di Nicolantonio F, Loupakis F
and Bardelli A: Liquid biopsy: Monitoring cancer-genetics in the
blood. Nat Rev Clin Oncol. 10:472–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Belchis DA, Tseng LH, Gniadek T, Haley L,
Lokhandwala P, Illei P, Gocke CD, Forde P, Brahmer J, Askin FB, et
al: Heterogeneity of resistance mutations detectable by
nextgeneration sequencing in TKI-treated lung adenocarcinoma.
Oncotarget. 7:45237–45248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chabon JJ, Simmons AD, Lovejoy AF,
Esfahani MS, Newman AM, Haringsma HJ, Kurtz DM, Stehr H, Scherer F,
Karlovich CA, et al: Circulating tumour DNA profiling reveals
heterogeneity of EGFR inhibitor resistance mechanisms in lung
cancer patients. Nat Commun. 7:118152016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morii E: Heterogeneity of tumor cells in
terms of cancer-initiating cells. J Toxicol Pathol. 30:1–6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rachiglio AM, Esposito Abate R, Sacco A,
Pasquale R, Fenizia F, Lambiase M, Morabito A, Montanino A, Rocco
G, Romano C, et al: Limits and potential of targeted sequencing
analysis of liquid biopsy in patients with lung and colon
carcinoma. Oncotarget. 7:66595–66605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao C, Qiu LX, Liao RY, Du FB, Ding H,
Yang WC, Li J and Chen Q: KRAS mutations and resistance to
EGFR-TKIs treatment in patients with non-small cell lung cancer: A
meta-analysis of 22 studies. Lung Cancer. 69:272–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Metro G, Chiari R, Duranti S, Siggillino
A, Fischer MJ, Giannarelli D, Ludovini V, Bennati C, Marcomigni L,
Baldi A, et al: Impact of specific mutant KRAS on clinical outcome
of EGFR-TKI-treated advanced non-small cell lung cancer patients
with an EGFR wild type genotype. Lung Cancer. 78:81–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bria E, Pilotto S, Amato E, Fassan M,
Novello S, Peretti U, Vavalà T, Kinspergher S, Righi L, Santo A, et
al: Molecular heterogeneity assessment by next-generation
sequencing and response to gefitinib of EGFR mutant advanced lung
adenocarcinoma. Oncotarget. 6:12783–12795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jung A and Kirchner T: Liquid biopsy in
tumor genetic diagnosis. Dtsch Arztebl Int. 115:169–174.
2018.PubMed/NCBI
|
|
39
|
Hiley C, de Bruin EC, McGranahan N and
Swanton C: Deciphering intratumor heterogeneity and temporal
acquisition of driver events to refine precision medicine. Genome
Biol. 15:4532014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yates LR, Gerstung M, Knappskog S, Desmedt
C, Gundem G, Van Loo P, Aas T, Alexandrov LB, Larsimont D, Davies
H, et al: Subclonal diversification of primary breast cancer
revealed by multiregion sequencing. Nat Med. 21:751–759. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Fujimoto J, Zhang J, Wedge DC,
Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, et al: Intratumor
heterogeneity in localized lung adenocarcinomas delineated by
multiregion sequencing. Science. 346:256–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taniguchi K, Okami J, Kodama K,
Higashiyama M and Kato K: Intratumor heterogeneity of epidermal
growth factor receptor mutations in lung cancer and its correlation
to the response to gefitinib. Cancer Sci. 99:929–935. 2008.
View Article : Google Scholar : PubMed/NCBI
|